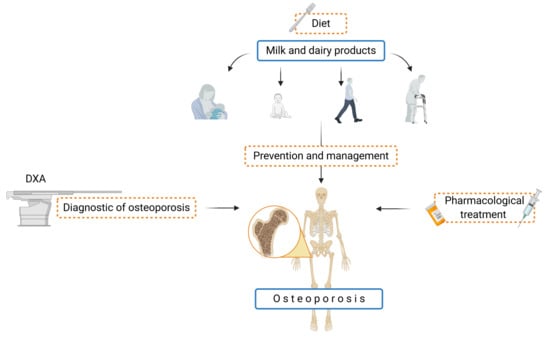
Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis
Abstract
1. Introduction
2. Milk and BMD
2.1. Breast Milk
2.2. Lactation and BMD
2.3. Cow’s Milk and Dairy Products and BMD
2.4. Plant Milk (Plant Beverages) and BMD
3. Intolerance and Allergy
3.1. Lactose Intolerance
3.2. Cow’s Milk Allergy
4. Milk and Dairy Products and Gut Microbiota-Modulation of BMD
5. Summary—Recommendation for Milk and Dairy Products in the Prevention and Treatment of Osteoporosis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janiszewska, M.; Kulik, T.; Dziedzic, M.; Żołnierczuk-Kieliszek, D.; Barańska, A. Osteoporosis as a Social Problem- Pathogenesis, Symptoms and Risk Factors of Postmenopausal Osteoporosis. Probl. Hig. Epidemiol. 2015, 96, 106–114. [Google Scholar]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A Comprehensive Overview on Osteoporosis and Its Risk Factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Hodges, J.K.; Cao, S.; Cladis, D.P.; Weaver, C.M. Lactose Intolerance and Bone Health: The Challenge of Ensuring Adequate Calcium Intake. Nutrients 2019, 11, 718. [Google Scholar] [CrossRef]
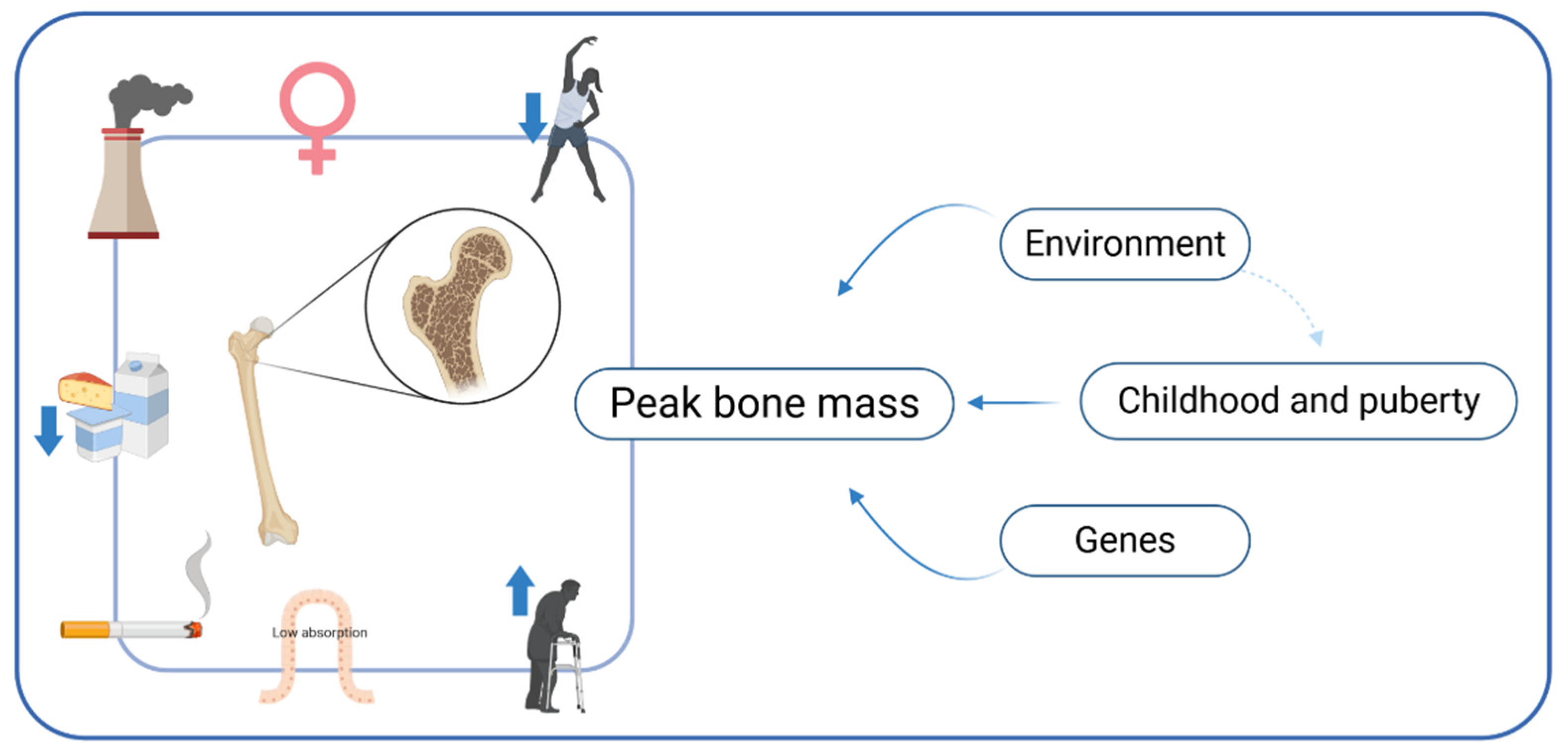
- Gordon, C.M.; Zemel, B.S.; Wren, T.A.L.; Leonard, M.B.; Bachrach, L.K.; Rauch, F.; Gilsanz, V.; Rosen, C.J.; Winer, K.K. The Determinants of Peak Bone Mass. J. Pediatrics 2017, 180, 261–269. [Google Scholar] [CrossRef]
- Osteoporosis: Peak Bone Mass in Women|NIH Osteoporosis and Related Bone Diseases National Resource Center. Available online: https://www.bones.nih.gov/health-info/bone/osteoporosis/bone-mass (accessed on 19 December 2020).
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s Position Statement on Peak Bone Mass Development and Lifestyle Factors: A Systematic Review and Implementation Recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, F.E.A.; Murray, L.; Gallagher, A.; Davey-Smith, G.; Neville, C.E.; Van’t Hof, R.; Boreham, C.; Ralston, S.H. Genetic and Environmental Determinants of Peak Bone Mass in Young Men and Women. J. Bone Miner. Res. 2002, 17, 1273–1279. