Sarcopenia and Vitamin D Deficiency in Patients with Crohn’s Disease: Pathological Conditions That Should Be Linked Together
Abstract
1. Introduction
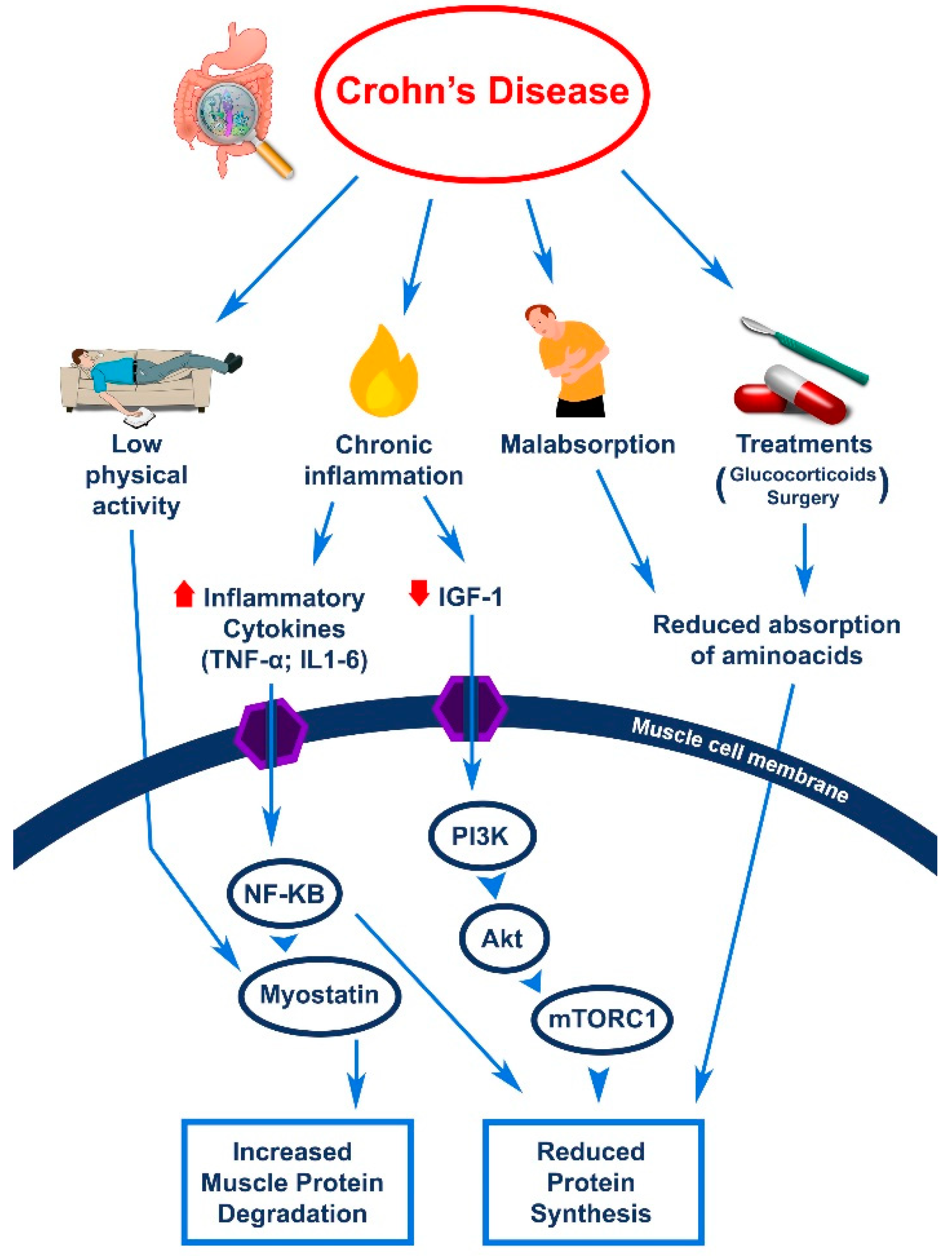
2. Sarcopenia and Crohn’s Disease
2.1. Definition of Sarcopenia
2.2. The Assessment of Sarcopenia
2.3. Sarcopenia in Patients with CD
3. Vitamin D Deficiency and Crohn’s Disease
3.1. Definition of Vitamin D Deficiency
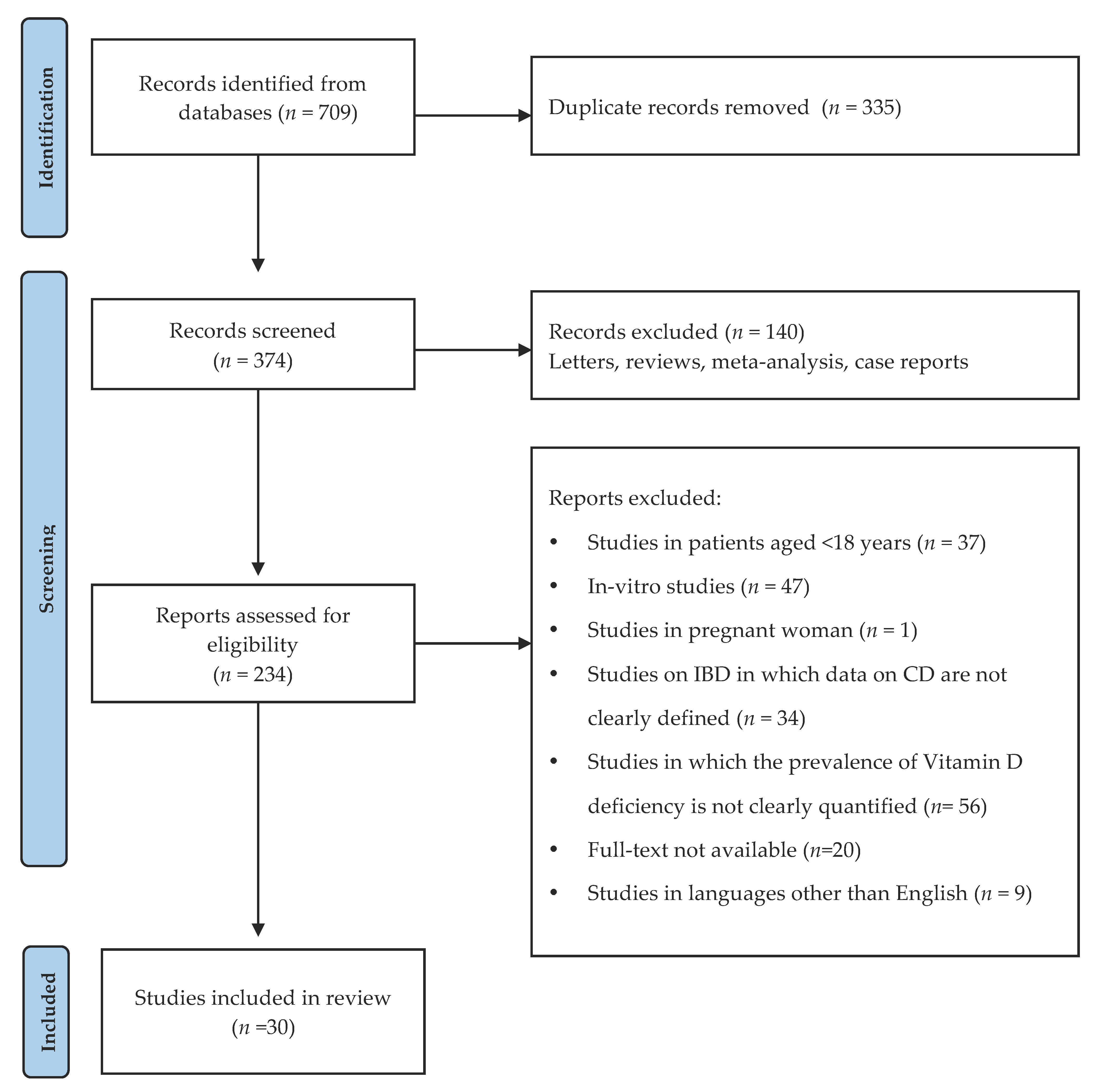
3.2. Prevalence of Vitamin D Deficiency in Patients with CD
4. Vitamin D and Sarcopenia
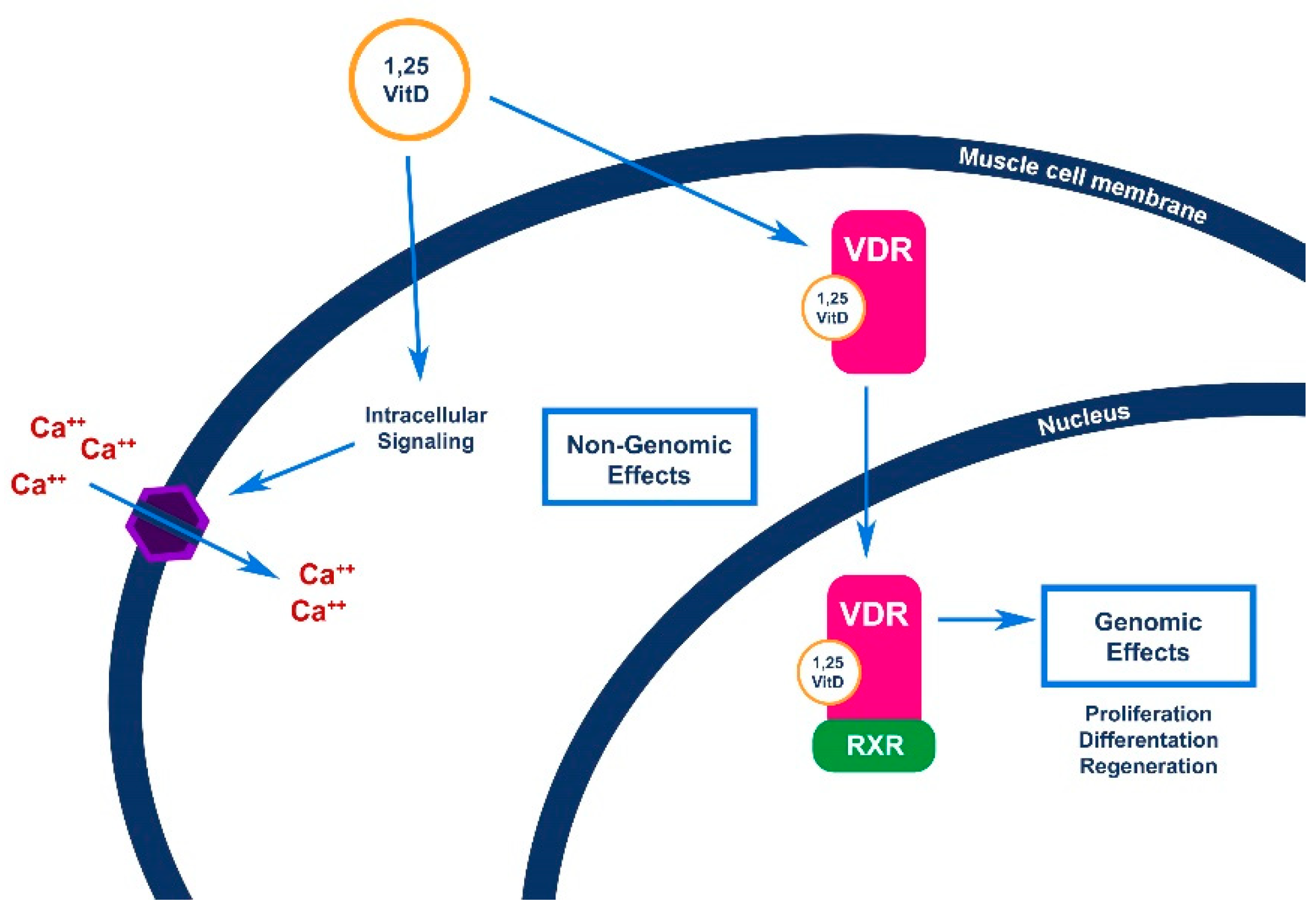
4.1. Effects of Vitamin D on Skeletal Muscle Function
4.2. Vitamin D and Sarcopenia: Evidence from Other Patients
5. The Missing Step: The Effect of Vitamin D Supplementation on Sarcopenia in Patients with CD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Loftus, E.V.; Colombel, J.F.; Sandborn, W.J. The natural history of adult crohn’s disease in population-based cohorts. Am. J. Gastroenterol. 2010, 105, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Grillot, J.; D’Engremont, C.; Parmentier, A.L.; Lakkis, Z.; Piton, G.; Cazaux, D.; Gay, C.; De Billy, M.; Koch, S.; Borot, S.; et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin. Nutr. 2020, 39, 3024–3030. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord. 2012, 13, 71–77. [Google Scholar] [CrossRef]
- Mager, D.R.; Carroll, M.W.; Wine, E.; Siminoski, K.; MacDonald, K.; Kluthe, C.L.; Medvedev, P.; Chen, M.; Wu, J.; Turner, J.M.; et al. Vitamin D status and risk for sarcopenia in youth with inflammatory bowel diseases. Eur. J. Clin. Nutr. 2018, 72, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Almurdhi, M.M.; Reeves, N.D.; Bowling, F.L.; Boulton, A.J.M.; Jeziorska, M.; Malik, R.A. Distal lower limb strength is reduced in subjects with impaired glucose tolerance and is related to elevated intramuscular fat level and vitamin D deficiency. Diabet. Med. 2017, 34, 356–363. [Google Scholar] [CrossRef]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Del Signore, S.; Donahue, S.; Morley, J.; et al. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; May, C.; Patel, H.P.; Baxter, M.; Sayer, A.A.; Roberts, H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): Study protocol. Pilot Feasibility Stud. 2016, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.; Beaudart, C.; Reginster, J.Y.; Petermans, J.; Bruyère, O. Comparison of the performance of five screening methods for sarcopenia. Clin. Epidemiol. 