Dietary Polyphenol Intake Is Associated with Biological Aging, a Novel Predictor of Cardiovascular Disease: Cross-Sectional Findings from the Moli-Sani Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Computation of Biological Age
2.3. Dietary Assessment
2.4. Ascertainment of Risk Factors
2.5. Statistical Analysis
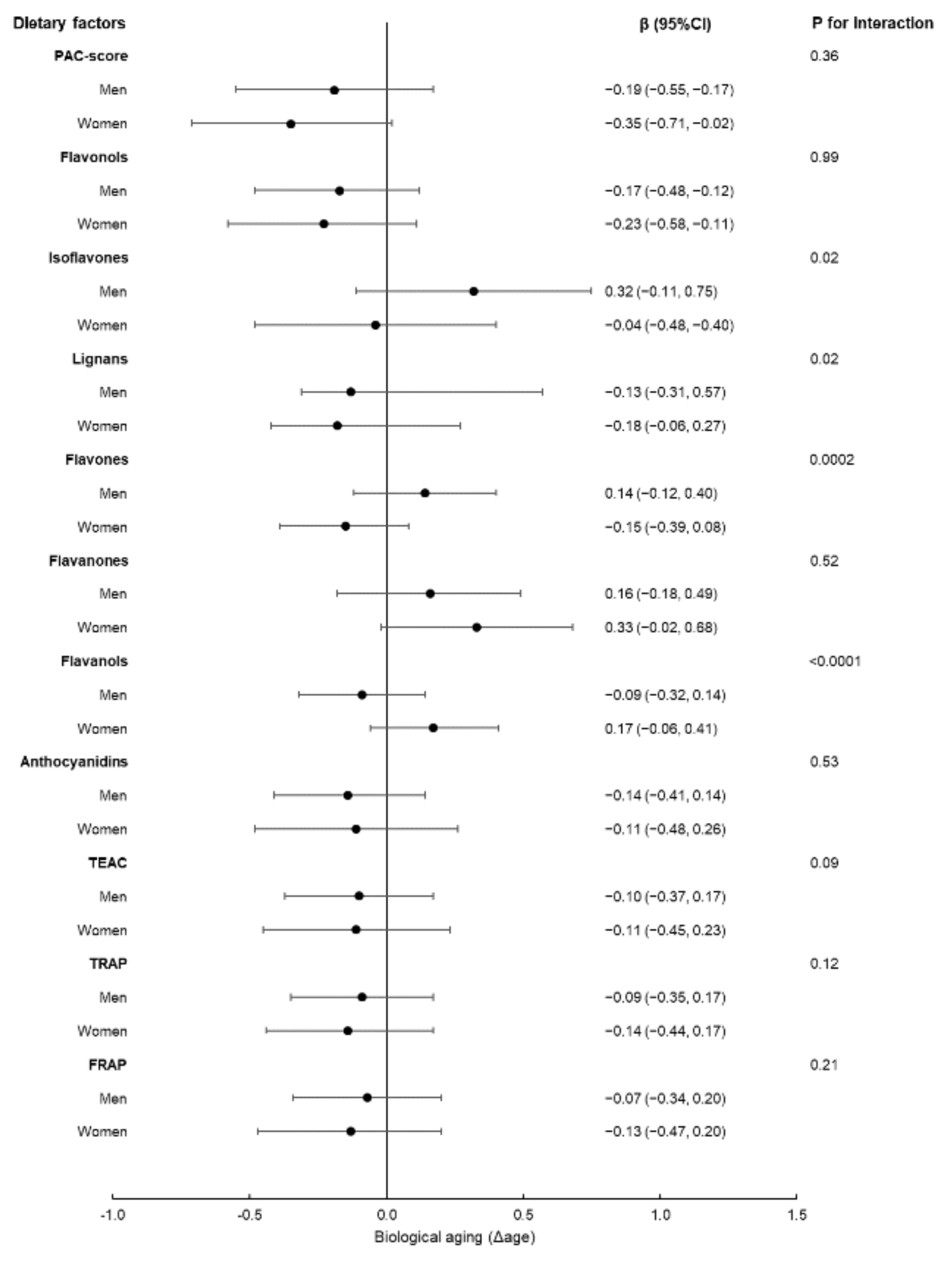
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Life Expectancy. 2016. Available online: http://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends/en/ (accessed on 9 November 2017).
