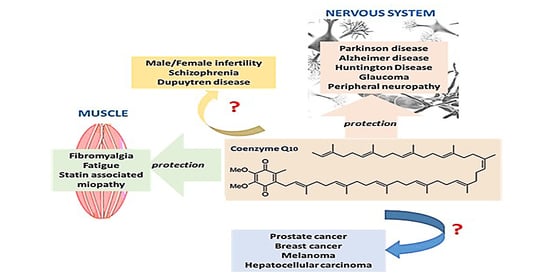
Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases
Abstract
1. Introduction
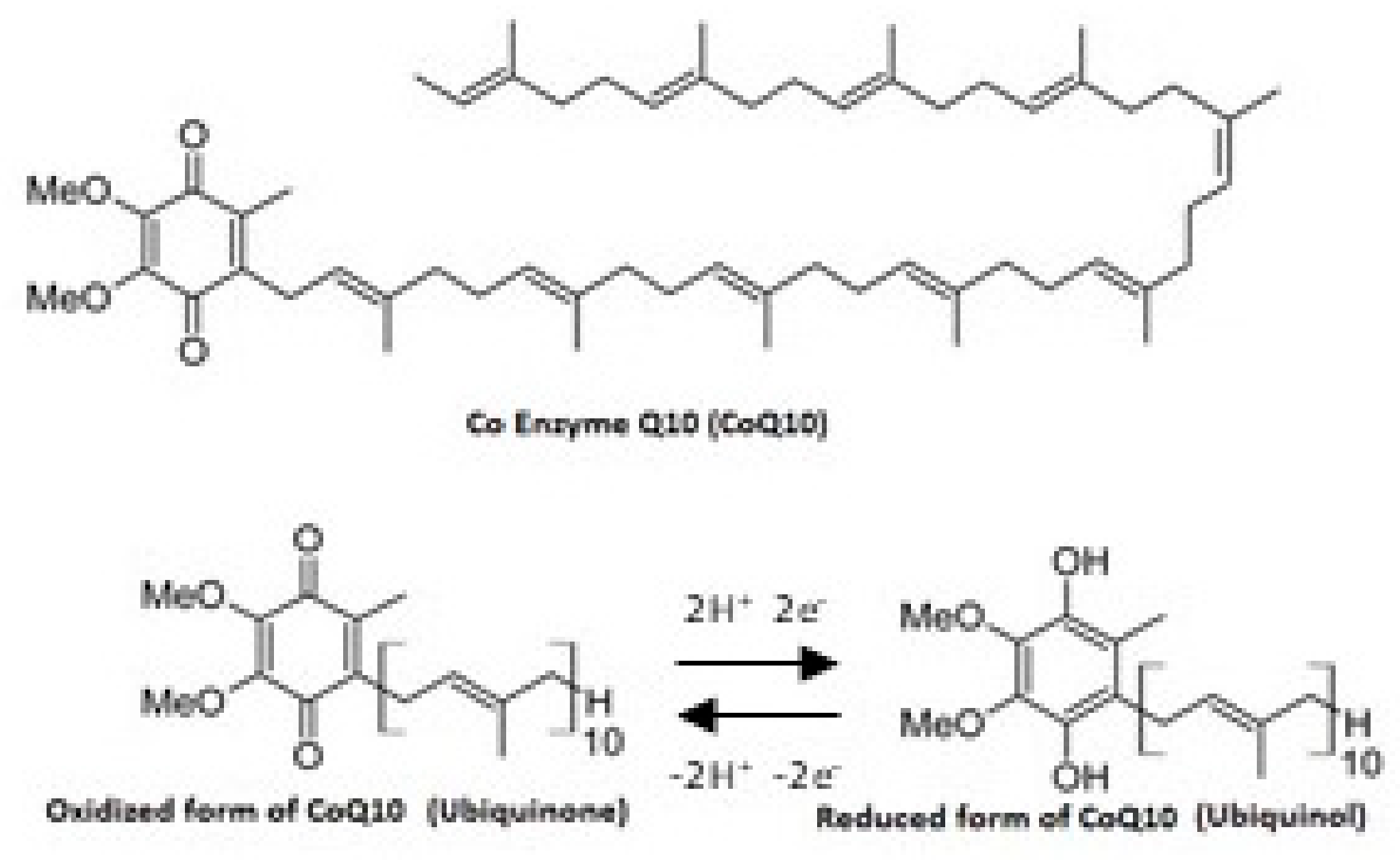
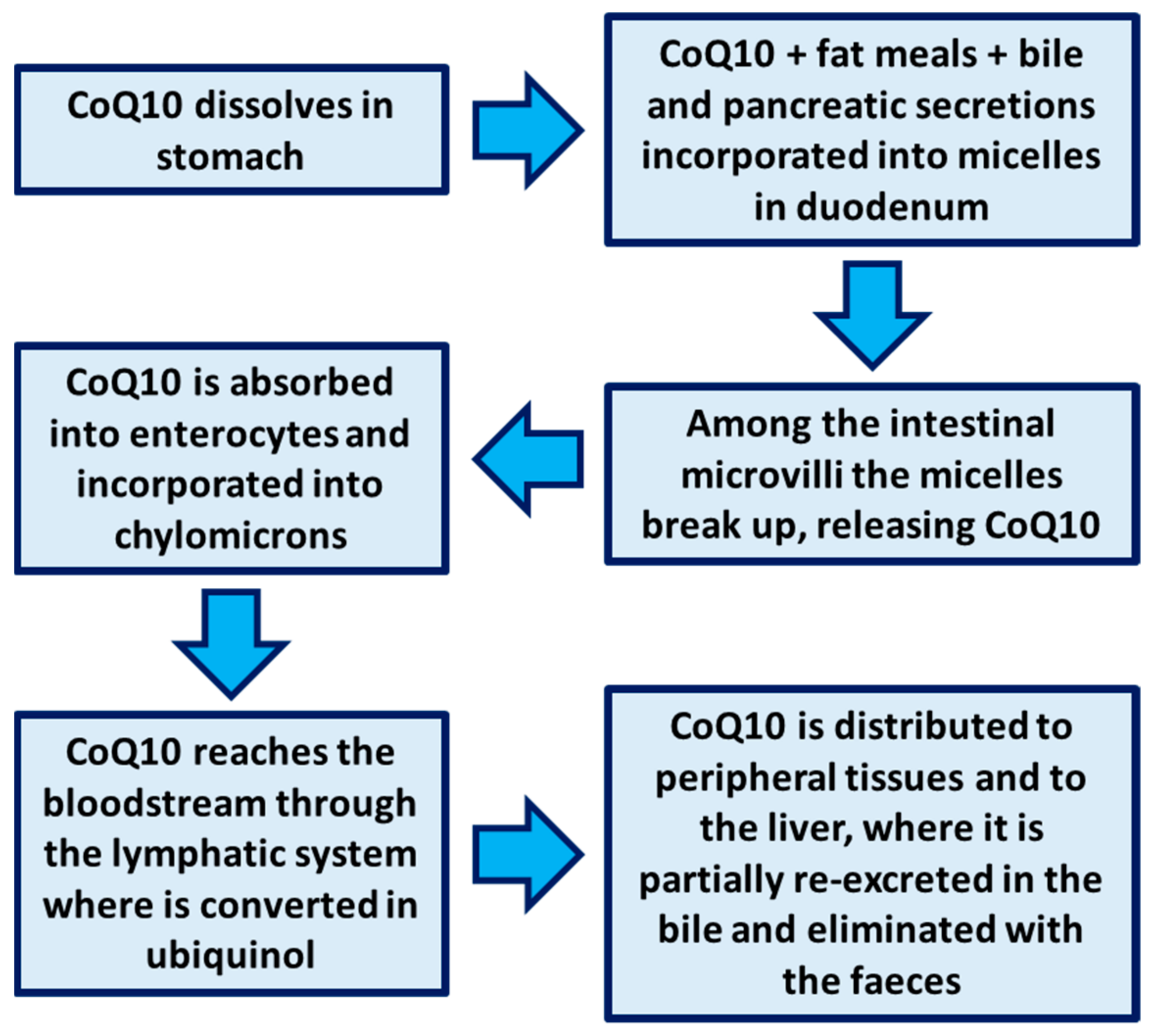
2. Bioavailability of CoQ10
3. Methods
4. Results
4.1. CoQ10 and Migraine
4.2. CoQ10 and Fatigue
4.2.1. CoQ10 in Patients with Chronic Fatigue Syndrome
4.2.2. CoQ10 in Patients with Fibromyalgia
4.2.3. CoQ10 in Patients with Statin-Associated Myopathy
4.2.4. CoQ10 and Fatigue in Healthy Volunteers
4.2.5. CoQ10 and Elite Athletes
4.2.6. CoQ10 in Patients with Other Fatigue-Related Diseases
4.3. CoQ10 and Neurodegenerative Diseases
4.3.1. CoQ10 and Parkinson Disease
4.3.2. CoQ10 and Huntington Disease
4.3.3. CoQ10 and Alzheimer Disease
4.3.4. CoQ10 and MS
4.4. CoQ10 and Neuropathy
4.4.1. CoQ10 and Diabetic Neuropathy
4.4.2. CoQ10 and Glaucoma
4.5. CoQ10 and Cancer
4.5.1. CoQ10 and Breast Cancer
4.5.2. CoQ10 and Hepatocellular Carcinoma (HCC)
4.5.3. CoQ10 and Prostatic Carcinoma
4.5.4. CoQ10 and Melanoma
4.6. CoQ10 and Fertility
4.7. CoQ10 and Dupuytren’s Disease (DD)
4.8. CoQ10 and Schizophrenia
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef]
- Crane, F.L. Biochemical Functions of Coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Haas, R.H. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion 2007, 7, S136–S145. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Yubero-Serrano, E.M.; Villalba, J.M.; Lopez-Miranda, J. Coenzyme Q10: From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2240–2257. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of coenzyme Q10in inflammation and gene expression. BioFactors 2008, 32, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Gonzalez-Guardia, L.; Rangel-Zuñiga, O.; Delgado-Lista, J.; Gutierrez-Mariscal, F.M.; Perez-Martinez, P.; Delgado-Casado, N.; Cruz-Teno, C.; Tinahones, F.J.; Villalba, J.M.; et al. Mediterranean Diet Supplemented With Coenzyme Q10 Modifies the Expression of Proinflammatory and Endoplasmic Reticulum Stress-Related Genes in Elderly Men and Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011, 67, 3–10. [Google Scholar] [CrossRef]
- Shukla, S.; Dubey, K.K. CoQ10 a super-vitamin: Review on application and biosynthesis. 3 Biotech 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Szkopińska, A. Ubiquinone. Biosynthesis of quinone ring and its isoprenoid side chain. Intracellular localization. Acta Biochim. Pol. 2000, 47, 469–480. [Google Scholar] [CrossRef]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1660, 171–199. [Google Scholar] [CrossRef]
- Weber, C.; Bysted, A.; Hłlmer, G. The coenzyme Q10 content of the average Danish diet. Int. J. Vitam. Nutr. Res. 1997, 67, 123–129. [Google Scholar] [PubMed]
- Zhang, Y.; Aberg, F.; Appelkvist, E.L.; Dallner, G.; Ernster, L. Uptake of dietary coenzyme Q supplement is limited in rats. J. Nutr. 1995, 125, 446–453. [Google Scholar] [PubMed]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Vega, A.F.; De La Mata, M.; Pavón, A.D.; De Miguel, M.; Calero, C.P.; Paz, M.V.; Cotán, D.; et al. Coenzyme q10 therapy. Mol. Syndr. 2014, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Appelkvist, E.-L.; Dallner, G.; Ernster, L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992, 295, 230–234. [Google Scholar] [CrossRef]
- Miles, M.V.; Horn, P.S.; Morrison, J.A.; Tang, P.H.; Degrauw, T.; Pesce, A.J. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin. Chim. Acta 2003, 332, 123–132. [Google Scholar] [CrossRef]
- Weis, M.; Mortensen, S.; Rassing, M.; Møller-Sonnergaard, J.; Poulsen, G.; Rasmussen, S. Bioavailability of four oral Coenzyme Q10 formulations in healthy volunteers. Mol. Asp. Med. 1994, 15, s273–s280. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Horn, P.; Miles, L.; Tang, P.; Steele, P.; Degrauw, T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutr. Res. 2002, 22, 919–929. [Google Scholar] [CrossRef]