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Altieri, B.; Di Somma, C.; Bhattoa, H.P.; Laudisio, D.; Duval, G.T.; Pugliese, G.; Annweiler, C.; Orio, F.; et al. Calcium and Vitamin D Supplementation. Myths and Realities with Regard to Cardiovascular Risk. Curr. Vasc. Pharmacol. 2019, 17, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M.; Amling, M.; Ignatius, A. Calcium and Vitamin D in Bone Fracture Healing and Post-Traumatic Bone Turnover. Eur. Cell Mater. 2018, 35, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Dadra, A.; Aggarwal, S.; Kumar, P.; Kumar, V.; Dibar, D.P.; Bhadada, S.K. High Prevalence of Vitamin D Deficiency and Osteoporosis in Patients with Fragility Fractures of Hip: A Pilot Study. J. Clin. Orthop. Trauma 2019, 10, 1097–1100. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.W. Prevalence of Vitamin D Deficiency in Postmenopausal High- and Low-Energy Fracture Patient. Arch. Osteoporos. 2018, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health Benefits of Physical Activity: The Evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.B.R.; Rizzoli, R.R.; Marchand, L.M.; Ferrari, S.; Beghetti, M.; Farpour-Lambert, N.J. Physical Activity Increases Bone Mineral Density in Children with Type 1 Diabetes. Med. Sci. Sports Exerc. 2012, 44, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.-C.; Chang, C.-B.; Han, D.-S.; Hong, C.-H.; Hwang, J.-S.; Tsai, K.-S.; Yang, R.-S. Effects of Exercise Improves Muscle Strength and Fat Mass in Patients with High Fracture Risk: A Randomized Control Trial. J. Formos. Med. Assoc. 2018, 117, 572–582. [Google Scholar] [CrossRef]
- Calcium Content of Common Foods | International Osteoporosis Foundation. Available online: https://www.osteoporosis.foundation/patients/prevention/calcium-content-of-common-foods (accessed on 14 December 2020).
- Office of Dietary Supplements-Calcium. Available online: https://ods.od.nih.gov/factsheets/Calcium-Consumer/ (accessed on 8 December 2020).