- Karasik, D.; Demissie, S.; Cupples, L.A.; Kiel, D.P. Disentangling the genetic determinants of human aging: Biological age as an alternative to the use of survival measures. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2005, 60, 574–587. [Google Scholar] [CrossRef]
- Cohen, J.E. Human Population: The Next Half Century. Science 2003, 302, 1172–1175. [Google Scholar] [CrossRef]
- De Lucia, C.; Murphy, T.; Steves, C.J.; Dobson, R.J.B.; Proitsi, P.; Thuret, S. Lifestyle mediates the role of nutrient-sensing pathways in cognitive aging: Cellular and epidemiological evidence. Commun. Biol. 2020, 3, 1–17. [Google Scholar] [CrossRef]
- Han, K.-T.; Kim, D.W.; Kim, S.J.; Kim, S.J. Biological Age Is Associated with the Active Use of Nutrition Data. Int. J. Environ. Res. Public Health 2018, 15, 2431. [Google Scholar] [CrossRef] [PubMed]
- Finkel, D.; Whitfield, K.; McGue, M. Genetic and Environmental Influences on Functional Age: A Twin Study. J. Gerontol. Ser. B 1995, 50, P104–P113. [Google Scholar] [CrossRef]
- Gialluisi, A.; Di Castelnuovo, A.; Donati, M.B.; De Gaetano, G.; Iacoviello, L.; the Moli-sani Study Investigators. Machine Learning Approaches for the Estimation of Biological Aging: The Road Ahead for Population Studies. Front. Med. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Mamoshina, P.; Kochetov, K.; Putin, E.; Cortese, F.; Aliper, A.; Lee, W.-S.; Ahn, S.-M.; Uhn, L.; Skjodt, N.; Kovalchuk, O.; et al. Population Specific Biomarkers of Human Aging: A Big Data Study Using South Korean, Canadian, and Eastern European Patient Populations. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1482–1490. [Google Scholar] [CrossRef]
- Klemera, P.; Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 2006, 127, 240–248. [Google Scholar] [CrossRef]
- Fiorito, G.; Polidoro, S.; Dugue, P.-A.; Kivimaki, M.; Ponzi, E.; Matullo, G.; Guarrera, S.; Assumma, M.B.; Georgiadis, P.; Kyrtopoulos, S.A.; et al. Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Park, J.; Cho, B.; Kwon, H.; Lee, C. Developing a biological age assessment equation using principal component analysis and clinical biomarkers of aging in Korean men. Arch. Gerontol. Geriatr. 2009, 49, 7–12. [Google Scholar] [CrossRef]
- Cole, J.H.; Franke, K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 2017, 40, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately Than Chronological Age? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 667–674. [Google Scholar] [CrossRef]
- Gialluisi, A.; Di Castelnuovo, A.; Costanzo, S.; Bonaccio, M.; Magnacca, S.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Capobianco, E.; et al. Exploring domains, clinical implications and environmental asso-ciations of a deep learning marker of biological ageing. medRxiv 2021. [Google Scholar] [CrossRef]
- Levine, M.; Crimmins, E. Evidence of accelerated aging among African Americans and its implications for mortality. Soc. Sci. Med. 2014, 118, 27–32. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, Y.; Cho, E.R.; Jee, S.H. Biological age as a useful index to predict seventeen-year survival and mortality in Koreans. BMC Geriatr. 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Mamoshina, P.; Kochetov, K.; Cortese, F.; Kovalchuk, A.; Aliper, A.; Putin, E.; Scheibye-Knudsen, M.; Cantor, C.R.; Skjodt, N.M.; Kovalchuk, O.; et al. Blood Biochemistry Analysis to Detect Smoking Status and Quantify Accelerated Aging in Smokers. Sci. Rep. 2019, 9, 142. [Google Scholar] [CrossRef]