- Kumar, S.; Rao, R.; Kumar, A.; Mahant, S.; Nanda, S. Novel Carriers for Coenzyme Q10 Delivery. Curr. Drug Deliv. 2016, 13, 1184–1204. [Google Scholar] [CrossRef]
- Hershey, A.D.; Powers, S.W.; Vockell, A.-L.B.; LeCates, S.L.; Ellinor, P.L.; Segers, A.; Burdine, D.; Manning, P.; Kabbouche, M.A. Coenzyme Q10 Deficiency and Response to Supplementation in Pediatric and Adolescent Migraine. Headache J. Head Face Pain 2007, 47, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Tarighat-Esfanjani, A.; Asghari-Jafarabadi, M.; Hashemilar, M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2018, 22, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Effect of coenzyme Q10 supplementation on clinical features of migraine: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Neurosci. 2019, 23, 868–875. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Lu, S.; Huang, W.; Di, W. Efficacy of CoQ10 as supplementation for migraine: A meta-analysis. Acta Neurol. Scand. 2018, 139, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Gaul, C.; Diener, H.C.; Danesch, U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain 2015, 16, 1–8. [Google Scholar] [CrossRef]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Foroushani, A.R.; Ranji-Burachaloo, S.; Djalali, M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Nutr. Neurosci. 2021, 24, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanaka, M.; Yamaguti, K.; Kajimoto, O.; Kuratsune, H.; Watanabe, Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav. Brain Funct. 2011, 7, 1–17. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Mizuma, H.; Ataka, S.; Tahara, T.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition 2008, 24, 293–299. [Google Scholar] [CrossRef]
- Mehrabani, S.; Askari, G.; Miraghajani, M.; Tavakoly, R.; Arab, A. Effect of coenzyme Q10 supplementation on fatigue: A systematic review of interventional studies. Complement. Ther. Med. 2019, 43, 181–187. [Google Scholar] [CrossRef]
- Yancey, J.R.; Thomas, S.M. Chronic fatigue syndrome: Diagnosis and treatment. Am. Fam. Physician 2012, 86, 741–746. [Google Scholar]
- Cooke, M.; Iosia, M.; Buford, T.; Shelmadine, B.; Hudson, G.; Kerksick, C.; Rasmussen, C.; Greenwood, M.; Leutholtz, B.; Willoughby, D.; et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J. Int. Soc. Sports Nutr. 2008, 5, 1–14. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009, 30, 470–476. [Google Scholar] [PubMed]
- Booth, N.E.; Myhill, S.; McLaren-Howard, J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int. J. Clin. Exp. Med. 2012, 5, 208–220. [Google Scholar] [PubMed]
- Castro-Marrero, J.; Cordero, M.D.; Segundo, M.J.; Sáez-Francàs, N.; Calvo, N.; Román-Malo, L.; Aliste, L.; De Sevilla, T.F.; Alegre, J. Does Oral Coenzyme Q10 Plus NADH Supplementation Improve Fatigue and Biochemical Parameters in Chronic Fatigue Syndrome? Antioxid. Redox Signal. 2015, 22, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab. Brain Dis. 2012, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. Clin. J. 2014, 13, 35. [Google Scholar]
- Alegre, J.; Rosés, J.; Javierre, C.; Ruiz-Baqués, A.; Segundo, M.; De Sevilla, T.F. Nicotinamida adenina dinucleótido (NADH) en pacientes con síndrome de fatiga crónica. Clín. Esp. 2010, 210, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Sáez-Francàs, N.; Segundo, M.J.; Calvo, N.; Faro, M.; Aliste, L.; de Sevilla, T.F.; Alegre, J. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome: A randomized, controlled, double-blind trial. Clin. Nutr. 2016, 35, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nojima, J.; Kajimoto, O.; Yamaguti, K.; Nakatomi, Y.; Kuratsune, H.; Watanabe, Y. Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. BioFactors 2016, 42, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Rossi, A.; Consensi, A.; Giacomelli, C.; Bazzichi, L. Role for a water-soluble form of CoQ10 in female subjects affected by fibromyalgia. A preliminary study. Clin. Exp. Rheumatol. 2016, 35, 20–27. [Google Scholar]
