Risk of Iron Overload in Obesity and Implications in Metabolic Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Dietary Assessment and Analysis
2.3. Assessment of Haem, Non-Haem and Fortified Iron Content of Foods
2.4. Measurements of Body Composition
2.5. Biochemical Analysis
2.5.1. Iron Homeostasis
2.5.2. Serum Lipids, Adipocytokines and Markers of Glucose Homeostasis
2.6. Assessment of Visceral Adiposity, Adipose Tissue Dysfunction and Lipid Accumulation
2.7. Assessment of Metabolic Health
2.8. Statistical Analysis
3. Results
3.1. Iron Store Status in Obesity
3.2. Associations between Measures of Body Composition, Iron Biomarkers and Adipocytokines with Serum Hepcidin and Serum Ferritin
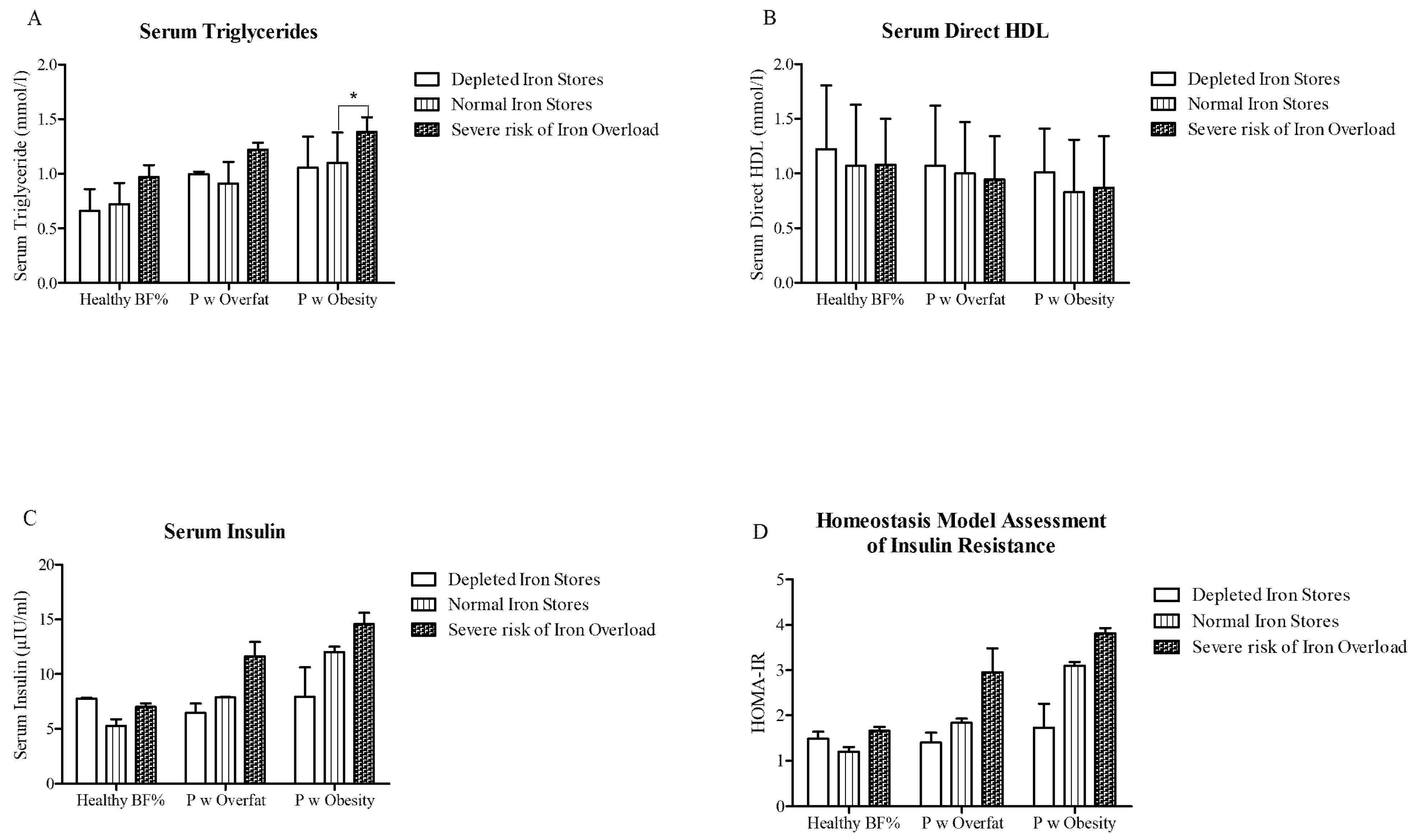
3.3. Relationship between Elevated Serum Ferritin and Metabolic Health
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E.; NASH Clinical Research Network. Serum ferritin is an inde-pendent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Mittelman, S.D.; Watanabe, R.M.; Coates, T.D.; Wood, J.C. Pancreatic iron and glucose dysregulation in thalas-semia major. Am. J. Hematol. 2012, 87, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Vari, I.S.; Balkau, B.; Kettaneh, A.; André, P.; Tichet, J.; Fumeron, F.; Caces, E.; Marre, M.; Grandchamp, B.; Ducimetière, P.; et al. Ferritin and Transferrin Are Associated With Metabolic Syndrome Abnormalities and Their Change Over Time in a General Population: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2007, 30, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, S.; Sharp, P.; Ramesh, B.; Srai, S.K. Inhibition of iron transport across human intestinal epithelial cells by hepcidin. Blood 2004, 104, 2178–2180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravasi, G.; Pelucchi, S.; Trombini, P.; Mariani, R.; Tomosugi, N.; Modignani, G.L.; Pozzi, M.; Nemeth, E.; Ganz, T.; Hayashi, H.; et al. Hepcidin Expression in Iron Overload Diseases Is Variably Modulated by Circulating Factors. PLoS ONE 2012, 7, e36425. [Google Scholar] [CrossRef]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Troutt, J.S.; Konrad, R.J.; Ghabril, M.; Saxena, R.; Bell, L.N.; Kowdley, K.V.; Chalasani, N. Serum hepcidin levels are associated with obesity but not liver disease. Obesity 2014, 22, 836–841. [Google Scholar] [CrossRef]
- Jehn, M.; Clark, J.M.; Guallar, E. Serum Ferritin and Risk of the Metabolic Syndrome in U.S. Adults. Diabetes Care 2004, 27, 2422–2428. [Google Scholar] [CrossRef]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini–Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased Adipose Tissue Expression of Hepcidin in Severe Obesity Is Independent From Diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef]