- Pyrkov, T.V.; Fedichev, P.O. Biological age is a universal marker of aging, stress, and frailty. bioRxiv 2019. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review. Adv. Nutr. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- García-Gavilán, J.; Bulló, M.; Canudas, S.; Martínez-González, M.; Estruch, R.; Giardina, S.; Fitó, M.; Corella, D.; Ros, E.; Salas-Salvadó, J. Extra virgin olive oil consumption reduces the risk of osteoporotic fractures in the PREDIMED trial. Clin. Nutr. 2018, 37, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X.; Meng, S.; Fung, T.; Chan, A.T.; Liang, G.; Giovannucci, E.; De Vivo, I.; Lee, J.H.; Nan, H. Fruit and vegetable consumption, cigarette smoke, and leukocyte mitochondrial DNA copy number. Am. J. Clin. Nutr. 2019, 109, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Meinilä, J.; Perälä, M.-M.; Kautiainen, H.; Männistö, S.; Kanerva, N.; Shivappa, N.; Hébert, J.R.; Iozzo, P.; Guzzardi, M.A.; Eriksson, J.G. Healthy diets and telomere length and attrition during a 10-year follow-up. Eur. J. Clin. Nutr. 2019, 73, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Molinuevo, J.-L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10, S296–S303. [Google Scholar] [CrossRef]
- Brighenti, F.; Valtueña, S.; Pellegrini, N.; Ardigò, D.; Del Rio, D.; Salvatore, S.; Piatti, P.; Serafini, M.; Zavaroni, I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br. J. Nutr. 2005, 93, 619–625. [Google Scholar] [CrossRef]
- Iacoviello, L.; Bonanni, A.; Costanzo, S.; De Curtis, A.; Di Castelnuovo, A.; Olivieri, M.; Zito, F.; Donati, M.B.; de Gaetano, G. The Moli-Sani Project, a randomized, prospective cohort study in the Molise region in Italy; design, rationale and objectives. Ital. J. Public Health 2007, 4, 110–118. [Google Scholar] [CrossRef]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26, S152–S160. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian Epic Cohorts: Presentation of Data and Methodological Issues. Tumori J. 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Italian Food Composition Tables. Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione. Available online: http://www.inran.it/646/tabelle_di_composizione_degli_alimenti. html (accessed on 1 May 2015).
- Di Giuseppe, R.; Arcari, A.; Serafini, M.; Di Castelnuovo, A.; Zito, F.; De Curtis, A.; Sieri, S.; Krogh, V.; Pellegrini, N.; Schünemann, H.J.; et al. Total dietary antioxidant capacity and lung function in an Italian population: A favorable role in premenopausal/never smoker women. Eur. J. Clin. Nutr. 2011, 66, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pounis, G.D.; Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Persichillo, M.; Krogh, V.; Donati, M.B.; De Gaetano, G.; Iacoviello, L. Flavonoid and lignan intake in a Mediterranean population: Proposal for a holistic approach in polyphenol dietary analysis, the Moli-sani Study. Eur. J. Clin. Nutr. 2015, 70, 338–345. [Google Scholar] [CrossRef]
- BioActive Substances in Food Information System, eBASIS. Eurofir. Available online: http://ebasis.eurofir.org/Default.asp (accessed on 1 March 2015).