2018, 10, 71–82. [Google Scholar] [CrossRef]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional aspects in inflammatory bowel diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and inflammatory bowel disease: A systematic review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Adams, D.W.; Gurwara, S.; Silver, H.J.; Horst, S.N.; Beaulieu, D.B.; Schwartz, D.A.; Seidner, D.L. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm. Bowel Dis. 2017, 23, 1182–1186. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Quinlan, J.I.; Overthrow, K.; Greig, C.; Lord, J.M.; Armstrong, M.J.; Cooper, S.C. Sarcopenia in inflammatory bowel disease: A narrative overview. Nutrients 2021, 13, 656. [Google Scholar] [CrossRef]
- Tigas, S.; Tsatsoulis, A. Endocrine and metabolic manifestations in inflammatory bowel disease. Ann. Gastroenterol. 2012, 25, 37–44. [Google Scholar]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Boparai, G.; Kedia, S.; Kandasamy, D.; Sharma, R.; Madhusudhan, K.S.; Dash, N.R.; Sahu, P.; Pal, S.; Sahni, P.; Panwar, R.; et al. Combination of sarcopenia and high visceral fat predict poor outcomes in patients with Crohn’s disease. Eur. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Celentano, V.; Kamil-Mustafa, L.; Beable, R.; Ball, C.; Flashman, K.G.; Jennings, Z.; O’Leary, D.P.; Higginson, A.; Luxton, S. Preoperative assessment of skeletal muscle mass during magnetic resonance enterography in patients with Crohn’s disease. Updates Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Yoon, H.; Oh, D.J.; Lee, J.M.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn’s disease. Intest. Res. 2020, 18, 79–84. [Google Scholar] [CrossRef]
- Thiberge, C.; Charpentier, C.; Gillibert, A.; Modzelewski, R.; Dacher, J.N.; Savoye, G.; Savoye-Collet, C. Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn’s disease. J. Crohns Colitis 2018, 12, 1429–1437. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, C.; Xie, T.; Yang, J.; Dai, X.; Lv, T.; Li, Y.; Gu, L.; Wei, Y.; Gong, J.; et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin. Nutr. 2017, 36, 1586–1592. [Google Scholar] [CrossRef]
- Csontos, Á.A.; Molnár, A.; Piri, Z.; Pálfi, E.; Miheller, P. Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease. Rev. Esp. Enfermedades Dig. 2017, 109, 26–32. [Google Scholar] [CrossRef][Green Version]
- Holt, D.Q.; Moore, G.T.; Strauss, B.J.G.; Hamilton, A.L.; De Cruz, P.; Kamm, M.A. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment. Pharmacol. Ther. 2017, 45, 1255–1264. [Google Scholar] [CrossRef]