- Tussing-Humphreys, L.M.; Nemeth, E.; Fantuzzi, G.; Freels, S.; Guzman, G.; Holterman, A.X.L.; Braunschweig, C. Elevated systemic hep-cidin and iron depletion in obese premenopausal females. Obesity 2010, 18, 1449–1456. [Google Scholar] [CrossRef]
- Amato, A.; Santoro, N.; Calabrò, P.; Grandone, A.; Swinkels, D.W.; Perrone, L.; Del Giudice, E.M. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int. J. Obes. 2010, 34, 1772–1774. [Google Scholar] [CrossRef]
- Alam, F.; Memon, A.S.; Fatima, S.S. Increased body mass index may lead to hyperferritinemia irrespective of body iron stores. Pakistan J. Med. Sci. 2015, 31, 1521–1526. [Google Scholar] [CrossRef]
- Moschonis, G.; Chrousos, G.P.; Lionis, C.; Mougios, V.; Manios, Y. Association of total body and visceral fat mass with iron deficiency in preadolescents: The Healthy Growth Study. Br. J. Nutr. 2011, 108, 710–719. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Isabelle, C.E.; Bissani, H.N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The effect of central obesity on in fl ammation, hepcidin and iron metabolism in young women. Int. J. Obes. 2020, 44, 1291–1300. [Google Scholar] [CrossRef]
- IUNA. National Adult Nutrition Survey Summary Report; IUNA: Dublin, Ireland, 2011. [Google Scholar]
- Hol-land, V.; Unwin, I.D.; Buss, H.D. Cereals and Cereal Products. The Third Supplement to McCance and Widdowson’s The Composition of Foods. Nutr. Bull. 1988, 13, 169–170. [Google Scholar] [CrossRef]
- Halliday, A. Milk Products and Eggs 4th Supplement to McCance and Widdowson’s The Composition of Foods. Nutr. Bull. 1990, 15, 133–134. [Google Scholar] [CrossRef]
- Holland, B.; Widdowson, E.M.; Unwin, I.D.; Buss, D.H. Vegetables, Herbs and Spices: Fifth Supplement to McCance and Widdowson’s The Composition of Foods. Vegetables, Herbs and Spices: Fifth Supplement to McCance and Widdowson’s The Composition of Foods. R. Soc. Chem. 1991, 36, 432. [Google Scholar] [CrossRef]
- Johnsen, D.B.; Holland, I.D. Unwin und D. H. Bus: Fruit and Nuts. First Supplement to the Fifth Edition of McCance and Widdowson’s The Composition of Foods; The Royal Society of Chemistry: Cambridge, UK, 1992; Volume 37, p. 107. [Google Scholar] [CrossRef]
- Behnke, U.B.; Holland, J.; Brown, D.H. Buss: Fish and Fish Products. Third Supplement to the Fifth Edition of McCance and Widdowson’s The Composition of Foods. 135 Seiten, zahlr. Tab. R. Soc. Chem. Minist. Agric. Fish. Food Nahr. 1994, 38, 451–452. [Google Scholar] [CrossRef]
- Behnke, U. Meat, Poultry and Game. Fifth supplement to the fifth Edition of McCance and Widdowson’s The Composition of Foods; VII and 161 pages, 3 figures and numerous tables. R. Soc. Chem. Minist. Agric. Fish. Food Nahr. 1996, 40, 226. [Google Scholar] [CrossRef]
- Behnke, U.W.; Chan, J.; Brown, S.M. Church and D. H. Buss: Meat Products and Dishes. Sixth supplement to the Fifth Edition of McCance and Widdowson’s The Composition of Foods;VII and 162 pages, numerous tables. R. Soc. Chem. Minist. Agric. Fish. Food Nahr. 1997. [Google Scholar] [CrossRef]