- Pellegrini, N.; Salvatore, S.; Valtueña, S.; Bedogni, G.; Porrini, M.; Pala, V.; Del Rio, D.; Sieri, S.; Miglio, C.; Krogh, V.; et al. Development and Validation of a Food Frequency Questionnaire for the Assessment of Dietary Total Antioxidant Capacity. J. Nutr. 2007, 137, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.L.; Belsky, D.W.; Knodt, A.R.; Ireland, D.; Melzer, T.R.; Poulton, R.; Ramrakha, S.; Caspi, A.; Moffitt, T.E.; Hariri, A.R. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Hibler, E.; Huang, L.; Andrade, J.; Spring, B. Impact of a diet and activity health promotion intervention on regional patterns of DNA methylation. Clin. Epigenet. 2019, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.; Medina-Remón, A.; Martínez-González, M.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Pounis, G.; Costanzo, S.; Bonaccio, M.; Di Castelnuovo, A.; De Curtis, A.; Ruggiero, E.; Persichillo, M.; Cerletti, C.; Donati, M.B.; De Gaetano, G.; et al. Reduced mortality risk by a polyphenol-rich diet: An analysis from the Moli-sani study. Nutrition 2018, 48, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Sacanella, E.; Badia, E.; Antúnez, E.; Nicolás, J.M.; Fernández-Solá, J.; Rotilio, D.; de Gaetano, G.; Rubin, E.; Urbano-Márquez, A. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: A prospective randomized crossover trial. Atherosclerosis 2004, 175, 117–123. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The Effects of Dietary Polyphenols on Circulating Cardiovascular Disease Biomarkers and Iron Status: A Systematic Review. Nutr. Metab. Insights 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and Aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Stefanadis, C. Longevity and Diet. Myth or pragmatism? Maturitas 2013, 76, 303–307. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrition 2018, 11, 53. [Google Scholar] [CrossRef]
- Barbour, K.E.; Boudreau, R.; Danielson, M.E.; Youk, A.O.; Wactawski-Wende, J.; Greep, N.C.; Lacroix, A.Z.; Jackson, R.D.; Wallace, R.B.; Bauer, D.C.; et al. Inflammatory markers and the risk of hip fracture: The women’s health initiative. J. Bone Miner. Res. 2012, 27, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Quach, A.; Levine, M.E.; Tanaka, T.; Lu, A.T.; Chen, B.H.; Ferrucci, L.; Ritz, B.; Bandinelli, S.; Neuhouser, M.L.; Beasley, J.M.; et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 2017, 9, 419–446. [Google Scholar] [CrossRef]
- Pounis, G.; Costanzo, S.; Di Giuseppe, R.; De Lucia, F.; Santimone, I.; Sciarretta, A.; Barisciano, P.; Persichillo, M.; De Curtis, A.; Zito, F.; et al. Consumption of healthy foods at different content of antioxidant vitamins and phytochemicals and metabolic risk factors for cardiovascular disease in men and women of the Moli–sani study. Eur. J. Clin. Nutr. 2012, 67, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Chisari, E.; Shivappa, N.; Vyas, S. Polyphenol-Rich Foods and Osteoporosis. Curr. Pharm. Des. 2019, 25, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Zhao, L.-G.; Shu, X.-O.; Li, H.-L.; Zhang, W.; Gao, J.; Sun, J.-W.; Zheng, W.; Xiang, Y.-B. Dietary antioxidant vitamins intake and mortality: A report from two cohort studies of Chinese adults in Shanghai. J. Epidemiol. 2017, 27, 89–97. [Google Scholar] [CrossRef]