- Bamba, S.; Sasaki, M.; Takaoka, A.; Takahashi, K.; Imaeda, H.; Nishida, A.; Inatomi, O.; Sugimoto, M.; Andoh, A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS ONE 2017, 12, e0180036. [Google Scholar] [CrossRef]
- Cravo, M.L.; Velho, S.; Torres, J.; Costa Santos, M.P.; Palmela, C.; Cruz, R.; Strecht, J.; Maio, R.; Baracos, V. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: An exploratory study. Clin. Nutr. ESPEN 2017, 21, 79–85. [Google Scholar] [CrossRef]
- Bryant, R.V.; Ooi, S.; Schultz, C.G.; Goess, C.; Grafton, R.; Hughes, J.; Lim, A.; Bartholomeusz, F.D.; Andrews, J.M. Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, L.; Cao, T.; Yang, J.; Gong, J.; Zhu, W.; Li, N.; Li, J. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients with Crohn’s Disease Undergoing Bowel Resection. J. Parenter. Enter. Nutr. 2017, 41, 592–600. [Google Scholar] [CrossRef]
- Schneider, S.; Al-Jaouni, R.; Filippi, J.; Wiroth, J.B.; Zeanandin, G.; Arab, K.; Hébuterne, X. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm. Bowel Dis. 2008, 14, 1562–1568. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- MF, H. Vitamina D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar]
- Janssen, C.E.; Globig, A.M.; Grawitz, A.B.; Bettinger, D.; Hasselblatt, P. Seasonal variability of vitamin D status in patients with inflammatory bowel disease—A retrospective cohort study. PLoS ONE 2019, 14, e0217238. [Google Scholar] [CrossRef] [PubMed]
- Burrelli Scotti, G.; Afferri, M.T.; De Carolis, A.; Vaiarello, V.; Fassino, V.; Ferrone, F.; Minisola, S.; Nieddu, L.; Vernia, P. Factors affecting vitamin D deficiency in active inflammatory bowel diseases. Dig. Liver Dis. 2019, 51, 657–662. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The association of disease activity, BMI and phase angle with vitamin D deficiency in patients with IBD. Nutrients 2019, 11, 2583. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.L.; Jahnsen, J.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Bernklev, T.; Moum, B.; et al. Fatigue is not associated with Vitamin D deficiency in inflammatory bowel disease patients. World J. Gastroenterol. 2018, 24, 3293–3301. [Google Scholar] [CrossRef]
- Torella, M.C.; Rausch, A.; Lasa, J.; Zubiaurre, I. Vitamin D deficiency among inflammatory bowel disease patients in Argentina: A cross-sectional study. Arq. Gastroenterol. 2018, 55, 216–220. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Li, L.; Chen, P.; Mao, R.; Feng, R.; Qiu, Y.; He, Y.; Chen, B.; Zeng, Z.; et al. A new model based on 25-hydroxyvitamin D3 for predicting active Crohn’s disease in Chinese patients. Mediat. Inflamm. 2018, 2018, 3275025. [Google Scholar] [CrossRef]
