Anti-Fatigue Effect of a Dietary Supplement from the Fermented By-Products of Taiwan Tilapia Aquatic Waste and Monostroma nitidum Oligosaccharide Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Material Origin
2.1.2. Hot Extraction Process of Fish Bone Powder
2.1.3. Preparation of Fish Bone Powder Fermentation Broth
2.1.4. Preparation of Monostroma Nitidum Polysaccharide Hydrolysates
2.2. Test Animals and Study Design
2.3. Swimming Exercise Training Mode
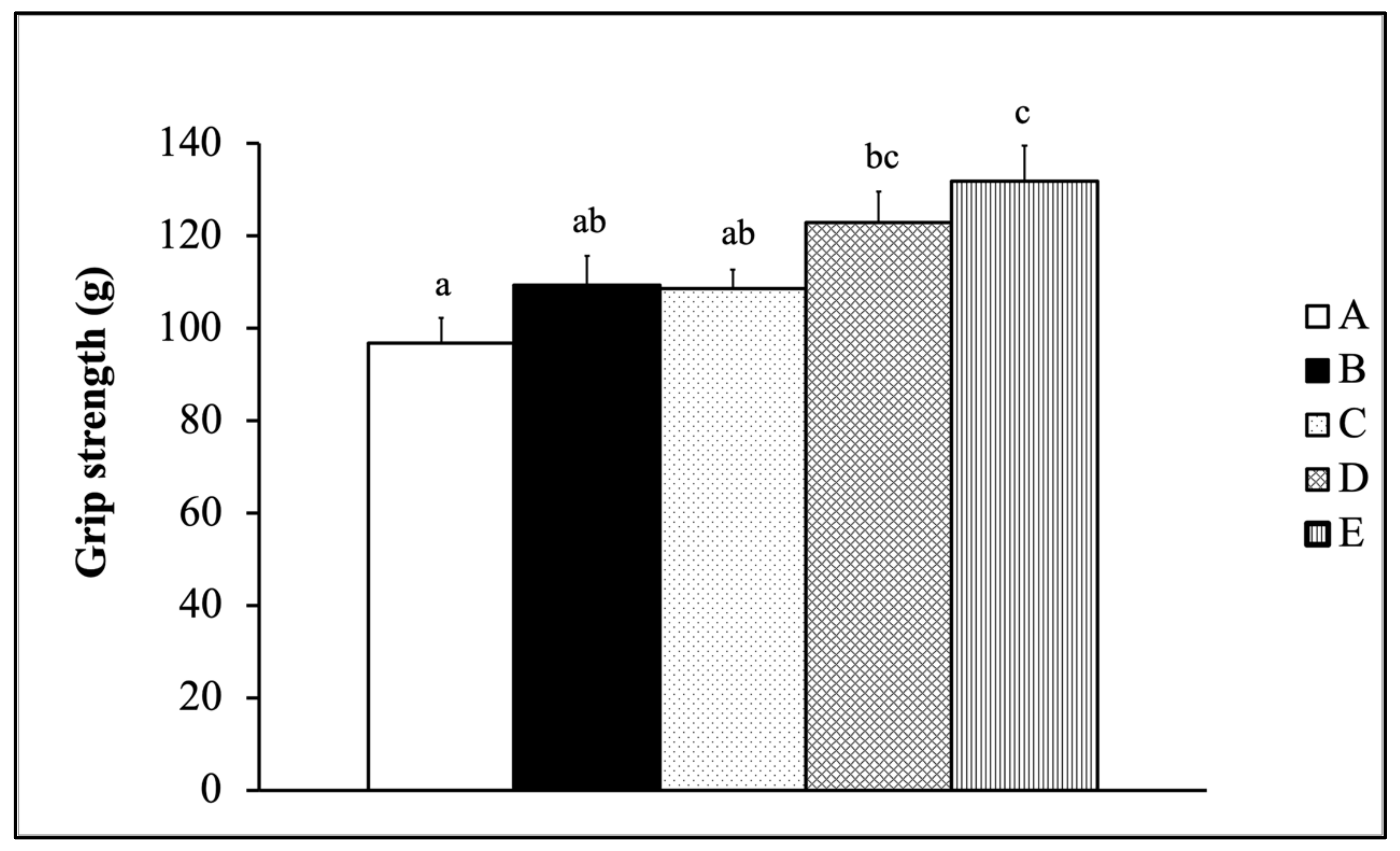
2.4. Forelimb Grip Strength Test
2.5. Exhaustive Swimming Test
2.6. Biochemistry Tests
2.7. Analysis of Fatigue-Related Biomarkers
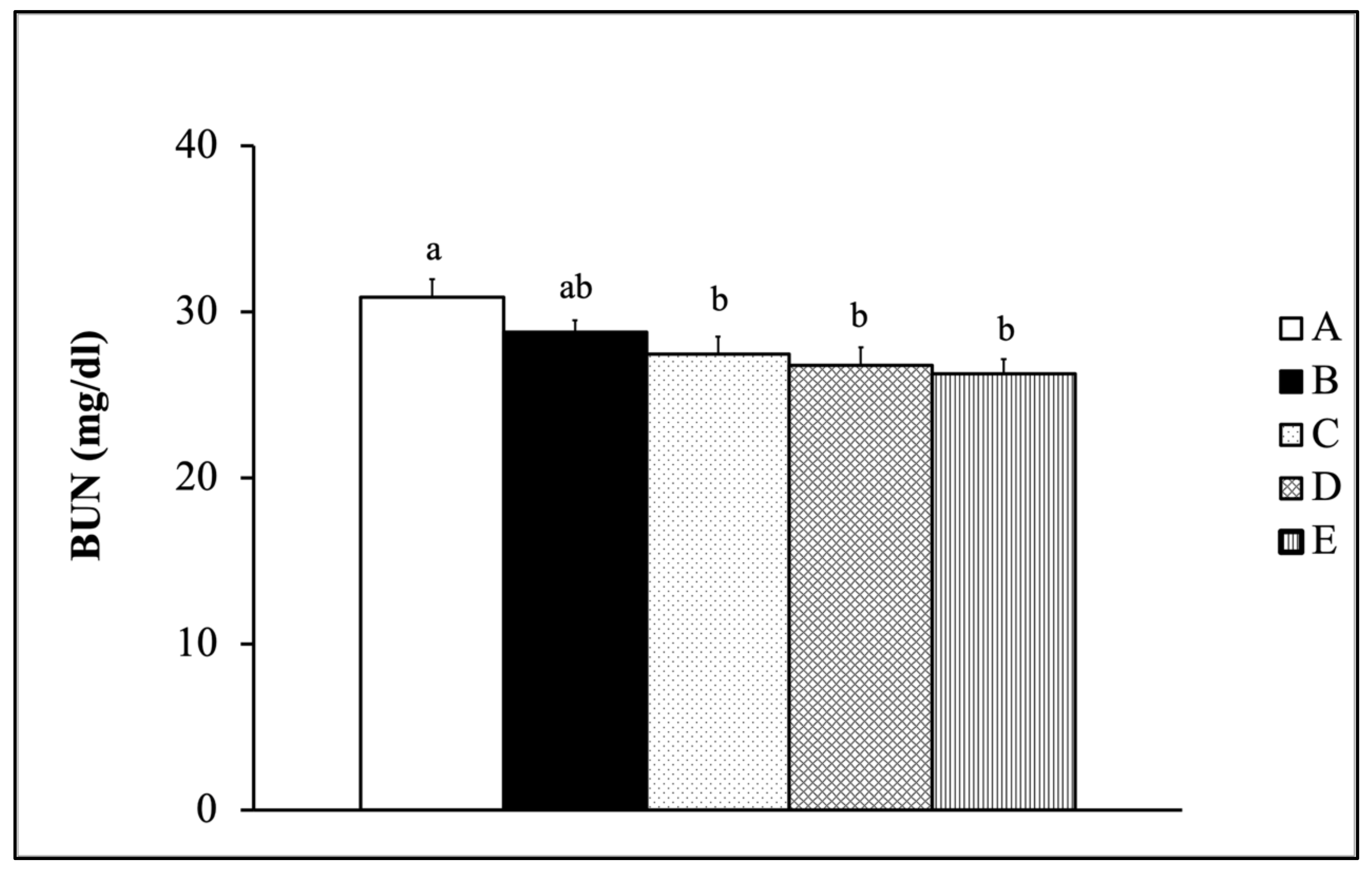
2.7.1. Blood Urea Nitrogen Test
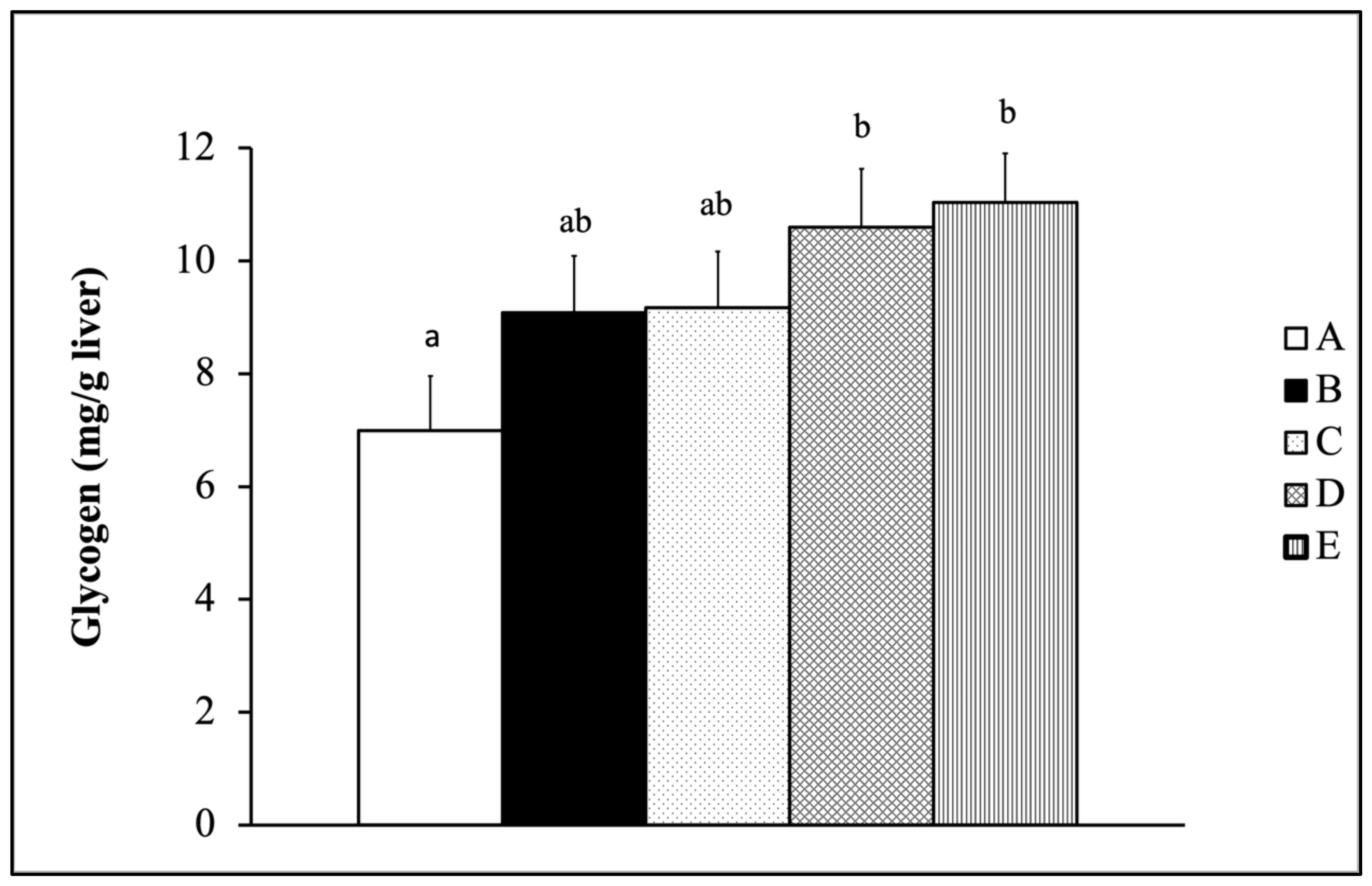
2.7.2. Hepatic Glycogen Test
2.7.3. Blood Lactate Test
2.8. Statistical Analysis
3. Results
3.1. Impact of Body Weight, Intake Amount, and Water Consumption Volume After Six Weeks of Supplementation with a Complex of Fermented Tilapia By-Products and Monostroma Nitidum Oligosaccharides Combined with Physical Training
3.2. Evaluation of Exercise Performance After Six Weeks of Supplementing with a Mixture of Fermented Tilapia By-Products and Monostroma Nitidum Oligosaccharides Combined with Exercise Training
3.3. Biochemical Blood Analysis
3.4. Fatigue-Related Biomarker Analysis
3.4.1. BUN Concentration
3.4.2. Glycogen Level
3.4.3. Blood Lactate Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, R.K.; Agnew, M.J. Influence of mental workload on muscle endurance, fatigue, and recovery during intermittent static work. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 112, 2891–2902. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus an-guillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Chen, H.-C.; Huang, C.-C.; Lin, T.-J.; Hsu, M.-C.; Hsu, Y.-J. Ubiquinol supplementation alters exercise induced fatigue by increasing lipid utilization in mice. Nutrients 2019, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Kawamura, T.; Muraoka, I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef]