- Pennington, J.A. McCance and Widdowson’s the composition of foods. J. Food Compos. Anal. 1992, 5, 264. [Google Scholar] [CrossRef]
- Pennington, J.A.T.; Holland, B.; Welch, A.A.; Buss, D.H. Vegetable Dishes, (The second supplement to McCance & Widdowson′s The Composition of Foods. J. Food Compos. Anal. 1993, 242. [Google Scholar]
- Robertson, C. McCance and Widdowson’s The Composition of Foods–Sixth. Nutr. Bull. 2003, 18, 1–13. [Google Scholar]
- Lombardi-Boccia, G.; Martínez-Domínguez, B.; Aguzzi, A.; Rincón-León, F. Optimization of heme iron analysis in raw and cooked red meat. Food Chem. 2002, 78, 505–510. [Google Scholar] [CrossRef]
- Skolmowska, D.; Głąbska, D. Analysis of Heme and Non-Heme Iron Intake and Iron Dietary Sources in Adolescent Menstruating Females in a National Polish Sample. Nutrition 2019, 11, 1049. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, F.; Lu, Y.; Wu, C.; Wang, Z.; Zang, J.; Guo, C.; Jia, X.; Yao, J.; Peng, H.; et al. Total and Nonheme Dietary Iron Intake Is Associated with Metabolic Syndrome and Its Components in Chinese Men and Women. Nutrition 2018, 10, 1663. [Google Scholar] [CrossRef]
- Buffini, M.; Goscinny, S.; Van Loco, J.; Nugent, A.P.; Walton, J.; Flynn, A.; Gibney, M.J.; McNulty, B.A. Dietary intakes of six intense sweeteners by Irish adults. Food Addit. Contam. Part A 2017, 35, 425–438. [Google Scholar] [CrossRef]
- Linares, C.L.; Ciangura, C.; Bouillot, J.-L.; Coupaye, M.; Declèves, X.; Poitou, C.; Basdevant, A.; Oppert, J.-M. Validity of Leg-to-Leg Bioelectrical Impedance Analysis to Estimate Body Fat in Obesity. Obes. Surg. 2010, 21, 917–923. [Google Scholar] [CrossRef]
- Masih, D.; Rakhra, G.; Vats, A.; Verma, S.; Sharma, Y.; Singh, S. Assessing body composition by bioelectric impedance analysis and dual-energy X-ray absorptiometry in physically active normal and overweight Indian males. Natl. J. Physiol. Pharm. Pharmacol. 2018, 1. [Google Scholar] [CrossRef]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serum Ferritin Concentrations for Assessment of Iron Status and Iron Deficiency in Populations; WHO Vitamin and Mineral Nutrition Information System: Geneva, Switzerland, 2011. [Google Scholar]
- Adams, P.C.; Reboussin, D.M.; Barton, J.C.; McLaren, C.E.; Eckfeldt, J.H.; McLaren, G.D.; Dawkins, F.W.; Acton, R.T.; Harris, E.L.; Gordeuk, V.R.; et al. Hemochromatosis and Iron-Overload Screening in a Racially Diverse Population. N. Engl. J. Med. 2005, 352, 1769–1778. [Google Scholar] [CrossRef]