- Alrefai, D.; Jones, J.; El-Matary, W.; Whiting, S.J.; Aljebreen, A.; Mirhosseini, N.; Vatanparast, H. The association of vitamin D status with disease activity in a cohort of crohn′s disease patients in Canada. Nutrients 2017, 9, 1112. [Google Scholar] [CrossRef]
- Venkata, K.V.R.; Arora, S.S.; Xie, F.L.; Malik, T.A. Impact of Vitamin D on the hospitalization rate of Crohn’s disease patients seen at a tertiary care center. World J. Gastroenterol. 2017, 23, 2539–2544. [Google Scholar] [CrossRef]
- Pallav, K.; Riche, D.; May, W.L.; Sanchez, P.; Gupta, N.K. Predictors of Vitamin D deficiency in inflammatory bowel disease and health: A Mississippi perspective Retrospective Study. World J. Gastroenterol. 2017, 23, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Kotze, L.M.; Costa, C.T.; Cavassani, M.F.; Nisihara, R.M. Alert for bone alterations and low serum concentrations of Vitamin D in patients with intestinal in?ammatory disease. Rev. Assoc. Med. Bras. 2017, 63, 13–17. [Google Scholar] [CrossRef]
- Reich, K.M.; Fedorak, R.N.; Madsen, K.; Kroeker, K.I. Role of Vitamin D in Infliximab-induced Remission in Adult Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, P.C.; Netinho, J.G.; Cunrath, G.S.; Ronchi, L.S.; de Melo, M.M.C.; Gonçalves Filho, F.; Gonçalves Filho, F.d.A.; Muniz, R.C.C.; Martins, A.T.S.; de Oliveira, R.A.; et al. Association between vitamin D serum levels and disease activity markers in patients with Crohn’s Disease. Int. J. Colorectal Dis. 2016, 31, 1495–1496. [Google Scholar] [CrossRef]
- Xia, S.L.; Lin, X.X.; Guo, M.D.; Zhang, D.G.; Zheng, S.Z.; Jiang, L.J.; Jin, J.; Lin, X.Q.; Ding, R.; Jiang, Y. Association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with Crohn’s disease in Chinese patients. J. Gastroenterol. Hepatol. 2016, 31, 795–801. [Google Scholar] [CrossRef]
- Dias de Castro, F.; Magalhães, J.; Boal Carvalho, P.; Moreira, M.J.; Mota, P.; Cotter, J. Lower levels of vitamin D correlate with clinical disease activity and quality of life in inflammatory bowel disease. Arq. Gastroenterol. 2015, 52, 260–265. [Google Scholar] [CrossRef]
- Raftery, T.; Merrick, M.; Healy, M.; Mahmud, N.; O’Morain, C.; Smith, S.; McNamara, D.; O’Sullivan, M. Vitamin D Status Is Associated with Intestinal Inflammation as Measured by Fecal Calprotectin in Crohn’s Disease in Clinical Remission. Dig. Dis. Sci. 2015, 60, 2427–2435. [Google Scholar] [CrossRef]