- Lambert, E.V.; St Clair Gibson, A.; Noakes, T.D. Complex systems model of fatigue: Integrative homoeostatic control of pe-ripheral physiological systems during exercise in humans. Br. J. Sports Med. 2005, 39, 52–62. [Google Scholar] [CrossRef]
- Vasilaki, A.; Mansouri, A.; Van Remmen, H.; Van Der Meulen, J.H.; Larkin, L.; Richardson, A.G.; McArdle, A.; Faulkner, J.A.; Jackson, M.J. Free radical generation by skeletal muscle of adult and old mice: Effect of contractile activity. Aging Cell 2006, 5, 109–117. [Google Scholar] [CrossRef]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free. Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef]
- Zheng, X.; Long, W.; Liu, G.; Zhang, X.; Yang, X. Effect of seabuckthorn (Hippophae rhamnoides ssp. sinensis) leaf extract on the swimming endurance and exhaustive exercise-induced oxidative stress of rats. J. Sci. Food Agric. 2011, 92, 736–742. [Google Scholar] [CrossRef]
- Filho, P.R.C.D.O.; Netto, F.M.; Ramos, K.K.; Trindade, M.A.; Viegas, E.M.M. Elaboration of sausage using minced fish of Nile tilapia filleting waste. Braz. Arch. Biol. Technol. 2010, 53, 1383–1391. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports 2006, 16, 3–63. [Google Scholar] [CrossRef]
- Chen, G.-W.; Tsai, J.-S.; Pan, B.S. Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007, 17, 641–647. [Google Scholar] [CrossRef]
- Wu, S.C.; Zen, K.C.; Wu, X.H.; Pan, C.L. Purification and characterization of agarase PV-2 from Pseudomonas vesicularis MA103. J. Fish. Soc. Taiwan 2012, 39, 91–105. [Google Scholar]
