High and Low Haemoglobin Levels in Early Pregnancy Are Associated to a Higher Risk of Miscarriage: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Outcome
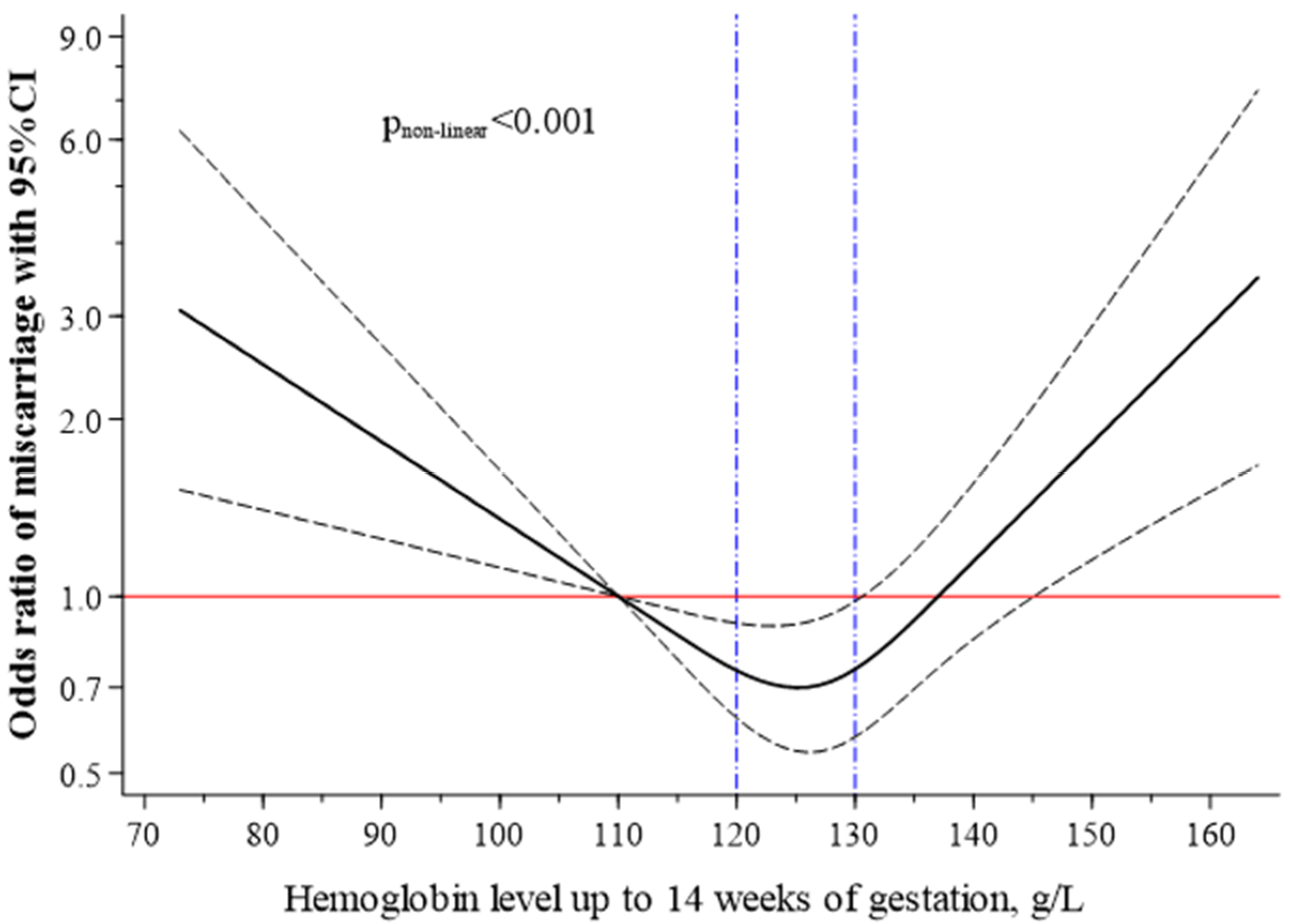
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Rouse, C.E.; Eckert, L.O.; Babarinsa, I.; Fay, E.; Gupta, M.; Harrison, M.S.; Kawai, A.; Alison, T.; Kharbanda, E.; Elyse, O.; et al. Spontaneous abortion and ectopic pregnancy: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6563–6574. [Google Scholar] [PubMed]
- García-Enguídanos, A.; Calle, M.E.; Valero, J.; Luna, S.; Domínguez-Rojas, V. Risk factors in miscarriage: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 102, 111–119. [Google Scholar] [CrossRef]
- Garrido-Gimenez, C.; Alijotas-Reig, J. Recurrent miscarriage: Causes, evaluation and management. Postgrad. Med. J. 2015, 91, 151–162. [Google Scholar] [CrossRef]
- Sheth, F.J.; Liehr, T.; Kumari, P.; Akinde, R.; Sheth, H.J.; Sheth, J.J. Chromosomal abnormalities in couples with repeated fetal loss: An Indian retrospective study. Indian J. Hum. Genet. 2013, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.C.; Christiansen, O.B.; Kolte, A.M.; Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 2013, 11, 154. [Google Scholar] [CrossRef]
- Nilsson, S.F.; Andersen, P.; Strandberg-Larsen, K.; Nybo Andersen, A.-M. Risk factors for miscarriage from a prevention perspective: A nationwide follow-up study. Int. J. Obstet. Gynaecol. 2014, 121, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Dey, D.; Roy, S.S. Molecular mechanism of insulin resistance. J. Biosci. 2007, 32, 405–413. [Google Scholar] [CrossRef][Green Version]
- Khalifa El-Saidy, T.M.; El-Sayed Amr, T.-S. The modifiable and nonmodifiable risk factors for miscarriage. Egypt. Nurs. J. 2016, 13, 169. [Google Scholar]
- Zhou, H.; Liu, Y.; Liu, L.; Zhang, M.; Chen, X.; Qi, Y. Maternal pre-pregnancy risk factors for miscarriage from a prevention perspective: A cohort study in China. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 57–63. [Google Scholar] [CrossRef]
- Maconochie, N.; Doyle, P.; Prior, S.; Simmons, R. Risk factors for first trimester miscarriage—Results from a UK-population-based case-control study. Int. J. Obstet. Gynaecol. 2007, 114, 170–186. [Google Scholar] [CrossRef]