- De Bruyn, J.R.; van Heeckeren, R.; Ponsioen, C.Y.; van den Brink, G.R.; Löwenberg, M.; Bredenoord, A.J.; Frijstein, G.; D’Haens, G.R. Vitamin D deficiency in Crohn’s disease and healthy controls: A prospective case-control study in the Netherlands. J. Crohns Colitis 2014, 8, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, G.; Mihai, C.; Dranga, M.; Prelipcean, C.C. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J. Gastroenterol. 2014, 20, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, T.; Krajcovicova, A.; Koller, T.; Toth, J.; Nevidanska, M.; Huorka, M.; Payer, J. Higher vitamin D serum concentration increases health related quality of life in patients with inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 15787–15796. [Google Scholar] [CrossRef]
- Veit, L.E.; Maranda, L.; Fong, J.; Nwosu, B.U. The vitamin D status in inflammatory bowel disease. PLoS ONE 2014, 9, e101583. [Google Scholar] [CrossRef] [PubMed]
- Salacinski, A.J.; Regueiro, M.D.; Broeder, C.E.; McCrory, J.L. Decreased neuromuscular function in Crohn’s disease patients is not associated with low serum vitamin D levels. Dig. Dis. Sci. 2013, 58, 526–533. [Google Scholar] [CrossRef]
- Fu, Y.T.N.; Chatur, N.; Cheong-Lee, C.; Salh, B. Hypovitaminosis D in adults with inflammatory bowel disease: Potential role of ethnicity. Dig. Dis. Sci. 2012, 57, 2144–2148. [Google Scholar] [CrossRef]
- Nic Suibhne, T.; Cox, G.; Healy, M.; O’Morain, C.; O’Sullivan, M. Vitamin D deficiency in Crohn’s disease: Prevalence, risk factors and supplement use in an outpatient setting. J. Crohns Colitis 2012, 6, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Atia, A.; Murthy, R.; Bailey, B.A.; Manning, T.; Garrett, L.L.; Youssef, D.; Peiris, A.N. Vitamin D Status in Veterans with inflammatory bowel disease: Relationship to Health care costs and services. Mil. Med. 2011, 176, 711–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jørgensen, S.P.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, A.; Tanaka, K.; Tsugawa, N.; Nakase, H.; Tsuji, H.; Shide, K.; Kamao, M.; Chiba, T.; Inagaki, N.; Okano, T.; et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos. Int. 2009, 20, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.; Shanahan, F.; Cashman, K.D. Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur. J. Clin. Nutr. 2006, 60, 889–896. [Google Scholar] [CrossRef]
- McCarthy, D.; Duggan, P.; O’Brien, M.; Kiely, M.; McCarthy, J.; Shanahan, F.; Cashman, K.D. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2005, 21, 1073–1083. [Google Scholar] [CrossRef]