- Beaton, M.D.; Adams, P.C. Treatment of hyperferritinemia. Ann. Hepatol. 2012, 11, 294–300. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C. Visceral Adiposity Index: An Indicator of Adipose Tissue Dysfunction. Int. J. Endocrinol. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Chiang, J.K.; Koo, M. Lipid accumulation product: A simple and accurate index for predicting metabolic syndrome in Tai-wanese people aged 50 and over. BMC Cardiovasc. Disord. 2012, 12, 78. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults, E. Executive summary of the third report of the national cholesterol (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Moreno-Navarrete, J.M.; Moreno, M.; Puig, J.; Blasco, G.; Ortega, F.; Xifra, G.; Ricart, W.; Fernández-Real, J.M. Hepatic iron content is independently associated with serum hepcidin levels in subjects with obesity. Clin. Nutr. 2017, 36, 1434–1439. [Google Scholar] [CrossRef]
- Tussing-Humphreys, L.M.; Nemeth, E.; Fantuzzi, G.; Freels, S.; Holterman, A.-X.L.; Galvani, C.; Ayloo, S.; Vitello, J.; Braunschweig, C. Decreased Serum Hepcidin and Improved Functional Iron Status 6 Months After Restrictive Bariatric Surgery. Obesety 2010, 18, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Bryant, C.E.; Rooney, K.B.; Steinbeck, K.S.; Griffin, H.J.; Petocz, P.; O’Connor, H.T. Iron, Hepcidin and Inflammatory Status of Young Healthy Overweight and Obese Women in Australia. PLoS ONE 2013, 8, e68675. [Google Scholar] [CrossRef] [PubMed]
- Manolov, V.E.; Atanasova, B.D.; Velizarova, M.G.; Vasilev, V.G.; Tzatchev, K.N. Serum hepcidin levels in Bulgarian popula-tion. Clin. Lab. 2014, 60, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a putative mediator of anemia of in-flammation, is a type II acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, But Not Tumor Necrosis Factor-α,in Vivo1. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Chung, B.; Matak, P.; McKie, A.T.; Sharp, P. Leptin Increases the Expression of the Iron Regulatory Hormone Hepcidin in HuH7 Human Hepatoma Cells. J. Nutr. 2007, 137, 2366–2370. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of Nonalcoholic Fatty Liver Disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef]
- Sivitz, W.I.; Walsh, S.A.; Morgan, D.A.; Thomas, M.J.; Haynes, W.G. Effects of Leptin on Insulin Sensitivity in Normal Rats*. Endocrinology 1997, 138, 3395–3401. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Zinkernagel, A.S.; Datta, V.; Lauth, X.; Johnson, R.S.; Nizet, V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood 2006, 107, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Findik, D.D. Gender-related variations in iron metabolism and liver diseases. World J. Hepatol. 2010, 2, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—The Third National Health and Nutrition Examination Survey. Int. J. Obes. 2001, 25, 639–645. [Google Scholar] [CrossRef]