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. North. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Hytten, F.E. Clinical and Chemical Studies in Human Lactation. VIII. Relationship of the Age, Physique, and Nutritional Status of the Mother to the Yield and Composition of Her Milk. Br. Med. J. 1954, 2, 844–845. [Google Scholar] [CrossRef][Green Version]
- Thomson, A.M.; Black, A.E. Nutritional Aspects of Human Lactation. Bull. World Health Organ. 1975, 52, 163–177. [Google Scholar]
- Mosca, F.; Giannì, M.L. Human Milk: Composition and Health Benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef]
- Gopalan, C. Effect of Nutrition on Pregnancy and Lactation. Bull. World Health Organ. 1962, 26, 203–211. [Google Scholar]
- Pietrzak-Fiećko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Patin, R.V.; Vítolo, M.R.; Valverde, M.A.; Carvalho, P.O.; Pastore, G.M.; Lopez, F.A. The Influence of Sardine Consumption on the Omega-3 Fatty Acid Content of Mature Human Milk. J. Pediatr. 2006, 82, 63–69. [Google Scholar] [CrossRef]
- Loughrill, E.; Wray, D.; Christides, T.; Zand, N. Calcium to Phosphorus Ratio, Essential Elements and Vitamin D Content of Infant Foods in the UK: Possible Implications for Bone Health. Matern. Child. Nutr. 2017, 13, e12368. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Brown, R.B.; Razzaque, M.S. Osteoporosis in Populations with High Calcium Intake: Does Phosphate Toxicity Explain the Paradox? Ind. J. Clin. Biochem. 2015, 30, 365–367. [Google Scholar] [CrossRef]
- Burgess, K. Milk and Dairy Products in Human Nutrition; Muehlhoff, E., Bennett, A., McMahon, D., Eds.; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2013; ISBN 978-92-5-107864-8. [Google Scholar] [CrossRef]
- Aparicio, M.; Browne, P.D.; Hechler, C.; Beijers, R.; Rodríguez, J.M.; de Weerth, C.; Fernández, L. Human Milk Cortisol and Immune Factors over the First Three Postnatal Months: Relations to Maternal Psychosocial Distress. PLoS ONE 2020, 15, e0233554. [Google Scholar] [CrossRef] [PubMed]
- Al-Agha, A.E.; Kabli, Y.O.; AlBeiruty, M.G.; Milyani, A.A. Determinants of Bone Mineral Density through Quantitative Ultrasound Screening of Healthy Children Visiting Ambulatory Paediatric Clinics. Saudi Med. J. 2019, 40, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Burrows, R.; Reyes, M.; Lozoff, B.; Gahagan, S.; Albala, C. Breastfeeding as the Sole Source of Milk for 6 Months and Adolescent Bone Mineral Density. Osteoporos. Int. 2017, 28, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- van den Hooven, E.H.; Gharsalli, M.; Heppe, D.H.M.; Raat, H.; Hofman, A.; Franco, O.H.; Rivadeneira, F.; Jaddoe, V.W.V. Associations of Breast-Feeding Patterns and Introduction of Solid Foods with Childhood Bone Mass: The Generation R Study. Br. J. Nutr. 2016, 115, 1024–1032. [Google Scholar] [CrossRef]
- Hwang, I.R.; Choi, Y.K.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Kim, S.W.; Park, K.G. Association between Prolonged Breastfeeding and Bone Mineral Density and Osteoporosis in Postmenopausal Women: KNHANES 2010–2011. Osteoporos. Int. 2016, 27, 257–265. [Google Scholar] [CrossRef]
- Tsvetov, G.; Levy, S.; Benbassat, C.; Shraga-Slutzky, I.; Hirsch, D. Influence of Number of Deliveries and Total Breast-Feeding Time on Bone Mineral Density in Premenopausal and Young Postmenopausal Women. Maturitas 2014, 77, 249–254. [Google Scholar] [CrossRef]
- Bolzetta, F.; Veronese, N.; De Rui, M.; Berton, L.; Carraro, S.; Pizzato, S.; Girotti, G.; De Ronch, I.; Manzato, E.; Coin, A.; et al. Duration of Breastfeeding as a Risk Factor for Vertebral Fractures. Bone 2014, 68, 41–45. [Google Scholar] [CrossRef]