- World Health Organization. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2015; Available online: https://www.who.int/nutrition/publications/micronutrients/global_prevalence_anaemia_2011/en/ (accessed on 20 January 2021).
- Young, M.F.; Oaks, B.M.; Tandon, S.; Martorell, R.; Dewey, K.G.; Wendt, A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 47–68. [Google Scholar] [CrossRef]
- Jung, J.; Rahman, M.; Rahman, S.; Swe, K.T.; Islam, R.; Rahman, O.; Akter, S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 69–82. [Google Scholar] [CrossRef]
- Sukrat, B.; Wilasrusmee, C.; Siribumrungwong, B.; McEvoy, M.; Okascharoen, C.; Attia, J.; Thakkinstian, A. Hemoglobin concentration and pregnancy outcomes: A systematic review and meta-analysis. BioMed Res. Int. 2013, 2013, 769057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Q.; Yang, Y.; Wang, L.; Liu, F.; Li, Q.; Ji, M.; He, Y.; Wang, Y.; Zhang, Y.; et al. Preconception Hb concentration and risk of preterm birth in over 2·7 million Chinese women aged 20-49 years: A population-based cohort study. Br. J. Nutr. 2018, 120, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Oaks, B.M. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am. J. Clin. Nutr. 2017, 106, 1694S–1702S. [Google Scholar] [CrossRef]
- Hämäläinen, H.; Hakkarainen, K.; Heinonen, S. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin. Nutr. 2003, 22, 271–275. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Liu, F.; Wang, L.; Wang, Q.; Shen, H.; Xu, Z.; Zhang, Y.; Yan, D.; He, Y.; et al. Preconception Hb concentration with risk of spontaneous abortion: A population-based cohort study in over 3·9 million women across rural China. Public Health Nutr. 2020, 23, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Abeysena, C.; Jayawardana, P.; Seneviratne, R.D.A. Maternal haemoglobin level at booking visit and its effect on adverse pregnancy outcome. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Departament de Salut; Agència de Salut Pública de Catalunya. Protocol de seguiment de l’embaraç a Catalunya. In Revisada. Generalitat de Catalunya, 3rd ed.; Departament de Salut: Barcelona, Spain, 2018. Available online: http://salutpublica.gencat.cat/web/.content/minisite/aspcat/promocio_salut/embaras_part_puerperi/protocol_seguiment_embaras/protocol-seguiment-embaras-2018.pdf (accessed on 20 January 2021).