- Ruivard, M.; Lainé, F.; Ganz, T.; Olbina, G.; Westerman, M.; Nemeth, E.; Rambeau, M.; Mazur, A.; Gerbaud, L.; Tournilhac, V.; et al. Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin. J. Hepatol. 2009, 50, 1219–1225. [Google Scholar] [CrossRef]
- Orban, E.; Schwab, S.; Thorand, B.; Huth, C. Association of iron indices and type 2 diabetes: A meta-analysis of observational studies. Diabetes/Metabolism Res. Rev. 2014, 30, 372–394. [Google Scholar] [CrossRef]
- Kernan, K.F.; A Carcillo, J. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Fujita, N.; Sugimoto, R.; Takeo, M.; Urawa, N.; Mifuji, R.; Tanaka, H.; Kobayashi, Y.; Iwasa, M.; Watanabe, S.; Adachi, Y.; et al. Hepcidin Expression in the Liver: Relatively Low Level in Patients with Chronic Hepatitis C. Mol. Med. 2007, 13, 97–104. [Google Scholar] [CrossRef]
- Sachinidis, A.; Doumas, M.; Imprialos, K.; Stavropoulos, K.; Katsimardou, A.; Athyros, V.G. Dysmetabolic Iron Overload in Metabolic Syndrome. Curr. Pharm. Des. 2020, 26, 1019–1024. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Cross-Talk Between Iron Metabolism and Diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef]
- Bozzini, C.; Girelli, D.; Olivieri, O.; Martinelli, N.; Bassi, A.; De Matteis, G.; Tenuti, I.; Lotto, V.; Friso, S.; Pizzolo, F.; et al. Prevalence of Body Iron Excess in the Metabolic Syndrome. Diabetes Care 2005, 28, 2061–2063. [Google Scholar] [CrossRef]
- Rumberger, J.M.; Peters, T.; Burrington, C.; Green, A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes 2004, 53, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Homko, C.J.; Cheung, P.; Boden, G. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes 2003, 52, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Datz, C.; Felder, T.K.; Niederseer, D.; Aigner, E. Iron homeostasis in the Metabolic Syndrome. Eur. J. Clin. Investig. 2013, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.T.; Schioedt, F.V.; Junker, A.E.; Magnussen, K. Diagnosis and Treatment of Genetic HFE-Hemochromatosis: The Danish Aspect. Gastroenterol. Res. 2019, 12, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Franco, O.H.; Hu, F.B.; Cai, L.; Yu, Z.; Li, H.; Ye, X.; Qi, Q.; Xingwang, Y.; Pan, A.; et al. Ferritin Concentrations, Metabolic Syndrome, and Type 2 Diabetes in Middle-Aged and Elderly Chinese. J. Clin. Endocrinol. Metab. 2008, 93, 4690–4696. [Google Scholar] [CrossRef]
- Upala, S.; Jaruvongvanich, V.; Riangwiwat, T.; Sanguankeo, A. Outcome of phlebotomy for treating nonalcoholic fatty liver disease: A systematic review and meta-analysis. Saudi J. Gastroenterol. 2016, 22, 407–414. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Later, W.; Hitze, B.; Sato, T.; Kossel, E.; Glüer, C.C. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray ab-sorptiometry. Obes. Facts. 2008, 1, 319–324. [Google Scholar] [CrossRef]