- Kubota, Y.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Wada, Y.; Inaba, Y.; Tamakoshi, A.; THE JACC Study Group. Dietary Intakes of Antioxidant Vitamins and Mortality from Cardiovascular Disease. Stroke 2011, 42, 1665–1672. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S.; Three-generation Study of Women on Diets and Health Study Groups. Inverse association between dietary habits with high total antioxidant capacity and prevalence of frailty among elderly Japanese women: A multicenter cross-sectional study. J. Nutr. Health Aging 2014, 18. [Google Scholar] [CrossRef]
- García-Calzón, S.; Moleres, A.; Martínez-González, M.A.; Martínez, J.A.; Zalba, G.; Marti, A. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin. Nutr. 2015, 34, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Feskens, E.; Ikram, M.A.; Heijer, T.D.; Vernooij, M.; Van Der Lijn, F.; Hofman, A.; Niessen, W.J.; Breteler, M.M. Total antioxidant capacity of the diet and major neurologic outcomes in older adults. Neurology 2013, 80, 904–910. [Google Scholar] [CrossRef]
- Cipolletti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the Antioxidant Activity of Dietary Polyphenols in Cancer: The Modulation of Estrogen Receptors (ERs) Signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Sánchez, P.; Sanchez-Villegas, A.; Ruano-Rodríguez, C.; Gea, A.; Lamuela-Raventos, R.M.; Estruch, R.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Schroder, H.; et al. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur. J. Nutr. 2015, 55, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Szcześniewska, D.; Pająk, A.; Stepaniak, U.; Drygas, W. Dietary Polyphenol Intake, but Not the Dietary Total Antioxidant Capacity, Is Inversely Related to Cardiovascular Disease in Postmenopausal Polish Women: Results of WOBASZ and WOBASZ II Studies. Oxidative Med. Cell. Longev. 2017, 2017, 5982809. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrition 2019, 11, 1355. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pająk, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017, 56, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Brunelleschi, S.; Steardo, L.; Cuomo, V. Gender differences in drug responses. Pharmacol. Res. 2007, 55, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Spoletini, I.; Vitale, C. Cardiovascular disease in women, is it different to men? The role of sex hormones. Climacteric 2017, 20, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Putin, E.; Mamoshina, P.; Aliper, A.; Korzinkin, M.; Moskalev, A.; Kolosov, A.; Ostrovskiy, A.; Cantor, C.; Vijg, J.; Zhavoronkov, A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging 2016, 8, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Rielli, R.; Demattè, L.; Donfrancesco, C.; Ciccarelli, P.; Caiola, P.D.S.; Dima, F.; Noce, C.L.; Brignoli, O.; Cuffari, A.; et al. CUORE project: Implementation of the 10-year risk score. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 642–649. [Google Scholar] [CrossRef] [PubMed]
| PAC-Score (Quartiles) | ||||||
|---|---|---|---|---|---|---|
| All | Q1 | Q2 | Q3 | Q4 | p Value | |
| N of subjects | 4592 | 1169 | 1109 | 1122 | 1192 | - |
| PAC-score (mean (SD)) | 0.77 (13.2) | −16.2 (5.2) | −4.0 (2.5) | 5.2 (2.8) | 17.7 (4.7) | <0.0001 |