- Ribot, B.; Ruiz-Díez, F.; Abajo, S.; March, G.; Fargas, F.; Arija, V. Prevalence of anaemia, risk of haemoconcentration and risk factors during the three trimesters of pregnancy. Nutr. Hosp. 2018, 35, 123–130. [Google Scholar]
- Scanlon, K.S.; Yip, R.; Schieve, L.A.; Cogswell, M.E. High and low hemoglobin levels during pregnancy: Differential risks for preterm birth and small for gestational age. Obstet. Gynaecol. 2000, 96, 741–748. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Steenland, K.; Tapia, V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1477–R1485. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Zhang, D.; Ren, Q.; Ganz, T.; Liu, S.; Nemeth, E. Iron homeostasis in pregnancy and spontaneous abortion. Am. J. Hematol 2019, 94, 184–188. [Google Scholar] [CrossRef]
- Allen, L.H. Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J. Nutr. 2001, 131, 581S–589S. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Maeng, H.-Y.; Sun, Y.-K.; Kim, Y.-A.; Park, D.-W.; Park, T.S.; Lee, S.T.; Choi, J.-R. Oxidative status in iron-deficiency anemia. J. Clin. Lab. Anal. 2009, 23, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef]
- Arezes, J.; Nemeth, E. Hepcidin and iron disorders: New biology and clinical approaches. Int. J. Lab. Hematol. 2015, 37, 92–98. [Google Scholar] [CrossRef]
- Iglesias Vázquez, L.; Arija, V.; Aranda, N.; Aparicio, E.; Serrat, N.; Fargas, F.; Ruiz, F.; Palleja, M.; Coronel, P.; Gimeno, M.; et al. The effectiveness of different doses of iron supplementation and the prenatal determinants of maternal iron status in pregnant spanish women: ECLIPSES study. Nutrients 2019, 11, 2418. [Google Scholar] [CrossRef]
- Heilmann, L.; Siekmann, U.; Schmid-Schönbein, H.; Ludwig, H. Hemoconcentration and pre-eclampsia. Arch. Gynecol. 1981, 231, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Koller, O.; Sandvei, R.; Sagen, N. High hemoglobin levels during pregnancy and fetal risk. Int. J. Gynaecol. Obstet. 1980, 18, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Stephansson, O.; Dickman, P.W.; Johansson, A.; Cnattingius, S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. J. Am. Med. Assoc. 2000, 284, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef]
- Goonewardene, M.; Shehata, M.; Hamad, A. Anaemia in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Pena-Rosas, J.P.; Viteri, F.E. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database Syst. Rev. 2006, 3, CD004736. [Google Scholar]
- Magnus, M.C.; Wilcox, A.J.; Morken, N.-H.; Weinberg, C.R.; Håberg, S.E. Role of maternal age and pregnancy history in risk of miscarriage: Prospective register based study. Br. Med. J. 2019, 364, l869. [Google Scholar] [CrossRef] [PubMed]