| Depleted Iron Stores (n = 69) | Normal Iron Stores (n = 849) | Severe Risk of Iron Overload (n = 202) | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | p† | ηp2 | |
| Age (years) | ||||||||
| Total | 43.00 a | 15 | 39.00 a | 26 | 52.00 b | 21 | <0.001 | 0.051 |
| Male | 53.00 a,b | 25 | 36.00 a | 25 | 48.00 b | 23 | <0.001 | 0.042 |
| Female | 42.50 a | 14 | 41.00 a | 26 | 57.00 b | 16.5 | <0.001 | 0.063 |
| Body mass index (kg/m2) | ||||||||
| Total | 25.42 a,b | 5.21 | 25.81 a | 5.82 | 28.82 b | 5.65 | <0.001 | 0.031 |
| Male | 27.06 a,b | 3.54 | 26.61 a | 5.17 | 28.81 b | 5.16 | <0.001 | 0.032 |
| Female | 25.14 a,b | 5.57 | 25.25 a | 5.8 | 29.04 b | 6.93 | 0.032 | 0.023 |
| Waist circumference (cm) | ||||||||
| Total | 83.15 a,b | 14.86 | 88.50 a | 19.7 | 99.50 b | 17.7 | 0.035 | 0.011 |
| Male | 92.2 | 15.8 | 93.63 | 18 | 101.05 | 16.2 | 0.368 | - |
| Female | 82.5 | 13.98 | 84.05 | 17 | 94.05 | 18.23 | 1.00 | - |
| Waist:hip ratio | ||||||||
| Total | 0.84 | 0.11 | 0.87 | 0.13 | 0.92 | 0.11 | 0.480 | - |
| Male | 0.9 | 0.1 | 0.9 | 0.12 | 0.95 | 0.1 | 0.400 | - |
| Female | 0.82 | 0.11 | 0.84 | 0.11 | 0.86 | 0.11 | 1.00 | - |
| % Body fat | ||||||||
| Total | 32.70 a,b | 9.5 | 27.88 a | 13.38 | 29.28 b | 14.59 | <0.001 | 0.025 |
| Male | 22.10 a,b | 8.63 | 22.05 a | 10.35 | 26.55 b | 7.9 | 0.008 | 0.028 |
| Female | 33.7 | 7.55 | 34.25 | 10.5 | 40.4 | 9.13 | 0.400 | - |
| Lipid accumulation product index | ||||||||
| Total | 23.06 a,b | 25.19 | 26.95 b | 31.12 | 46.46 b | 45.62 | <0.001 | 0.022 |
| Male | 38.61 | 61.82 | 32.15 | 33.37 | 44.25 | 51.53 | 0.440 | - |
| Female | 22.47 | 24.57 | 24.31 | 24.47 | 48.36 | 46.26 | 0.064 | - |
| Visceral adiposity index | ||||||||
| Total | 0.96 a | 0.67 | 1.06 a | 0.87 | 1.39 b | 1.28 | <0.001 | 0.025 |
| Male | 1.29 | 1.34 | 1.09 | 0.97 | 1.24 | 1.11 | 0.712 | - |
| Female | 0.09 a | 0.62 | 1.05 a | 0.7 | 1.45 b | 1.46 | <0.001 | 0.044 |
| % population displaying adipose tissue dysfunction | ||||||||
| Total | 7.8% a | 12.7% a | 25% b | <0.001 | ||||
| Male | 14.30% | 13.90% | 21.60% | 1.00 | ||||
| Female | 6.8% a | 11.6% a | 31.9% b | <0.001 | ||||
| People with Healthy % BF (n = 407) | People with Overfat (n = 342) | People with Obesity (n = 237) | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | p† | ηp2 | |
| Serum Hepcidin (ng/mL) | ||||||||
| Total | 5.62 a | 4.46 | 6.78 b | 4.91 | 7.73 b | 5.1 | <0.001 | 0.017 |
| Male | 6.45 a | 4.42 | 7.49 ab | 5.28 | 9.06 b | 4.67 | 0.048 | 0.021 |
| Female | 4.94 | 4.16 | 5.76 | 4.86 | 7.21 | 4.83 | 0.270 | - |
| Serum Ferritin (ng/mL) | ||||||||
| Total | 75.91 a | 93.94 | 98.65 b | 120.63 | 132.86 b | 137.23 | <0.001 | 0.026 |
| Male | 120.50 a | 91.93 | 149.68 a | 112.52 | 175.91 b | 138.04 | <0.001 | 0.051 |
| Female | 39.69 | 52.46 | 70.27 | 84.00 | 67.55 | 116.31 | 0.984 | - |
| Hepcidin:Ferritin Ratio | ||||||||