| PAC-score (min, max) | −28, 28 | −28.0, −9.0 | −8.0, 0 | 1.0, 10.0 | 11.0, 28.0 | <0.0001 |
| Chronological age (y; mean (SD)) | 55.6 (11.7) | 55.9 (12.4) | 56.0 (11.8) | 55.0 (11.3) | 55.4 (11.1) | <0.0001 |
| Biological age (y; mean (SD)) | 54.8 (8.6) | 55.5 (9.0) | 55.0 (8.6) | 54.5 (8.3) | 54.2 (8.4) | <0.0001 |
| Δage (y; mean (SD)) | −0.75 (7.72) | −0.4 (7.8) | −1.0 (7.7) | −0.5 (7.7) | −1.2 (7.6) | <0.0001 |
| Men | 2211 (48.2) | 42.0 | 46.9 | 49.3 | 54.3 | <0.0001 |
| Education | 0.06 | |||||
| Up to lower school | 2437 (53.1) | 56.0 | 52.7 | 52.9 | 50.7 | |
| Upper secondary | 1604 (34.9) | 33.4 | 34.8 | 34.8 | 36.7 | |
| Postsecondary education | 551 (12.0) | 10.6 | 12.5 | 12.3 | 12.6 | |
| Unascertained | 1 (0.1) | |||||
| Smoking status | 0.0002 | |||||
| Never smoker | 2294 (50.0) | 53.5 | 48.0 | 49.9 | 48.4 | |
| Current smokers | 1032 (22.5) | 24.7 | 22.4 | 22.7 | 20.0 | |
| Former smoker | 1266 (27.6) | 21.8 | 29.6 | 27.4 | 31.5 | |
| Unascertained | 5 (0.1) | |||||
| Body mass index | 0.01 | |||||
| Normal weight (≤25 kg/m2) | 1217 (26.5) | 28.5 | 27.2 | 27.6 | 22.8 | |
| Overweight (25–30 kg/m2) | 1932 (42.1) | 41.5 | 41.2 | 41.8 | 43.7 | |
| Obese (≥30 kg/m2) | 1443 (31.4) | 30.0 | 31.6 | 30.6 | 33.5 | |
| Unascertained | 4 (0.1) | |||||
| Leisure-time physical activity | <0.0001 | |||||
| <30 min/d | 1589 (34.6) | 40.4 | 36.8 | 31.4 | 30.0 | |
| ≥30 min/d | 3003 (65.4) | 59.6 | 63.2 | 68.6 | 70.0 | |
| Cardiovascular disease | 0.76 | |||||
| No | 4270 (93.0) | 93.0 | 92.0 | 93.6 | 93.4 | |
| Yes | 253 (5.5) | 5.1 | 6.3 | 5.5 | 5.1 | |
| Unascertained | 69 (1.5) | 1.9 | 1.7 | 0.9 | 1.5 | |
| Cancer | 0.96 | |||||
| No | 4425 (96.4) | 95.9 | 96.3 | 96.9 | 96.4 | |
| Yes | 148 (3.2) | 3.4 | 3.2 | 2.8 | 3.5 | |
| Unascertained | 19 (0.4) | 0.7 | 0.5 | 0.4 | 0.1 | |
| Diabetes | 0.42 | |||||
| No | 4305 (93.8) | 94.3 | 94.2 | 92.7 | 93.8 | |
| Yes | 222 (4.8) | 4.5 | 4.3 | 5.6 | 4.9 | |
| Unascertained | 65 (1.4) | 1.2 | 1.4 | 1.7 | 1.3 | |
| Hypertension | 0.12 | |||||
| No | 3232 (70.4) | 69.7 | 69.2 | 70.3 | 72.2 | |
| Yes | 1317 (28.7) | 29.4 | 29.9 | 28.8 | 26.7 | |
| Unascertained | 43 (0.9) | 0.9 | 0.9 | 0.9 | 1.1 | |
| Hyperlipidaemia | 0.74 | |||||
| No | 4187 (91.2) | 92.1 | 89.6 | 91.9 | 91.0 | |
| Yes | 360 (7.8) | 7.0 | 9.2 | 7.1 | 8.0 | |
| Unascertained | 45 (1.0) | 0.9 | 1.2 | 1.0 | 0.9 | |
| Menopausal status | 0.95 | |||||
| No | 965 (40.5) | 41.0 | 39.7 | 41.8 | 40.1 | |
| Yes | 1410 (59.2) | 59.0 | 60.3 | 58.2 | 60.0 | |
| Unascertained | 6 (0.3) | |||||
| Hormone replacement therapy | 0.02 | |||||
| No | 2246 (94.3) | 96.7 | 94.0 | 92.7 | 93.8 | |
| Yes | 135 (5.7) | 3.3 | 6.0 | 7.0 | 6.2 | |
| Polyphenol classes/sub-classes (mg/d) | ||||||
| Flavonols (mean (SD)) | 19.3 (10.4) | 10.3 (4.2) | 15.5 (4.8) | 20.1 (5.9) | 31.0 (10.8) | <0.0001 |
| Isoflavones (mean (SD)) | 25.2 (11.4) | 14.5 (4.4) | 20.8 (3.8) | 26.3 (4.9) | 38.8 (11.3) | <0.0001 |
| Lignans (mean (SD)) | 89.4 (42.2) | 49.2 (14.0) | 72.3 (12.3) | 92.6 (16.0) | 141.8 (41.1) | <0.0001 |
| Flavones (mean (SD)) | 0.8 (0.5) | 0.5 (0.3) | 0.7 (0.4) | 0.9 (0.4) | 1.2 (0.7) | <0.0001 |
| Flavanones (mean (SD)) | 35.1 (17.0) | 19.5 (7.1) | 29.0 (6.5) | 37.4 (9.6) | 54.1 (17.3) | <0.0001 |
| Flavanols (mg/d; mean (SD)) | 73.6 (83.6) | 38.0 (70.3) | 56.5 (52.0) | 81.5 (70.5) | 116.9 (106.9) | <0.0001 |