- Wu, S.C.; Kang, S.K.; Pan, C.L. Purification and characterization of Aeromonas salmonicida MF108 agarose CAS-II. J. Fish. Soc. Taiwan 2012, 39, 57–74. [Google Scholar]
- Takeshita, H.; Yamamoto, K.; Nozato, S.; Inagaki, T.; Tsuchimochi, H.; Shirai, M.; Yamamoto, R.; Imaizumi, Y.; Hongyo, K.; Yokoyama, S.; et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci. Rep. 2017, 7, srep42323. [Google Scholar] [CrossRef]
- Elia, D.; Stadler, K.; Horváth, V.; Jakus, J. Effect of soy- and whey protein-isolate supplemented diet on the redox parameters of trained mice. Eur. J. Nutr. 2006, 45, 259–266. [Google Scholar] [CrossRef]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and bio-chemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-fatigue effects of small molecule oligopeptides isolated from panax ginseng C. A. meyer in mice. Nutrients 2016, 8, 807. [Google Scholar] [CrossRef]
- Kan, N.-W.; Ho, C.-S.; Chiu, Y.-S.; Huang, W.-C.; Chen, P.-Y.; Tung, Y.-T.; Huang, C.-C. Effects of resveratrol supplementation and exercise training on exercise performance in middle-aged mice. Molecules 2016, 21, 661. [Google Scholar] [CrossRef]
- Wu, R.-E.; Huang, W.-C.; Liao, C.-C.; Chang, Y.-K.; Kan, N.-W.; Huang, C.-C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef]
- Shimomura, Y.; Yamamoto, Y.; Bajotto, G.; Sato, J.; Murakami, T.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J. Nutr. 2006, 136, 529S–532S. [Google Scholar] [CrossRef]
- Huang, W.C.; Lin, C.I.; Chiu, C.C.; Lin, Y.T.; Huang, W.K.; Huang, H.Y.; Huang, C.C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lin, C.-L.; Wei, L.; Hsu, Y.-J.; Chen, K.-N.; Huang, C.-C.; Kao, C.-H. Sake protein supplementation affects exercise performance and biochemical profiles in power-exercise-trained mice. Nutrients 2016, 8, 106. [Google Scholar] [CrossRef]