- Cordero, M.D.; Santos-García, R.; Bermejo-Jover, D.; Sánchez-Domínguez, B.; Jaramillo-Santos, M.R.; Bullon, P. Coenzyme Q10 in salivary cells correlate with blood cells in Fibromyalgia: Improvement in clinical and biochemical parameter after oral treatment. Clin. Biochem. 2012, 45, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; De Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullón, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcazar, J.A. Can Coenzyme Q10 Improve Clinical and Molecular Parameters in Fibromyalgia? Antioxid. Redox Signal. 2013, 19, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Tsai, K.L.; Chen, L.H.; Chiou, S.H.; Chiou, G.Y.; Chen, Y.C.; Chou, H.Y.; Chen, L.K.; Chen, H.Y.; Chiu, T.H.; Tsai, C.S.; et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011, 55, S227–S240. [Google Scholar] [CrossRef] [PubMed]
- Langsjoen, P.H.; Langsjoen, J.O.; Langsjoen, A.M.; Lucas, L.A. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. BioFactors 2005, 25, 147–152. [Google Scholar] [CrossRef]
- Cordero, M.D.; Cano-García, F.J.; Alcocer-Gómez, E.; De Miguel, M.; Sánchez-Alcázar, J.A. Oxidative Stress Correlates with Headache Symptoms in Fibromyalgia: Coenzyme Q10 Effect on Clinical Improvement. PLoS ONE 2012, 7, e35677. [Google Scholar] [CrossRef]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef]
- Corsini, A. Statin-Related Muscle Complaints: An Underestimated Risk. Cardiovasc. Drugs Ther. 2005, 19, 379–381. [Google Scholar] [CrossRef]
- Deichmann, R.; Lavie, C.; Andrews, S. Coenzyme Q10 and Statin-Induced Mitochondrial Dysfunction. Ochsner J. 2010, 10, 16–21. [Google Scholar]
- Fedacko, J.; Pella, D.; Fedackova, P.; Hänninen, O.; Tuomainen, P.; Jarcuska, P.; Lopuchovsky, T.; Jedlickova, L.; Merkovska, L.; Littarru, G.P. Coenzyme Q10and selenium in statin-associated myopathy treatment. Can. J. Physiol. Pharmacol. 2013, 91, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Gharahdaghi, N.; Shabkhiz, F.; Azarboo, E.; Keyhanian, A. The effects of daily coenzyme Q10 supplementation on VO2max, vVO2max and Intermittent Exercise performance in soccer players. Life Sci. J. 2013, 10, 22–28. [Google Scholar]
- Lee, Y.J.; Cho, W.J.; Kim, J.K.; Lee, D.C. Effects of Coenzyme Q10 on Arterial Stiffness, Metabolic Parameters, and Fatigue in Obese Subjects: A Double-Blind Randomized Controlled Study. J. Med. Food 2011, 14, 386–390. [Google Scholar] [CrossRef]
- Gökbel, H.; Gül, I.; Belviranl, M.; Okudan, N. The Effects Of Coenzyme Q10 Supplementation on Performance During Repeated Bouts of Supramaximal Exercise in Sedentary Men. J. Strength Cond. Res. 2010, 24, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ylikoski, T.; Piirainen, J.; Hanninen, O.; Penttinen, J. The effect of coenzyme Q10 on the exercise performance of cross-country skiers. Mol. Asp. Med. 1997, 18, 283–290. [Google Scholar] [CrossRef]
- Macdonald, J.; Galley, H.; Webster, N. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003, 90, 221–232. [Google Scholar] [CrossRef]
- Kreher, J.B.; Schwartz, J.B. Overtraining Syndrome. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef]
- Bonetti, A.; Solito, F.; Carmosino, G.; Bargossi, A.M.; Fiorella, P.L. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J. Sports Med. Phys. Fit. 2000, 40, 51. [Google Scholar]
- Malm, C.; Svensson, M.; Ekblom, B.; Sjödin, B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol. Scand. 1997, 161, 379–384. [Google Scholar] [CrossRef]