| Anthocyanidins (mg/d; mean (SD)) | 170.2 (104.4) | 82.4 (42.8) | 135.3 (57.8) | 176.8 (67.2) | 282.7 (107.0) | <0.0001 |
| Total antioxidant capacity | ||||||
| FRAP | 19.0 (8.6) | 15.3 (7.2) | 17.9 (7.7) | 19.9 (8.2) | 22.9 (9.3) | <0.0001 |
| TEAC | 6.3 (2.9) | 4.9 (2.3) | 5.9 (2.5) | 6.6 (2.7) | 7.7 (3.1) | <0.0001 |
| TRAP | 9.2 (4.4) | 7.6 (3.8) | 8.8 (4.0) | 9.6 (4.3) | 10.9 (4.8) | <0.0001 |
| Dietary fiber (g/d) | 20.6 (7.0) | 14.6 (3.6) | 18.3 (3.6) | 21.2 (4.1) | 28.2 (7.0) | <0.0001 |
| Energy intake (kcal/d) | 2113.4 (634.9) | 1773.3 (512.9) | 1998.3 (513.8) | 2181.1 (561.3) | 2490.5 (693.8) | <0.0001 |
| Biological Aging (Δage) | ||||
|---|---|---|---|---|
| Dietary Factors | β (95%CI) 1 | p Value | β (95%CI) 2 | p Value |
| PAC-score | −0.30 (−0.49, −0.12) | 0.001 | −0.27 (−0.52, −0.02) | 0.03 |
| Polyphenol classes/sub-classes (mg/d) | ||||
| Flavonols | −0.28 (−0.47, −0.10) | 0.003 | −0.21 (−0.44, 0.01) | 0.06 |
| Isoflavones | −0.12 (−0.30, 0.06) | 0.18 | 0.13 (−0.17, 0.44) | 0.40 |
| Lignans | −0.17 (−0.35, 0.01) | 0.06 | −0.02 (−0.34, 0.29) | 0.89 |
| Flavones | −0.02 (−0.19, 0.15) | 0.84 | −0.02 (−0.19, 0.16) | 0.84 |
| Flavanones | −0.02 (−0.20, 0.16) | 0.82 | 0.23 (−0.01, 0.47) | 0.06 |
| Flavanols | −0.04 (−0.22, 0.13) | 0.60 | 0.02 (−0.14, 0.19) | 0.78 |
| Anthocyanidins | −0.25 (−0.43, −0.09) | 0.05 | −0.15 (−0.37, 0.07) | 0.17 |
| Total antioxidant capacity | ||||
| FRAP | −0.01 (−0.21, 0.20) | 0.93 | −0.13 (−0.34, 0.07) | 0.20 |
| TEAC | −0.03 (−0.24, 0.17) | 0.74 | −0.14 (−0.34, 0.07) | 0.19 |
| TRAP | −0.01 (−0.20, 0.18) | 0.94 | −0.14 (−0.34, 0.05) | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S.; Gialluisi, A.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Dietary Polyphenol Intake Is Associated with Biological Aging, a Novel Predictor of Cardiovascular Disease: Cross-Sectional Findings from the Moli-Sani Study. Nutrients 2021, 13, 1701. https://doi.org/10.3390/nu13051701
Esposito S, Gialluisi A, Costanzo S, Di Castelnuovo A, Ruggiero E, De Curtis A, Persichillo M, Cerletti C, Donati MB, de Gaetano G, et al. Dietary Polyphenol Intake Is Associated with Biological Aging, a Novel Predictor of Cardiovascular Disease: Cross-Sectional Findings from the Moli-Sani Study. Nutrients. 2021; 13(5):1701. https://doi.org/10.3390/nu13051701
Chicago/Turabian StyleEsposito, Simona, Alessandro Gialluisi, Simona Costanzo, Augusto Di Castelnuovo, Emilia Ruggiero, Amalia De Curtis, Mariarosaria Persichillo, Chiara Cerletti, Maria Benedetta Donati, Giovanni de Gaetano, and et al. 2021. "Dietary Polyphenol Intake Is Associated with Biological Aging, a Novel Predictor of Cardiovascular Disease: Cross-Sectional Findings from the Moli-Sani Study" Nutrients 13, no. 5: 1701. https://doi.org/10.3390/nu13051701
APA StyleEsposito, S., Gialluisi, A., Costanzo, S., Di Castelnuovo, A., Ruggiero, E., De Curtis, A., Persichillo, M., Cerletti, C., Donati, M. B., de Gaetano, G., Iacoviello, L., Bonaccio, M., & on behalf of the Investigators for the Moli-Sani Study. (2021). Dietary Polyphenol Intake Is Associated with Biological Aging, a Novel Predictor of Cardiovascular Disease: Cross-Sectional Findings from the Moli-Sani Study. Nutrients, 13(5), 1701. https://doi.org/10.3390/nu13051701