- Wagenmakers, A.J.; Beckers, E.J.; Brouns, F.; Kuipers, H.; Soeters, P.B.; Van Der Vusse, G.J.; Saris, W.H. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am. J. Physiol. Metab. 1991, 260, E883–E890. [Google Scholar] [CrossRef]
- Wang, J.-J.; Shieh, M.-J.; Kuo, S.-L.; Lee, C.-L.; Pan, T.-M. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl. Microbiol. Biotechnol. 2006, 70, 247–253. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Coyle, E.F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Jeong, W.-S.; Lee, H.-Y. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J. Exerc. Nutr. Biochem. 2013, 17, 169–180. [Google Scholar] [CrossRef]
- Hübner-Woźniak, E.; Lerczak, K.; Sendecki, W. Effect of marathon run on changes in some biochemical variables in plasma of amateur long-distance runners. Biol. Sport 1993, 10, 173–181. [Google Scholar]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol. Pharm. Bull. 2006, 29, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M. Muscle glycogen and metabolic regulation. Proc. Nutr. Soc. 2004, 63, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ferraz, P.L.; Bozza, T.; Nicastro, H.; Lancha, A.H., Jr. Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition 2013, 29, 1388–1394. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir supplementation modifies gut micro-biota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef]
- Kim, K.M.; Yu, K.W.; Kang, D.H.; Suh, H.J. Anti-stress and anti-fatigue effect of fermented rice bran. Phytother. Res. 2002, 16, 700–702. [Google Scholar] [CrossRef]