- Stear, S.J.; Castell, L.M.; Burke, L.M.; Jeacocke, N.; Ekblom, B.; Shing, C.; Calder, P.C.; Lewis, N. A-Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance—part 10. Br. J. Sports Med. 2010, 44, 688–690. [Google Scholar] [CrossRef]
- Liao, P.; Zhang, Y.; Liao, Y.; Zheng, N.-J.; Zhang, X. Effects of coenzyme Q10 supplementation on liver mitochondrial function and aerobic capacity in adolescent athletes. J. Appl. Physiol. 2007, 23, 491–494. [Google Scholar]
- Weston, S.B.; Zhou, S.; Weatherby, R.P.; Robson, S.J. Does Exogenous Coenzyme Q10 Affect Aerobic Capacity in Endurance Athletes? Int. J. Sport Nutr. 1997, 7, 197–206. [Google Scholar] [CrossRef]
- Imai, H.; Hayashi, T.; Negawa, T.; Nakamura, K.; Tomida, M.; Koda, K.; Tajima, T.; Koda, Y.; Suda, K.; Era, S. Strenuous Exercise-Induced Change in Redox State of Human Serum Albumin during Intensive Kendo Training. Jpn. J. Physiol. 2002, 52, 135–140. [Google Scholar] [CrossRef]
- Kon, M.; Tanabe, K.; Akimoto, T.; Kimura, F.; Tanimura, Y.; Shimizu, K.; Okamoto, T.; Kono, I. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q10. Br. J. Nutr. 2008, 100, 903–909. [Google Scholar] [CrossRef]
- Shimizu, K.; Kon, M.; Tanimura, Y.; Hanaoka, Y.; Kimura, F.; Akama, T.; Kono, I. Coenzyme Q10 supplementation downregulates the increase of monocytes expressing toll-like receptor 4 in response to 6-day intensive training in kendo athletes. Appl. Physiol. Nutr. Metab. 2015, 40, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Armanfar, M.; Jafari, A.; Dehghan, G.R. Effect of coenzyme Q10 Supplementation on Exercise-Induced Response of Oxidative Stress and Muscle Damage Indicators in Male Runners. Med. J. Islam. Repub. Iran 2015, 17, 202. [Google Scholar] [CrossRef]
- Emami, A. The Impact of Pre-Cooling and CoQ10Supplementation on Mediators of Inflammatory Cytokines in Elite Swimmers. Nutr. Cancer 2020, 72, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.; Erman, A.; Ben-Gal, T.; Dvir, D.; Georghiou, G.P.; Stamler, A.; Vered, Y.; Vidne, B.A.; Aravot, D. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: A randomized, placebo-controlled study. Clin. Cardiol. 2004, 27, 295–299. [Google Scholar] [CrossRef]
- Peel, M.M.; Cooke, M.; Lewis-Peel, H.J.; Lea, R.A.; Moyle, W. A randomized controlled trial of coenzyme Q10 for fatigue in the late-onset sequelae of poliomyelitis. Complement. Ther. Med. 2015, 23, 789–793. [Google Scholar] [CrossRef]
- Lesser, G.J.; Case, D.; Stark, N.; Williford, S.; Giguere, J.; Garino, L.A.; Naughton, M.J.; Vitolins, M.Z.; Lively, M.O.; Shaw, E.G. A Randomized, Double-Blind, Placebo-Controlled Study of Oral Coenzyme Q10 to Relieve Self-Reported Treatment-Related Fatigue in Newly Diagnosed Patients with Breast Cancer. J. Support. Oncol. 2012, 11, 31–42. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.N.; Zhan, S.Y.; Xia, Y. Coenzyme Q10 for Parkinson’s disease. Cochrane Database Syst. Rev. 2012, CD008150. [Google Scholar] [CrossRef]
- Shults, C.W.; Haas, R.H.; Passov, D.; Beal, M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann. Neurol. 1997, 42, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T. Serum coenzyme Q-10 level in parkinson syndrome. In Biomedical and Clinical Aspects of Coenzyme Q; Folkers, K., Littarru, G.P., Yamagami, T., Eds.; Elsevier Science Publishers: New York, NY, USA, 1991; pp. 159–166. [Google Scholar]
- Müller, T.; Büttner, T.; Gholipour, A.-F.; Kuhn, W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci. Lett. 2003, 341, 201–204. [Google Scholar] [CrossRef]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of Coenzyme Q10 in Early Parkinson Disease. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef]
- Horstink, M.W.I.M.; Van Engelen, B.G. The effect of coenzyme Q10 therapy in Parkinson disease could be symptomatic. Arch. Neurol. 2003, 60, 1170–1172. [Google Scholar] [CrossRef]