- Tajika, M.; Matsuura, A.; Nakamura, T.; Suzuki, T.; Sawaki, A.; Kato, T.; Hara, K.; Ookubo, K.; Yamo, K.; Kato, M.; et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J. Gastroenterol. 2004, 39, 527–533. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Siminoski, K.; Steinhart, H.; Greenberg, G.; Fedorak, R.N. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can. J. Gastroenterol. 2003, 17, 473–478. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2002, 37, 192–199. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Bikle, D.D. Editorial: Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2020, 11, 317. [Google Scholar] [CrossRef]
- Agergaard, J.; Trøstrup, J.; Uth, J.; Iversen, J.V.; Boesen, A.; Andersen, J.L.; Schjerling, P.; Langberg, H. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men?—A randomized controlled trial. Nutr. Metab. 2015, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2 vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Angeline, M.E.; Gee, A.O.; Shindle, M.; Warren, R.F.; Rodeo, S.A. The effects of vitamin d deficiency in athletes. Am. J. Sports Med. 2013, 41, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D deficiency and sarcopenia in older persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, K.V.; Brekke, M.; Gjelstad, S.; Lagerløv, P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: A cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand. J. Prim. Health Care 2010, 28, 166–171. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Boettger, S.F.; Angersbach, B.; Klimek, C.N.; Wanderley, A.L.M.; Shaibekov, A.; Sieske, L.; Wang, B.; Zuchowski, M.; Wirth, R.; Pourhassan, M. Prevalence and predictors of vitamin D-deficiency in frail older hospitalized patients. BMC Geriatr. 2018, 18, 219. [Google Scholar] [CrossRef]
- Granic, A.; Hil, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.L.; Mathers, J.C.; Sayer, A.A. Vitamin d status, muscle strength and physical performance decline in very old adults: A prospective study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D Receptor Expression in Human Muscle Tissue Decreases with Age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Hill, T.R.; Aspray, T.J.; Francis, R.M. Vitamin D and bone health outcomes in older age. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2013; Volume 72, pp. 372–380. [Google Scholar]