- MacRae, H.S.; Dennis, S.C.; Bosch, A.N.; Noakes, T.D. Effects of training on lactate production and removal during progres-sive exercise in humans. J. Appl. Physiol. 1992, 72, 1649–1656. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lee, H.C.; Chen, M.T.; Huang, C.C.; Chen, W.C. Dehydroepiandrosterone supplementation combined with weight-loading whole-body vibration training (WWBV) affects exercise performance and muscle glycogen storage in mid-dle-aged C57BL/6 mice. Int. J. Med. Sci. 2018, 15, 564–573. [Google Scholar] [CrossRef]

| Group (n = 15) | Body Weight (g) | Food Intake (g/Day) | Water Consumption (mL/Day) | |
|---|---|---|---|---|
| Initial | Final | |||
| A | 30.30 ± 0.44 | 31.55 ± 0.57 | 5.74 ± 0.14 | 6.69 ± 0.13 |
| B | 29.68 ± 0.68 | 32.09 ± 0.42 | 5.54 ± 0.13 | 6.93 ± 0.10 |
| C | 29.94 ± 0.51 | 31.27 ± 0.52 | 5.31 ± 0.09 | 7.15 ± 0.11 |
| D | 29.20 ± 0.43 | 31.21 ± 0.51 | 5.47 ± 0.18 | 6.95 ± 0.12 |
| E | 30.05 ± 0.46 | 31.94 ± 0.45 | 5.23 ± 0.15 | 7.10 ± 0.13 |
| Group | A | B | C | D | E |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 106.18 ± 8.40 | 122.32 ± 4.67 | 122.93 ± 2.66 | 121.76 ± 5.94 | 123.35 ± 5.60 |
| Total protein (g/dL) | 5.40 ± 0.15 | 5.42 ± 0.29 | 5.47 ± 0.29 | 5.55 ± 0.33 | 5.59 ± 0.29 |
| Albumin (g/dL) | 3.28 ± 0.20 | 3.37 ± 0.18 | 3.34 ± 0.22 | 3.53 ± 0.14 | 3.49 ± 0.19 |
| Triglyceride (mg/dL) | 98.60 ± 6.11 | 105.47 ± 5.09 | 112.13 ± 3.50 | 108.27 ± 6.59 | 110.80 ± 3.64 |
| CHOL (mg/dL) | 119.73 ± 4.28 | 120.20 ± 2.88 | 113.27 ± 2.79 | 122.73 ± 4.20 | 115.80 ± 3.01 |
| ALP (U/L) | 76.60 ± 4.30 | 79.00 ± 2.78 | 81.13 ± 1.52 | 76.87 ± 4.02 | 78.20 ± 2.87 |
| GOT (U/L) | 135.92 ± 4.90 | 133.54 ± 4.31 | 130.90 ± 2.19 | 124.85 ± 2.08 | 125.03 ± 4.89 |
| GPT (U/L) | 57.47 ± 3.13 | 56.80 ± 4.29 | 53.20 ± 1.71 | 56.67 ± 2.65 | 54.93 ± 3.04 |
| Creatine kinase (U/L) | 275.53 ± 7.30 | 287.40 ± 8.83 | 278.27 ± 5.29 | 260.60 ± 9.56 | 263.40 ± 6.41 |
| Group | A | B | C | D | E |
|---|---|---|---|---|---|
| Lactate—Before swimming (mmol/L) | 3.16 ± 0.60 | 3.21 ± 0.52 | 3.00 ± 0.43 | 3.17 ± 0.34 | 3.13 ± 0.39 |