- Boots, C.; Stephenson, M.D. Does obesity increase the risk of miscarriage in spontaneous conception: A systematic review. Semin. Reprod. Med. 2011, 29, 507–513. [Google Scholar] [CrossRef] [PubMed]
| Maternal Hb Concentrations Categories, n (%) a | |||||||
|---|---|---|---|---|---|---|---|
| N | Mean Hb ± SD | Anaemia <110 g/L n = 358 (3.8) | Normal Hb 110–140 g/L n = 8505 (90.0) | High Hb ≥140 g/L n = 590 (6.2) | p-Value | ||
| Hb concentration up to 14 weeks of gestation (g/L), mean ± SD | 9453 | 126.3 ± 9.1 | 103.0 ± 6.3 † | 126.1 ± 6.8 | 143.2 ± 3.3 0 # | <0.001 | |
| Miscarriage, n (%) | 520 | 30 (8.4) † | 430 (5.1) | 60 (10.2) # | <0.001 | ||
| Age of mother at conception (years), mean ± SD | 9453 | 29.9 ± 5.5 | 29.8 ± 6.2 | 29.9 ± 5.5 | 30.2 ± 5.4 | 0.51 | |
| <20, n (%) | 331 | 125.3 ± 8.4 | 16 (4.7) | 306 (3.8) | 9 (1.6) | 0.003 | |
| 20–24, n (%) | 1357 | 126.1 ± 9.7 | 69 (20.4) † | 1199 (14.9) | 89 (15.8) | ||
| 25–29, n (%) | 2498 | 126.5 ± 8.9 | 84 (24.8) | 2263 (28.2) | 151 (26.7) | ||
| 30–34, n (%) | 2713 | 126.7 ± 8.8 | 87 (25.7) | 2442 (30.4) | 184 (32.6) | ||
| 35–39, n (%) | 1685 | 126.0 ± 9.2 | 62 (18.3) | 1519 (18.9) | 104 (18.4) | ||
| ≥40, n (%) | 337 | 125.9 ± 10.3 * | 21 (6.2) † | 289 (3.6) | 27 (4.8) | ||
| Weight in the first trimester (Kg) | 9008 | 65.2 ± 12.5 | 64.6 ± 12.6 | 65.2 ± 12.4 | 66.7 ± 12.6 # | 0.017 | |
| Missing, n | 445 | 25 | 384 | 36 | |||
| BMI in the first trimester (Kg/m2), mean ± SD | 8935 | 24.8 ± 4.5 | 24.3 ± 4.4 | 24.7 ± 4.5 | 25.3 ± 4.6 # | 0.002 | |
| <18.5, n (%) | 249 | 124.6 ± 9.4 | 16 (4.9) | 220 (2.7) | 13 (1.3) | 0.001 | |
| 18.5-24.9, n (%) | 5127 | 125.9 ± 8.8 | 189 (57.3) | 4663 (57.9) | 275 (50.4) | ||
| ≥25, n (%) | 3559 | 127.0 ± 9.2 * | 125 (37.9) | 3176 (39.4) | 258 (47.3) # | ||
| Missing, n | 518 | 28 | 446 | 44 | |||
| Smoking habit, n (%) | |||||||
| No | 6926 | 126.0 ± 9.2 | 283 (85.2) | 6252 (79.6) | 391 (74.5) | 0.001 | |
| Yes | 1781 | 127.1 ± 8.7 * | 49 (14.8) † | 1598 (20.4) | 134 (25.5) # | ||
| Missing, n | 640 | 106 | 106 | ||||
| Previous births (number), mean ± SD | 9323 | 0.8 ± 0.9 | 1.1 ± 1.1 † | 0.8 ± 0.9 | 0.7 ± 0.9 # | <0.001 | |
| Missing, n | 130 | 6 | 112 | 12 | |||
| Parity, n (%) | |||||||
| Nulliparous | 4064 | 126.9 ± 8.9 | 122 (34.7) | 3650 (43.5) | 292 (50.5) | <0.001 | |
| Parous | 5259 | 125.8 ± 9.2 * | 230 (65.3) † | 4743 (56.5) | 286 (49.5) # | ||
| Missing, n | 130 | 6 | 112 | 12 | |||
| Pregnancies (number), mean ± SD | 9323 | 2.2 ± 1.2 | 2.6 ± 1.5 † | 2.2 ± 1.2 | 2.1 ± 1.2 | <0.001 | |
| Missing, n | 130 | 6 | 112 | 12 | |||
| Previous miscarriage, n (%) | |||||||
| 0 | 6609 | 126.4 ± 8.9 | 223 (63.4) | 5989 (71.4) | 397 (68.7) | 0.003 | |
| 1 | 2067 | 126.2 ± 9.5 | 90 (25.6) | 1839 (21.9) | 138 (23.8) | ||
| ≥2 | 647 | 125.6 ± 9.8 | 39 (11.1) † | 565 (6.7) | 43 (7.4) | ||
| Missing, n | 130 | 6 | 112 | 12 | |||