- Shults, C.W.; Beal, M.F.; Song, D.; Fontaine, D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp. Neurol. 2004, 188, 491–494. [Google Scholar] [CrossRef]
- Storch, A.; Jost, W.H.; Vieregge, P.; Spiegel, J.; Greulich, W.; Durner, J.; Müller, T.; Kupsch, A.; Henningsen, H.; Oertel, W.H.; et al. Randomized, Double-blind, Placebo-Controlled Trial on Symptomatic Effects of Coenzyme Q10 in Parkinson Disease. Arch. Neurol. 2007, 64, 938–944. [Google Scholar] [CrossRef]
- Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; Ravina, B.; et al. A Randomized Clinical Trial of High-Dosage Coenzyme Q10 in Early Parkinson Disease. JAMA Neurol. 2014, 71, 543–552. [Google Scholar] [CrossRef]
- Cleren, C.; Yang, L.; Lorenzo, B.; Calingasan, N.Y.; Schomer, A.; Sireci, A.; Wille, E.J.; Beal, M.F. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 2007, 104, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Kawajiri, S.; Yamamoto, Y.; Nakahara, T.; Ando, M.; Hashimoto, K.; Nagase, M.; Saito, Y.; Hattori, N. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Park. Relat. Disord. 2015, 21, 911–916. [Google Scholar] [CrossRef]
- The Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology 2001, 57, 397–404. [Google Scholar] [CrossRef]
- McGarry, A.; McDermott, M.; Kieburtz, K.; De Blieck, E.A.; Beal, F.; Marder, K.; Ross, C.; Shoulson, I.; Gilbert, P.; Mallonee, W.M.; et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology 2017, 88, 152–159. [Google Scholar] [CrossRef]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer Disease. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, V.; Bakshi, R. Quality of life in patients with multiple sclerosis. J. Neurol. Sci. 2002, 205, 51–58. [Google Scholar] [CrossRef]
- Kos, D.; Kerckhofs, E.; Nagels, G.; D’Hooghe, M.B.; Ilsbroukx, S. Origin of Fatigue in Multiple Sclerosis: Review of the Literature. Neurorehabilit. Neural Repair 2007, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vercoulen, J.H.M.M.; Hommes, O.R.; Swanink, C.M.A.; Jongen, P.J.H.; Fennis, J.F.M.; Galama, J.M.D.; Van Der Meer, J.W.M.; Bleijenberg, G. The Measurement of Fatigue in Patients with Multiple Sclerosis. Arch. Neurol. 1996, 53, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Irwin, M.R. Depression and Immunity: Inflammation and Depressive Symptoms in Multiple Sclerosis. Neurol. Clin. 2006, 24, 507–519. [Google Scholar] [CrossRef]
- Bilici, M.; Efe, H.; Köroğlu, M.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001, 64, 43–51. [Google Scholar] [CrossRef]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2015, 19, 138–143. [Google Scholar] [CrossRef]
- Fakhrabadi, M.A.; Ghotrom, A.Z.; Mozaffari-Khosravi, H.; Nodoushan, H.H.; Nadjarzadeh, A.; Information, R. Effect of Coenzyme Q10 on Oxidative Stress, Glycemic Control and Inflammation in Diabetic Neuropathy: A Double Blind Randomized Clinical Trial. Int. J. Vitam. Nutr. Res. 2014, 84, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Gandolfi, S.; Marangoni, D.; Rossetti, L.; Tanga, L.; Tardini, M.; Traina, S.; Ungaro, N.; Vetrugno, M.; et al. Effects of Coenzyme Q10 in Conjunction With Vitamin E on Retinal-evoked and Cortical-evoked Responses in Patients With Open-angle Glaucoma. J. Glaucoma 2014, 23, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Ozates, S.; Elgin, K.U.; Yilmaz, N.S.; Demirel, O.O.; Sen, E.; Yilmazbas, P. Evaluation of oxidative stress in pseudo-exfoliative glaucoma patients treated with and without topical coenzyme Q10 and vitamin E. Eur. J. Ophthalmol. 2019, 29, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.G.; Yuvaraj, S.; Vijayasarathy, K.; Gangadaran, S.G.D.; Sachdanandam, P. Effect of Coenzyme Q10, Riboflavin and Niacin on Serum CEA and CA 15-3 Levels in Breast Cancer Patients Undergoing Tamoxifen Therapy. Biol. Pharm. Bull. 2007, 30, 367–370. [Google Scholar] [CrossRef]