| Lactate—After swimming (mmol/L) | 5.87 ± 0.56 a | 4.67 ± 0.49 b | 4.39 ± 0.46 b | 4.36 ± 0.19 b | 4.23 ± 0.30 b |
| Lactate—After 20 min rest (mmol/L) | 5.07 ± 0.55 | 3.87 ± 0.47 | 3.71 ± 0.44 | 3.51 ± 0.26 | 3.50 ± 0.35 |
| Production rate of serum lactate | 2.79 ± 0.90 a | 1.37 ± 0.61 ab | 1.03 ± 0.42 b | 0.58 ± 0.15 b | 0.61 ± 0.19 b |
| Clearance rate of serum lactate | 0.15 ± 0.03 | 0.18 ± 0.04 | 0.17 ± 0.02 | 0.20 ± 0.04 | 0.18 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Kuo, C.-Y.; Kong, Z.-L.; Lai, C.-Y.; Chen, G.-W.; Yang, A.-J.; Lin, L.-H.; Wang, M.-F. Anti-Fatigue Effect of a Dietary Supplement from the Fermented By-Products of Taiwan Tilapia Aquatic Waste and Monostroma nitidum Oligosaccharide Complex. Nutrients 2021, 13, 1688. https://doi.org/10.3390/nu13051688
Chen Y-J, Kuo C-Y, Kong Z-L, Lai C-Y, Chen G-W, Yang A-J, Lin L-H, Wang M-F. Anti-Fatigue Effect of a Dietary Supplement from the Fermented By-Products of Taiwan Tilapia Aquatic Waste and Monostroma nitidum Oligosaccharide Complex. Nutrients. 2021; 13(5):1688. https://doi.org/10.3390/nu13051688
Chicago/Turabian StyleChen, Ying-Ju, Chun-Yen Kuo, Zwe-Ling Kong, Chin-Ying Lai, Guan-Wen Chen, An-Jen Yang, Liang-Hung Lin, and Ming-Fu Wang. 2021. "Anti-Fatigue Effect of a Dietary Supplement from the Fermented By-Products of Taiwan Tilapia Aquatic Waste and Monostroma nitidum Oligosaccharide Complex" Nutrients 13, no. 5: 1688. https://doi.org/10.3390/nu13051688
APA StyleChen, Y.-J., Kuo, C.-Y., Kong, Z.-L., Lai, C.-Y., Chen, G.-W., Yang, A.-J., Lin, L.-H., & Wang, M.-F. (2021). Anti-Fatigue Effect of a Dietary Supplement from the Fermented By-Products of Taiwan Tilapia Aquatic Waste and Monostroma nitidum Oligosaccharide Complex. Nutrients, 13(5), 1688. https://doi.org/10.3390/nu13051688