- Rigterink, T.; Appleton, L.; Day, A.S. Vitamin D therapy in children with inflammatory bowel disease: A systematic review. World J. Clin. Pediatr. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hradsky, O.; Soucek, O.; Maratova, K.; Matyskova, J.; Copova, I.; Zarubova, K.; Bronsky, J.; Sumnik, Z. Supplementation with 2000 IU of Cholecalciferol is Associated with Improvement of Trabecular Bone Mineral Density and Muscle Power in Pediatric Patients with IBD. Inflamm. Bowel Dis. 2017, 23, 514–523. [Google Scholar] [CrossRef] [PubMed]

| Variable | Parameter | Test | Tool | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Skeletal Muscle Strength | GS | GST | Dynamometer | Simple and inexpensive. |
|
| CST | None | Simple and inexpensive. |
| ||
| Skeletal Muscle Quantity | SMM | ASMI | BIA; DXA |
|
|
| SMM | MCSA | CT; MRI | The gold-standard methods. |
|
| Authors | Year | n | Age (Years) | Variable | Test | Tool | Sarcopenia (%) |
|---|---|---|---|---|---|---|---|
| Boparai [23] | 2021 | 44 | 34 ± 14.1 * | SMQ | MCSA | CT | 43 |
| Celentano et al. [24] | 2020 | 31 | 46 (49–72) † | SMQ | MCSA | MRI | 38 |
| Lee et al. [25] | 2020 | 79 | 29 ± 11.3 * | SMQ | MCSA | CT | 50 |
| Grillot et al. [3] | 2020 | 88 | 35 ± 12.4 * | SMQ | MCSA | CT | 58 |
| Thiberge et al. [26] | 2018 | 149 | 41 ± 17.5 * | SMQ | MCSA | CT | 33.6 |
| Zhang T. et al. [27] | 2017 | 105 | − | SMQ | MCSA | CT | 59 |
| Csontos et al. [28] | 2017 | 126 | 34 ± 11.5 * | SMQ | ASMI | BIA | 29.4 |
| Holt et al. [29] | 2017 | 44 | 38 ± 14.2 * | SMQ | MCSA | CT MRI | 41 |
| Bamba et al. [30] | 2017 | 43 | 29 (25–37) † | SMQ | MCSA | CT | 37 |
| Cravo et al. [31] | 2017 | 71 | 43 | SMQ | MCSA | CT | 31 |
| Bryant et al. [32] | 2015 | 95 | 31 (27–39) † | SMQ SMS | ASMI GST | BIA Dyn | 19 27 |
| Zhang T. et al. [33] | 2015 | 114 | 32 ± 11.5 * | SMQ | MCSA | CT | 61.4 |
| Schneider et al. [34] | 2008 | 82 | 36 ± 13.9 * | SMQ | ASMI | DXA | 60 |
| Authors | Year | n | Age (Years) | 25(OH)D Cut-Off (ng/mL) | Vitamin D Deficiency (%) |
|---|---|---|---|---|---|
| Janssen et al. [40] | 2019 | 256 | 43 (18–85) † | <20 20–30 | 63% 24% |
| Burrelli Scotti et al. [41] | 2019 | 33 78 | - | <20 | 39.6% 1 50% 2 |
| Mentella et al. [42] | 2019 | 101 | 37.9 ± 16.64 * | <20 <30 | 38.6% 25.7% |
| Frigstad et al. [43] | 2018 | 227 | 40 (18–77) † | <20 | 55% |
| Torella et al. [44] | 2018 | 14 | - | <30 | 78.6% |
| Lin et al. [45] | 2018 | 346 | - | <20 | 82.7% |
| Alrefai et al. [46] | 2017 | 201 | 40 ± 15.2 * | <12 12–20 | 18% 26% |
| Venkata et al. [47] | 2017 | 196 | − | <30 | 58.7% |
| Pallav et al. [48] | 2017 | 129 | − | <20 | 40.3% |