- Premkumar, V.G.; Yuvaraj, S.; Shanthi, P.; Sachdanandam, P. Co-enzyme Q10, riboflavin and niacin supplementation on alteration of DNA repair enzyme and DNA methylation in breast cancer patients undergoing tamoxifen therapy. Br. J. Nutr. 2008, 100, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.G.; Yuvaraj, S.; Sathish, S.; Shanthi, P.; Sachdanandam, P. Anti-angiogenic potential of CoenzymeQ10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vasc. Pharmacol. 2008, 48, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Huang, Y.C.; Cheng, S.B.; Huang, Y.T.; Lin, P.T. Effects of coenzyme Q10 supplementation on antioxidant capacity and inflammation in hepatocellular carcinoma patients after surgery: A randomized, placebo-controlled trial. Nutr. J. 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Iwase, S.; Kawaguchi, T.; Yotsumoto, D.; Doi, T.; Miyara, K.; Odagiri, H.; Kitamura, K.; Ariyoshi, K.; Miyaji, T.; Ishiki, H.; et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and l-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: A multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support. Care Cancer 2015, 24, 637–646. [Google Scholar] [CrossRef]
- Hoenjet, K.; Dagnelie, P.; Delaere, K.; Wijckmans, N.; Zambon, J.; Oosterhof, G. Effect of a Nutritional Supplement Containing Vitamin E, Selenium, Vitamin C and Coenzyme Q10 on Serum PSA in Patients with Hormonally Untreated Carcinoma of the Prostate: A Randomised Placebo-Controlled Study. Eur. Urol. 2005, 47, 433–440. [Google Scholar] [CrossRef]
- Rusciani, L.; Proietti, I.; Rusciani, A.; Paradisi, A.; Sbordoni, G.; Alfano, C.; Panunzi, S.; De Gaetano, A.; Lippa, S. Low plasma coenzyme Q10 levels as an independent prognostic factor for melanoma progression. J. Am. Acad. Dermatol. 2006, 54, 234–241. [Google Scholar] [CrossRef]
- Rusciani, L.; Proietti, I.; Paradisi, A.; Rusciani, A.; Guerriero, G.; Mammone, A.; De Gaetano, A.; Lippa, S. Recombinant interferon α-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: A 3-year trial with recombinant interferon-α and 5-year follow-up. Melanoma Res. 2007, 17, 177–183. [Google Scholar] [CrossRef]
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Eroglu, M.; Sahin, S.; Durukan, B.; Ozakpinar, O.B.; Erdinc, N.; Turkgeldi, L.; Sofuoglu, K.; Karateke, A. Blood Serum and Seminal Plasma Selenium, Total Antioxidant Capacity and Coenzyme Q10 Levels in Relation to Semen Parameters in Men with Idiopathic Infertility. Biol. Trace Elem. Res. 2014, 159, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Balercia, G.; Buldreghini, E.; Vignini, A.; Tiano, L.; Paggi, F.; Amoroso, S.; Ricciardo-Lamonica, G.; Boscaro, M.; Lenzi, A.; Littarru, G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: A placebo-controlled, double-blind randomized trial. Fertil. Steril. 2009, 91, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Safarinejad, S.; Shafiei, N.; Safarinejad, S. Effects of the Reduced Form of Coenzyme Q 10 (Ubiquinol) on Semen Parameters in Men with Idiopathic Infertility: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2012, 188, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Nadjarzadeh, A.; Sadeghi, M.R.; Amirjannati, N.; Vafa, M.R.; Motevalian, S.A.; Gohari, M.R.; Akhondi, M.A.; Yavari, P.; Shidfar, F. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: A randomized double-blind, placebo controlled trial. J. Endocrinol. Investig. 2011, 34, e224–e228. [Google Scholar]
- Pasqualotto, F.F.; Sundaram, A.; Sharma, R.K.; Borges, E.; Pasqualotto, E.B.; Agarwal, A. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil. Steril. 2008, 89, 602–607. [Google Scholar] [CrossRef]