| Total | 0.08 | 0.08 | 0.07 | 0.06 | 0.06 | 0.06 | 0.216 | - |
| Male | 0.06 a | 0.05 | 0.05 a,b | 0.04 | 0.048 b | 0.04 | 0.042 | 0.021 |
| Female | 0.10 | 0.10 | 0.09 | 0.08 | 0.10 | 0.08 | 1.00 | - |
| Haemoglobin (g/dL) | ||||||||
| Total | 14.10 a | 2.00 | 14.20 a,b | 1.90 | 14.50 b | 1.83 | 0.048 | 0.01 |
| Male | 15.10 | 1.50 | 15.10 | 1.40 | 15.30 | 1.4 | 0.066 | - |
| Female | 13.30 | 1.10 | 13.50 | 1.30 | 13.50 | 1.45 | 1.00 | - |
| Mean Corpuscular Volume (fL) | ||||||||
| Total | 91.20 | 5.10 | 90.50 | 5.00 | 90.30 | 5.2 | 0.132 | - |
| Male | 90.80 | 5.10 | 90.65 | 4.88 | 90.90 | 4.95 | 1.00 | - |
| Female | 91.55 a | 5.07 | 90.20 a,b | 5.30 | 89.90 b | 5.35 | 0.03 | 0.024 |
| Serum Total Iron-Binding Capacity (TIBC) (µmol/L) | ||||||||
| Total | 59.15 | 11.71 | 58.96 | 11.53 | 60.30 | 9.38 | 1.00 | - |
| Male | 56.84 | 8.16 | 58.20 | 10.32 | 59.10 | 11.3 | 1.00 | - |
| Female | 61.60 | 13.41 | 59.87 | 11.02 | 61.70 | 11.74 | 1.00 | - |
| Serum Hepcidin † | Serum Ferritin † | |||
|---|---|---|---|---|
| Males (β (95% CI)) | Females (β (95% CI)) | Males (β (95% CI)) | Females (β (95% CI)) | |
| Body composition | ||||
| BMI (kg/m2) † | 0.175 (0.31, 0.93) *** | 0.097 (0.04, 0.66) | 0.189 (0.51, 1.40) *** | 0.068 (−0.08, 0.75) |
| % Body fat † | 0.195 (0.15, 0.47) *** | 0.067 (−0.07, 0.45) | 0.228 (0.29, 0.74) *** | 0.064 (−0.10, 0.59) |
| Waist circumference (cm) † | 0.158 (0.24, 1.06) * | 0.065 (−0.10, 0.69) | 0.187 (0.50, 1.65) *** | 0.058 (−0.17, 0.87) |
| Waist:hip ratio † | 0.149 (0.29, 1.55) | 0.046 (−0.29, 0.94) | 0.160 (0.49, 2.27) * | 0.047 (−0.37, 1.26) |
| Visceral adiposity index † | 0.067 (−0.03, 0.15) | 0.084 (−0.01, 0.20) | 0.146 (0.06, 0.31) | 0.198 (0.17, 0.44) *** |
| Lipid accumulation product † | 0.105 (−0.001, 0.14) | 0.097 (0.001, 0.17) | 0.178 (0.07, 0.27) * | 0.149 (0.06, 0.29) * |
| Iron biomarkers | ||||
| Serum ferritin † | 0.509 (0.33, 0.43) *** | 0.532 (0.34, 0.46) *** | - | - |
| Serum hepcidin † | - | - | 0.516 (0.59, 0.79) *** | 0.521 (0.60, 0.79) *** |
| Hepcidin:ferritin ratio † | 0.246 (0.15, 0.28) *** | 0.246 (0.16, 0.30) *** | −0.677 (−0.86,−0.72) *** | −0.630 (−0.85, −0.70) *** |
| Adipocytokines | ||||
| Serum IL6 † | 0.055 (−0.02, 0.10) | 0.198 (0.09, 0.22) *** | −0.027 (−0.12, 0.06) | 0.145 (0.06, 0.24) * |
| IL6:IL10 ratio † | 0.041 (−0.03, 0.09) | 0.110 (0.01, 0.15) | −0.003 (−0.09, 0.08) | 0.111 (0.02, 0.20) |
| Serum TNFα † | 0.036 (−0.08, 0.22) | 0.136 (0.12, 0.48) * | −0.029 (−0.29, 0.14) | 0.112 (0.09, 0.57) |
| Serum Hs CRP † | 0.102 (0.005, 0.13) | 0.085 (−0.01, 0.12) | 0.006 (−0.08, 0.09) | 0.051 (−0.04, 0.14) |
| Serum leptin † | 0.126 (0.02, 0.18) | 0.008 (−0.06, 0.07) | 0.181 (0.09, 0.33) * | 0.025 (−0.06, 0.11) |
| Serum adiponectin † | −0.124 (−0.27, −0.06) * | −0.052 (−0.19, 0.04) | −0.136 (−0.41, −0.11) * | −0.043 (−0.24, 0.07) |