- Cooke-Hubley, S.; Gao, Z.; Mugford, G.; Kaiser, S.M.; Goltzman, D.; Leslie, W.D.; Davison, K.S.; Brown, J.P.; Probyn, L.; Lentle, B.; et al. Parity and Lactation Are Not Associated with Incident Fragility Fractures or Radiographic Vertebral Fractures over 16 Years of Follow-up: Canadian Multicentre Osteoporosis Study (CaMos). Arch. Osteoporos. 2019, 14, 49. [Google Scholar] [CrossRef]
- Kovacs, C.S. Calcium and Phosphate Metabolism and Related Disorders during Pregnancy and Lactation. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and Other Cultured Dairy Products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef]
- Kalkwarf, H.J.; Khoury, J.C.; Lanphear, B.P. Milk Intake during Childhood and Adolescence, Adult Bone Density, and Osteoporotic Fractures in US Women. Am. J. Clin. Nutr. 2003, 77, 257–265. [Google Scholar] [CrossRef]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and Dairy Products: Good or Bad for Human Health? An Assessment of the Totality of Scientific Evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef]
- Goulding, A.; Rockell, J.E.P.; Black, R.E.; Grant, A.M.; Jones, I.E.; Williams, S.M. Children Who Avoid Drinking Cow’s Milk Are at Increased Risk for Prepubertal Bone Fractures. J. Am. Diet. Assoc. 2004, 104, 250–253. [Google Scholar] [CrossRef]
- Wiley, A.S. Does Milk Make Children Grow? Relationships between Milk Consumption and Height in NHANES 1999–2002. Am. J. Hum. Biol. 2005, 17, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, R.M.; dos S Vaz, J.; Domingues, M.R.; Matijasevich, A.; Santos, I.S.; Ekelund, U.; Horta, B.L. Are Consumption of Dairy Products and Physical Activity Independently Related to Bone Mineral Density of 6-Year-Old Children? Longitudinal and Cross-Sectional Analyses in a Birth Cohort from Brazil. Public Health Nutr. 2018, 21, 2654–2664. [Google Scholar] [CrossRef]
- Sioen, I.; Michels, N.; Polfliet, C.; De Smet, S.; D’Haese, S.; Roggen, I.; Deschepper, J.; Goemaere, S.; Valtueña, J.; De Henauw, S. The Influence of Dairy Consumption, Sedentary Behaviour and Physical Activity on Bone Mass in Flemish Children: A Cross-Sectional Study. BMC Public Health 2015, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Torres-Costoso, A.; López-Muñoz, P.; Ferri-Morales, A.; Bravo-Morales, E.; Martínez-Vizcaíno, V.; Garrido-Miguel, M. Body Mass Index, Lean Mass, and Body Fat Percentage as Mediators of the Relationship between Milk Consumption and Bone Health in Young Adults. Nutrients 2019, 11, 2500. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, L.H.; Kiel, D.P.; Soedamah-Muthu, S.S.; Bouxsein, M.L.; Hannan, M.T.; Sahni, S. Higher Dairy Food Intake Is Associated With Higher Spine Quantitative Computed Tomography (QCT) Bone Measures in the Framingham Study for Men But Not Women. J. Bone Miner. Res. 2018, 33, 1283–1290. [Google Scholar] [CrossRef]
- Mangano, K.M.; Noel, S.E.; Sahni, S.; Tucker, K.L. Higher Dairy Intakes Are Associated with Higher Bone Mineral Density among Adults with Sufficient Vitamin D Status: Results from the Boston Puerto Rican Osteoporosis Study. J. Nutr. 2019, 149, 139–148. [Google Scholar] [CrossRef]
- Hallkvist, O.M.; Johansson, J.; Nordström, A.; Nordström, P.; Hult, A. Dairy Product Intake and Bone Properties in 70-Year-Old Men and Women. Arch. Osteoporos. 2018, 13, 9. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Warensjö Lemming, E.; Melhus, H.; Byberg, L. Milk Intake and Risk of Mortality and Fractures in Women and Men: Cohort Studies. BMJ 2014, 349, g6015. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Body, J.-J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs—Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Infante, D.; Tormo, R. Risk of Inadequate Bone Mineralization in Diseases Involving Long-Term Suppression of Dairy Products. J. Pediatric Gastroenterol. Nutr. 2000, 30, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Matía-Martín, P.; Torrego-Ellacuría, M.; Larrad-Sainz, A.; Fernández-Pérez, C.; Cuesta-Triana, F.; Rubio-Herrera, M.Á. Effects of Milk and Dairy Products on the Prevention of Osteoporosis and Osteoporotic Fractures in Europeans and Non-Hispanic Whites from North America: A Systematic Review and Updated Meta-Analysis. Adv. Nutr. 2019, 10, S120–S143. [Google Scholar] [CrossRef]