- Festa, R.; Giacchi, E.; Raimondo, S.; Tiano, L.; Zuccarelli, P.; Silvestrini, A.; Meucci, E.; Littarru, G.P.; Mancini, A. Coenzyme Q10supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia 2013, 46, 805–807. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.F.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Agapova, S.E.; Cameo, T.; Sopher, A.B.; Oberfield, S.E. Diagnosis and Challenges of Polycystic Ovary Syndrome in Adolescence. Semin. Reprod. Med. 2014, 32, 194–201. [Google Scholar] [CrossRef]
- Azziz, R. Diagnosis of Polycystic Ovarian Syndrome: The Rotterdam Criteria Are Premature. J. Clin. Endocrinol. Metab. 2006, 91, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Effect of Coenzyme Q10 Supplementation on Testosterone. Biomolecules 2018, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Mehrizi, M.Z.; Foroozanfard, F.; Akbari, H.; Jamilian, M.; Ahmadi, S.; Asemi, Z. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2017, 86, 560–566. [Google Scholar] [CrossRef]
- Rahmani, E.; Jamilian, M.; Samimi, M.; Mehrizi, M.Z.; Aghadavod, E.; Akbari, E.; Tamtaji, O.R.; Asemi, Z. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2017, 34, 217–222. [Google Scholar] [CrossRef]
- Izadi, A.; Shirazi, S.; Taghizadeh, S.; Gargari, B.P. Independent and Additive Effects of Coenzyme Q10 and Vitamin E on Cardiometabolic Outcomes and Visceral Adiposity in Women with Polycystic Ovary Syndrome. Arch. Med Res. 2019, 50, 1–10. [Google Scholar] [CrossRef]
- Verjee, L.S.; Verhoekx, J.S.N.; Chan, J.K.K.; Krausgruber, T.; Nicolaidou, V.; Izadi, D.; Davidson, D.; Feldmann, M.; Midwood, K.S.; Nanchahal, J. Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target. Proc. Natl. Acad. Sci. USA 2013, 110, E928–E937. [Google Scholar] [CrossRef]
- Lourens, W. Could coenzyme Q10 be the treatment for Dupuytren’s disease? BMJ Case Rep. 2019, 12, e226419. [Google Scholar] [CrossRef]
- Rowland, L.M.; Pradhan, S.; Korenic, S.; Wijtenburg, S.; Hong, L.E.; Edden, R.A.; Barker, P.B. Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 2016, 6, e967. [Google Scholar] [CrossRef]
- Do, K.Q.; Cabungcal, J.H.; Frank, A.; Steullet, P.; Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009, 19, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Bergman, O.; Ben-Shachar, D. Mitochondrial Oxidative Phosphorylation System (OXPHOS) Deficits in Schizophrenia. Can. J. Psychiatry 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Imagawa, M. Low Erythrocyte Coenzyme Q10Level in Schizophrenic Patients. Psychiatry Clin. Neurosci. 1989, 43, 143–145. [Google Scholar] [CrossRef]
- Maguire, Á.; Hargreaves, A.; Gill, M. Coenzyme Q10 and neuropsychiatric and neurological disorders: Relevance for schizophrenia. Nutr. Neurosci. 2018, 23, 756–769. [Google Scholar] [CrossRef]
- Kikkawa, K.; Takehara, I.; Miyakoshi, T.; Miyawaki, H. Safety of high dose supplementation of coenzyme Q10 in healthy human adults. Jpn. J. Food Chem. Saf. 2007, 14, 76–81. [Google Scholar] [CrossRef]
- Mortensen, A.L.; Rosenfeldt, F.; Filipiak, K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2013, 26, 147–156. [Google Scholar] [CrossRef] [PubMed]
| Organ | Ubiquinone Concentration (µg/g) | Ubiquinol Concentration (µg/g) | References |
|---|---|---|---|
| Heart | 132.0 | 61.0 | [14,15] |
| Kidneys | 77.0 | 75.0 | |
| Liver | 63.6 | 95.0 | |
| Muscle | 39.7 | 65.0 | |
| Brain | 13.4 | 23.0 | |
| Pancreas | 32.7 | ||
| Spleen | 24.6 | ||
| Lung | 7.9 | 25.0 | |
| Thyroid | 24.7 | ||
| Testis | 10.5 | ||
| Intestine | 11.5 | 95.0 | |
| Colon | 10.7 | ||
| Ventricle | 11.8 | ||
| Plasma (µmol/mL) | 1.1 | 96.0 |
| Study Design | Daily Doses | Effects on Symptoms | Effects on Lab. or Instrumental Parameters | Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| Migraine | RCTs | 100–400 mg/day | ↓ duration and severity of attacks | ↓ TNFα and GCPR levels | Not investigated |