| da Silva Kotze et al. [49] | 2017 | 38 | 40 (16–73) † | <20 20–30 | 10.5% 65.8% |
| Reich et al. [50] | 2016 | 28 | − | <30 | 53.6% |
| Rebouças et al. [51] | 2016 | 75 | 41 ± 15.6 * | <30 | 62.7% |
| Xia et al. [52] | 2016 | 124 | 27.6 ± 8.6 * | <20 | 67.8% |
| De Castro et al. [53] | 2015 | 57 | 33 ± 9.8 * | <20 <30 | 33% 72% |
| Raftery et al. [54] | 2015 | 119 | 45 ± 11.8 * | <20 | 36.1% |
| de Bruyn et al. [55] | 2014 | 101 | 41 (30–50) † | <20 | 54% |
| Dumitrescu et al. [56] | 2014 | 14 | 36 ± 9 * | <20 <30 | 36% 43% |
| Hlavaty et al. [57] | 2014 | 124 97 | - - | <12 | 60% 1 74% 2 |
| Veit et al. [58] | 2014 | 40 | 16.6 ± 2.2 * | <20 | 40% |
| Salacinski et al. [59] | 2013 | 19 | 44 ± 10.3 * | <20 20–30 | 10.5% 37% |
| Fu et al. [60] | 2012 | 40 | 40 ± 13.2 * | <20 | 42.5% |
| Suibhne et al. [61] | 2012 | 81 | 36 ± 11 * | <20 | 63% |
| Atia et al. [62] | 2011 | 43 | 61 ± 14.7 * | <20 <30 | 51.2% 83.7% |
| Jørgensen et al. [63] | 2010 | 94 | - | <20 | 30.9% |
| Kuwabara et al. [64] | 2009 | 29 | 32 ± 6.7 * | <20 | 100% |
| Gilman et al. [65] | 2006 | 58 | 38 ± 10.9 * | <20 | 19% 1 50% 2 |
| McCarthy et al. [66] | 2005 | 44 | 37 ± 11.1 * | <20 | 18.2% 1 50% 2 |
| Tajika et al. [67] | 2004 | 33 | 38 ± 7.5 * | ≤10 | 27.3% |
| Siffledeen et al. [68] | 2003 | 242 | - | <10 <16 | 8% 22% |
| Jahnsen et al. [69] | 2002 | 60 | - | <12 | 27% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmese, F.; Del Toro, R.; Di Marzio, G.; Cataleta, P.; Sama, M.G.; Domenicali, M. Sarcopenia and Vitamin D Deficiency in Patients with Crohn’s Disease: Pathological Conditions That Should Be Linked Together. Nutrients 2021, 13, 1378. https://doi.org/10.3390/nu13041378
Palmese F, Del Toro R, Di Marzio G, Cataleta P, Sama MG, Domenicali M. Sarcopenia and Vitamin D Deficiency in Patients with Crohn’s Disease: Pathological Conditions That Should Be Linked Together. Nutrients. 2021; 13(4):1378. https://doi.org/10.3390/nu13041378
Chicago/Turabian StylePalmese, Francesco, Rossella Del Toro, Giulia Di Marzio, Pierluigi Cataleta, Maria Giulia Sama, and Marco Domenicali. 2021. "Sarcopenia and Vitamin D Deficiency in Patients with Crohn’s Disease: Pathological Conditions That Should Be Linked Together" Nutrients 13, no. 4: 1378. https://doi.org/10.3390/nu13041378
APA StylePalmese, F., Del Toro, R., Di Marzio, G., Cataleta, P., Sama, M. G., & Domenicali, M. (2021). Sarcopenia and Vitamin D Deficiency in Patients with Crohn’s Disease: Pathological Conditions That Should Be Linked Together. Nutrients, 13(4), 1378. https://doi.org/10.3390/nu13041378