| Miscarriage | Unadjusted Model | Adjusted Model * | ||||||
|---|---|---|---|---|---|---|---|---|
| Maternal Characteristics | No. of Participants | n | % | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Hb concentration up to 14 weeks of gestation (g/L) | ||||||||
| <110 | 358 | 30 | 8.4 | 1.72 (1.17 to 2.52) | 0.006 | 2.08 (1.35 to 3.20) | <0.001 | |
| 110–139 | 8505 | 430 | 5.1 | 1.00 (ref.) | 1.00 (ref.) | |||
| ≥140 | 590 | 60 | 10.2 | 2.13 (1.60 to 2.82) | <0.001 | 1.78 (1.25 to 2.54) | 0.001 | |
| Maternal age at conception (years) | ||||||||
| <20 | 331 | 17 | 5.1 | 1.28 (0.76 to 2.18) | 0.35 | 1.10 (0.59 to 2.07) | 0.75 | |
| 20–24 | 1357 | 56 | 4.1 | 1.02 (0.73 to 1.43) | 0.90 | 0.80 (0.53 to 1.20) | 0.29 | |
| 25–29 | 2498 | 101 | 4.0 | 1.00 (ref) | 1.00 (ref) | |||
| 30–34 | 2716 | 148 | 5.5 | 1.37 (1.06 to 1.77) | 0.017 | 1.36 (1.01 to 1.84) | 0.041 | |
| 34–39 | 1685 | 122 | 7.2 | 1.85 (1.41 to 1.77) | <0.001 | 2.01 (1.47 to 2.75) | <0.001 | |
| ≥40 | 337 | 52 | 15.4 | 4.33 (3.03 to 6.18) | <0.001 | 4.83 (3.22 to 7.25) | <0.001 | |
| BMI in the first trimester (Kg/m2) | ||||||||
| <18.5 | 249 | 6 | 2.4 | 0.49 (0.22 to 1.11) | 0.09 | 0.67 (0.29 to 1.54) | 0.35 | |
| 18.5–24.9 | 5127 | 246 | 4.8 | 1.00 (ref) | 1.00 (ref) | |||
| ≥25 | 3559 | 217 | 6.1 | 1.29 (1.07 to 1.55) | 0.008 | 1.17 (0.94 to 1.45) | 0.154 | |
| Smoking habit | ||||||||
| No | 6926 | 340 | 4.9 | 1.00 (ref) | 1.00 (ref) | |||
| Yes | 1781 | 74 | 4.2 | 0.84 (0.65 to 1.09) | 0.18 | 0.84 (0.63 to 1.10) | 0.20 | |
| Parity | ||||||||
| Nulliparous | 4064 | 247 | 6.1 | 1.00 (ref) | 1.00 (ref) | |||
| Parous | 5259 | 261 | 4.9 | 0.81 (0.67 to 0.96) | 0.021 | 0.69 (0.55 to 0.86) | 0.001 | |
| Previous miscarriage | ||||||||
| 0 | 6609 | 354 | 5.4 | 1.00 (ref) | 1.00 (ref) | |||
| 1 | 2067 | 111 | 5.4 | 1.00 (0.81 to 1.24) | 0.98 | 0.87 (0.65 to 1.64) | 0.25 | |
| ≥2 | 647 | 43 | 6.7 | 1.26 (0.91 to 1.74) | 0.16 | 1.11 (0.75 to 1.64) | 0.61 | |
| Miscarriage | Adjusted Model * | |||||
|---|---|---|---|---|---|---|
| Maternal Characteristics | No. of Participants | n | % | OR (95% CI) | p-Value | |
| Only cases documented up to 14 weeks of gestation † | 9366 | 433 | 4.6 | |||
| Hb concentrations (g/L) | ||||||
| <110 | 353 | 25 | 7.1 | 2.11 (1.32 to 3.36) | 0.002 | |
| 110–139 | 8434 | 359 | 4.3 | 1.00 (ref.) | ||
| ≥140 | 579 | 49 | 8.5 | 1.66 (1.12 to 2.47) | 0.012 | |
| Only cases documented between 15 and 24 weeks of gestation ‡ | 9020 | 87 | 1.0 | |||
| Hb concentrations (g/L) | ||||||
| <110 | 333 | 5 | 1.5 | 1.93 (0.69 to 5.41) | 0.21 | |
| 110–139 | 8146 | 71 | 0.9 | 1.00 (ref.) | ||
| ≥140 | 541 | 11 | 2.0 | 2.38 (1.12 to 5.10) | 0.025 | |
| Excluding cases documented within 11 and 14 weeks of gestation with a period of fewer than 2 weeks from the date of the Hb tests § | 9299 | 366 | 3.9 | |||