| Study Design | Daily Doses | Effects on Symptoms | Effects on Lab or Instrumental Parameters | Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| Fatigue | RCTs | 200 mg/day, in association with NADH (20 mg/day) | ↓ FIS total score (CFS) | ↑ NAD+/NADH ratio and CoQ10, ATP, citrate synthase levels | Not investigated |
| RCTs | 300–400 mg | ↓ FIS total score | - | Not investigated | |
| Fibromyalgia | RCTs | 100–400 mg | ↓ fatigue (FIQ, VAS) | - | Not investigated |
| Statin-associated myopathy | Meta-analysis of RCTs | ≥200 mg | ↓ fatigue (VAS) | - | Not investigated |
| Other fatigue-related diseases | RCTs | 60–500 mg | ↓ fatigue (FSS) only in multiple sclerosis and in patients awaiting cardiac transplantation with end-stage heart failure | - | Not investigated |
| Study Design | Daily Doses | Effects on Symptoms | Effects on Lab or Instrumental Parameters | Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| PD | RCTs | 300–2400 mg | ↑significant mild symptomatic benefit, ↑ great improvements of patients everyday activities such as feeding, bathing, or dressing, ↑ effects on motor performance, = no significant changes in UPDRS | ↑ improvement in NADH-cytochrome c reductase activity, ↑ increase in CoQ10 plasmatic levels | Not investigated |
| HD | RCTs | 600–2400 mg | = no significant changes in: HDRS, in HDFCS, standardized neuropsychological measures and TFC scores | Not recorded | Not investigated |
| AD | RCTs | 400 mg | = MMSE scores and functional ability | = not significant differences in: CSF F-2-isoprostane levels, oxidative biomarkers, CSF Aβ42, tau, and P-tau (181) levels | Not investigated |
| MS | RCTs | 500 mg | ↑reduction of fatigue and depression | ↓ inflammatory markers TNF-α, IL-6 and MMP-9, = IL-4 and TGF-β levels | Not investigated |
| Glaucoma | RCTs | 100 mg | Not evaluated | ↑ inner retinal function, electroretinogram and visual cortical responses, ↓superoxide dismutase, = malondialdehyde levels | Not investigated |
| Neuropathy | RCTs | 200 mg | No significant improvement of neuropathic symptoms | = no significant differences on HbA1c, fasting blood glucose or lipid profile, ↑mean insulin sensitivity, ↑ total antioxidant capacity concentration, ↓C-protein level, = electromyography measurements | Not investigated |
| Study Design | Daily Doses | Effects on symptoms | Effects on Lab or Instrumental Parameters | Effects on Hard Outcomes | |
|---|---|---|---|---|---|
| Breast cancer | RCTs | 300–2400 mg | ↓ moderate-severe cancer-related fatigue (30 mg) | ↓ CEA, CA 15-3, IL-1β, IL-6, IL-8, TNF-α, vascular endothelial growth factor, pro-angiogenic marker levels, ↑ DNA repair enzymes (poly-ADP-ribose polymerase levels), a disappearance of DNA methylation patterns (RASSF1A DNA methylation pattern) | Not investigated |
| HCC | RCTs | 300 mg | Not investigated | ↓ hs-CRP, IL-6 ↑ SOD, CAT, GPx | Not investigated |
| Prostatic carcinoma | RCTs | 300 mg | Not investigated | ↑ CoQ10, vit E, selenium = PSA, testosterone, DHT, LH, SBHG | Not investigated |
| Melanoma | RCTs | 400 mg | Not investigated | Not investigated | ↓ rates of recurrence at 5 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testai, L.; Martelli, A.; Flori, L.; Cicero, A.F.G.; Colletti, A. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients 2021, 13, 1697. https://doi.org/10.3390/nu13051697
Testai L, Martelli A, Flori L, Cicero AFG, Colletti A. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients. 2021; 13(5):1697. https://doi.org/10.3390/nu13051697
Chicago/Turabian StyleTestai, Lara, Alma Martelli, Lorenzo Flori, Arrigo F. G. Cicero, and Alessandro Colletti. 2021. "Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases" Nutrients 13, no. 5: 1697. https://doi.org/10.3390/nu13051697
APA StyleTestai, L., Martelli, A., Flori, L., Cicero, A. F. G., & Colletti, A. (2021). Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients, 13(5), 1697. https://doi.org/10.3390/nu13051697