- Tang, A.L.; Walker, K.Z.; Wilcox, G.; Strauss, B.J.; Ashton, J.F.; Stojanovska, L. Calcium Absorption in Australian Osteopenic Post-Menopausal Women: An Acute Comparative Study of Fortified Soymilk to Cows’ Milk. Asia Pac. J. Clin. Nutr. 2010, 19, 243–249. [Google Scholar] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Rafferty, K.; Bierman, J. Bioavailability of the Calcium in Fortified Soy Imitation Milk, with Some Observations on Method. Am. J. Clin. Nutr. 2000, 71, 1166–1169. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Mølgaard, C.; Iuliano, S.; Rizzoli, R.; Manios, Y.; van Loon, L.J.C.; Lecerf, J.-M.; Moschonis, G.; Reginster, J.-Y.; Givens, I.; et al. Impact of Whole Dairy Matrix on Musculoskeletal Health and Aging–Current Knowledge and Research Gaps. Osteoporos. Int. 2020, 31, 601–615. [Google Scholar] [CrossRef]
- Lee, G.J.; Birken, C.S.; Parkin, P.C.; Lebovic, G.; Chen, Y.; L’Abbé, M.R.; Maguire, J.L. TARGet Kids! Collaboration Consumption of Non-Cow’s Milk Beverages and Serum Vitamin D Levels in Early Childhood. CMAJ 2014, 186, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.-C.; Brašić, J.R.; Liu, X.-D.; Gong, G.-Y.; Zhang, G.-M.; Liu, C.-J.; Gao, G.-Q. Bone Mineral Density in Postmenopausal Chinese Women Treated with Calcium Fortification in Soymilk and Cow’s Milk. Osteoporos. Int. 2012, 23, 1563–1570. [Google Scholar] [CrossRef]
- García-Martín, A.; Quesada Charneco, M.; Alvárez Guisado, A.; Jiménez Moleón, J.J.; Fonollá Joya, J.; Muñoz-Torres, M. Effect of milk product with soy isoflavones on quality of life and bone metabolism in postmenopausal Spanish women: Randomized trial. Med. Clin. 2012, 138, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lydeking-Olsen, E.; Beck-Jensen, J.-E.; Setchell, K.D.R.; Holm-Jensen, T. Soymilk or Progesterone for Prevention of Bone Loss—A 2 Year Randomized, Placebo-Controlled Trial. Eur. J. Nutr. 2004, 43, 246–257. [Google Scholar] [CrossRef]
- Yanaka, K.; Higuchi, M.; Ishimi, Y. Anti-Osteoporotic Effect of Soy Isoflavones Intake on Low Bone Mineral Density Caused by Voluntary Exercise and Food Restriction in Mature Female Rats. J. Nutr. Sci. Vitam. 2019, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-W.; Choi, S.-W.; Kim, H.-J.; Lee, K.-S.; Kim, S.-H.; Kim, S.-L.; Do, S.H.; Seo, W.-D. Germinated Soy Germ with Increased Soyasaponin Ab Improves BMP-2-Induced Bone Formation and Protects against in Vivo Bone Loss in Osteoporosis. Sci. Rep. 2018, 8, 12970. [Google Scholar] [CrossRef]
- Matthews, V.L.; Knutsen, S.F.; Beeson, W.L.; Fraser, G.E. Soy Milk and Dairy Consumption Are Independently Associated with Ultrasound Attenuation of the Heel Bone among Postmenopausal Women: The Adventist Health Study-2 (AHS-2). Nutr. Res. 2011, 31, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Klemarczyk, W.; Gajewska, J.; Chełchowska, M.; Franek, E.; Laskowska-Klita, T. The Influence of Vegan Diet on Bone Mineral Density and Biochemical Bone Turnover Markers. Pediatr. Endocrinol. Diabetes Metab. 2010, 16, 201–204. [Google Scholar]
- Vitoria, I. The Nutritional Limitations of Plant-Based Beverages in Infancy and Childhood. Nutr. Hosp. 2017, 34, 1205–1214. [Google Scholar] [CrossRef]