| Hb concentrations (g/L) | ||||||
| <110 | 347 | 19 | 5.5 | 1.95 (1.15 to 3.32) | 0.013 | |
| 110–139 | 8374 | 299 | 3.6 | 1.00 (ref.) | ||
| ≥140 | 578 | 48 | 8.3 | 2.20 (1.48 to 3.27) | <0.001 | |
| All pregnancy loss documented up to and beyond 24 weeks of gestation ¶ | 9488 | 555 | 5.9 | |||
| Hb concentrations (g/L) | ||||||
| <110 | 362 | 34 | 9.4 | 2.24 (1.50 to 3.36) | <0.001 | |
| 110–139 | 8536 | 461 | 5.4 | 1.00 (ref.) | ||
| ≥140 | 590 | 60 | 10.2 | 1.66 (1.17 to 2.37) | 0.005 | |
| Miscarriage | Adjusted Model * | ||||||
|---|---|---|---|---|---|---|---|
| Maternal Characteristics | No. of Participants | n | % | OR (95% CI) | p-Value | p for Interaction | |
| BMI <25 Kg/m2 | 5376 | 252 | 4.7 | 0.13 | |||
| Haemoglobin level (g/L) | |||||||
| <110 | 205 | 20 | 9.8 | 2.77 (1.66 to 4.62) | <0.001 | ||
| 110–139 | 4883 | 207 | 4.2 | 1.00 (ref.) | |||
| ≥140 | 288 | 25 | 8.7 | 2.25 (1.40 to 3.61) | 0.001 | ||
| BMI ≥25 Kg/m2 | 3559 | 217 | 6.1 | ||||
| Haemoglobin level (g/L) | |||||||
| <110 | 125 | 8 | 6.4 | 1.33 (0.63 to 2.78) | 0.45 | ||
| 110–139 | 3176 | 186 | 5.9 | 1.00 (ref.) | |||
| ≥140 | 258 | 23 | 8.9 | 1.45 (0.88 to 2.39) | 0.14 | ||
| Maternal age ≤ 29 years | 4187 | 175 | 4.2 | 0.55 | |||
| Haemoglobin level (g/L) | |||||||
| <110 | 169 | 11 | 6.5 | 2.14 (1.05 to 4.34) | 0.036 | ||
| 110–139 | 3768 | 142 | 3.8 | 1.00 (ref.) | |||
| ≥140 | 250 | 22 | 8.8 | 2.40 (1.47 to 4.24) | 0.002 | ||
| Maternal age > 29 years | 5266 | 345 | 6.6 | ||||
| Haemoglobin level (g/L) | |||||||
| <110 | 189 | 19 | 10.1 | 2.09 (1.24 to 3.52) | 0.006 | ||
| 110–139 | 4737 | 288 | 6.1 | 1.00 (ref.) | |||
| ≥140 | 340 | 38 | 11.2 | 1.56 (1.01 to 2.40) | 0.044 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-López, A.; Ribot, B.; Basora, J.; Arija, V. High and Low Haemoglobin Levels in Early Pregnancy Are Associated to a Higher Risk of Miscarriage: A Population-Based Cohort Study. Nutrients 2021, 13, 1578. https://doi.org/10.3390/nu13051578
Díaz-López A, Ribot B, Basora J, Arija V. High and Low Haemoglobin Levels in Early Pregnancy Are Associated to a Higher Risk of Miscarriage: A Population-Based Cohort Study. Nutrients. 2021; 13(5):1578. https://doi.org/10.3390/nu13051578
Chicago/Turabian StyleDíaz-López, Andrés, Blanca Ribot, Josep Basora, and Victoria Arija. 2021. "High and Low Haemoglobin Levels in Early Pregnancy Are Associated to a Higher Risk of Miscarriage: A Population-Based Cohort Study" Nutrients 13, no. 5: 1578. https://doi.org/10.3390/nu13051578
APA StyleDíaz-López, A., Ribot, B., Basora, J., & Arija, V. (2021). High and Low Haemoglobin Levels in Early Pregnancy Are Associated to a Higher Risk of Miscarriage: A Population-Based Cohort Study. Nutrients, 13(5), 1578. https://doi.org/10.3390/nu13051578