| Leptin: adiponectin ratio † | 0.158 (0.04, 0.17) * | 0.031 (−0.03, 0.07) | 0.238 (0.13, 0.32) *** | 0.042 (−0.03, 0.10) |
| Serum Ferritin | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| β (95% CI) | p† | β (95% CI) | p† | β (95% CI) | p† | |
| Total cholesterol | 0.064 (0.00, 0.03) | 0.248 | 0.04 (−0.01, 0.02) | 1.00 | 0.044 (−0.05, 0.03) | 1.00 |
| HDL cholesterol | −0.231 (−0.08,−0.05) | <0.001 | −0.267 (−0.09, −0.06) | <0.001 | −0.06 (−0.03, 0.001) | 0.496 |
| LDL cholesterol | 0.082 (0.01, 0.05) | 0.048 | 0.07 (0.003, 0.05) | 0.232 | 0.015 (−0.02, 0.03) | 1.00 |
| Triglycerides | 0.272 (0.11, 0.17) | <0.001 | 0.289 (0.12, 0.18) | <0.001 | 0.183 (0.06, 0.13) | <0.001 |
| Insulin | 0.137 (0.07, 0.17) | <0.001 | 0.187 (0.11, 0.21) | <0.001 | 0.087 (0.02, 0.12) | 0.040 |
| Glucose | 0.209 (0.03, 0.05) | <0.001 | 0.180 (0.02, 0.04) | <0.001 | 0.092 (0.004, 0.03) | 0.072 |
| HOMA-IR | 0.165 (0.10, 0.21) | <0.001 | 0.204 (0.13, 0.24) | <0.001 | 0.099 (0.04, 0.15) | 0.012 |
| QUICKI | −0.160 (−0.03, −0.01) | <0.001 | −0.203 (−0.04, −0.02) | <0.001 | −0.094 (−0.02, −0.004) | 0.024 |
| People with Healthy % BF | People with Overfat | People with Obesity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depleted Iron Stores | Normal Iron Stores | Severe Risk of Iron Overload | p | Depleted Iron Stores | Normal Iron Stores | Severe Risk of Iron Overload | p | Depleted Iron Stores | Normal Iron Stores | Severe Risk of Iron Overload | p | |
| No Risk Factors | 83.30% | 64.60% | 42.80% | 0.059 | 41.20% | 35.30% | 27.50% | 0.527 | 0.00% | 6.60% | 7.10% | 0.223 |
| 1–2 Risk Factors | 16.70% | 34.30% | 53.60% | 52.90% | 52.50% | 64.70% | 75.00% | 70.40% | 53.60% | |||
| ≥3 Risk Factors | 0.00% | 1.10% | 3.60% | 5.90% | 12.20% | 7.80% | 25.00% | 23.00% | 39.30% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore Heslin, A.; O’Donnell, A.; Buffini, M.; Nugent, A.P.; Walton, J.; Flynn, A.; McNulty, B.A. Risk of Iron Overload in Obesity and Implications in Metabolic Health. Nutrients 2021, 13, 1539. https://doi.org/10.3390/nu13051539
Moore Heslin A, O’Donnell A, Buffini M, Nugent AP, Walton J, Flynn A, McNulty BA. Risk of Iron Overload in Obesity and Implications in Metabolic Health. Nutrients. 2021; 13(5):1539. https://doi.org/10.3390/nu13051539
Chicago/Turabian StyleMoore Heslin, Aoibhín, Aisling O’Donnell, Maria Buffini, Anne P. Nugent, Janette Walton, Albert Flynn, and Breige A. McNulty. 2021. "Risk of Iron Overload in Obesity and Implications in Metabolic Health" Nutrients 13, no. 5: 1539. https://doi.org/10.3390/nu13051539
APA StyleMoore Heslin, A., O’Donnell, A., Buffini, M., Nugent, A. P., Walton, J., Flynn, A., & McNulty, B. A. (2021). Risk of Iron Overload in Obesity and Implications in Metabolic Health. Nutrients, 13(5), 1539. https://doi.org/10.3390/nu13051539