- Kowalska, D.; Gruczyńska, E.; Bryś, J. Mother’s Milk—First Food in Human Life. Probl. Hig. Epidemiol. 2015, 96, 387–398. [Google Scholar]
- Guetouache, M.; Guessas, B.; Medjekal, S. Composition and Nutritional Value of Raw Milk. Issues Biol. Sci. Pharm. Res. 2014, 2, 115–122. [Google Scholar]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant Based Alternatives to Conventional Milk, Production, Potential and Health Concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Skłądu I Wartości Odżywczej Żywności, 2nd ed.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2017. [Google Scholar]
- Heaney, R.P. Dairy Intake, Dietary Adequacy, and Lactose Intolerance12. Adv. Nutr. 2013, 4, 151–156. [Google Scholar] [CrossRef]
- Keith, J.N.; Nicholls, J.; Reed, A.; Kafer, K.; Miller, G.D. The Prevalence of Self-Reported Lactose Intolerance and the Consumption of Dairy Foods among African American Adults Are Less than Expected. J. Natl. Med. Assoc. 2011, 103, 36–45. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Lactose Intolerance in Patients with Inflammatory Bowel Diseases and Dietary Management in Prevention of Osteoporosis. Nutrition 2020, 82, 111043. [Google Scholar] [CrossRef] [PubMed]
- Treister-Goltzman, Y.; Friger, M.; Peleg, R. Does Primary Lactase Deficiency Reduce Bone Mineral Density in Postmenopausal Women? A Systematic Review and Meta-Analysis. Osteoporos. Int. 2018, 29, 2399–2407. [Google Scholar] [CrossRef]
- Klemm, P.; Dischereit, G.; Lange, U. Adult Lactose Intolerance, Calcium Intake, Bone Metabolism and Bone Density in German-Turkish Immigrants. J. Bone Miner. Metab. 2019, 38, 378–384. [Google Scholar] [CrossRef]
- Mnich, B.; Spinek, A.E.; Chyleński, M.; Sommerfeld, A.; Dabert, M.; Juras, A.; Szostek, K. Analysis of LCT-13910 Genotypes and Bone Mineral Density in Ancient Skeletal Materials. PLoS ONE 2018, 13, e0194966. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-García, V.; Flores-Merino, M.V.; Morales-Romero, J.; Bedolla-Pulido, A.; Mariscal-Castro, J.; Bedolla-Barajas, M. Allergy to cow’s milk protein, or lactose intolerance: A cross-sectional study in university students. Rev. Alerg. Mex. 2019, 66, 394–402. [Google Scholar] [CrossRef]
- Nachshon, L.; Goldberg, M.R.; Schwartz, N.; Sinai, T.; Amitzur-Levy, R.; Elizur, A.; Eisenberg, E.; Katz, Y. Decreased Bone Mineral Density in Young Adult IgE-Mediated Cow’s Milk-Allergic Patients. J. Allergy Clin. Immunol. 2014, 134, 1108–1113.e3. [Google Scholar] [CrossRef]
- Mailhot, G.; Perrone, V.; Alos, N.; Dubois, J.; Delvin, E.; Paradis, L.; Des Roches, A. Cow’s Milk Allergy and Bone Mineral Density in Prepubertal Children. Pediatrics 2016, 137, e20151742. [Google Scholar] [CrossRef]
- Yu, J.W.; Pekeles, G.; Legault, L.; McCusker, C.T. Milk Allergy and Vitamin D Deficiency Rickets: A Common Disorder Associated with an Uncommon Disease. Ann. Allergy Asthma Immunol. 2006, 96, 615–619. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef]
- Sakurama, H.; Kiyohara, M.; Wada, J.; Honda, Y.; Yamaguchi, M.; Fukiya, S.; Yokota, A.; Ashida, H.; Kumagai, H.; Kitaoka, M.; et al. Lacto- N -Biosidase Encoded by a Novel Gene of Bifidobacterium Longum Subspecies Longum Shows Unique Substrate Specificity and Requires a Designated Chaperone for Its Active Expression. J. Biol. Chem. 2013, 288, 25194–25206. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A Key Genetic Factor for Fucosyllactose Utilization Affects Infant Gut Microbiota Development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Yang, R.; Dai, Y.; Zeng, Y.; Tao, L.; Li, X.; Zeng, J.; Wang, Q. Gut Microbiota Composition and Bone Mineral Loss—Epidemiologic Evidence from Individuals in Wuhan, China. Osteoporos. Int. 2019, 30, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Levast, B.; Li, Z.; Madrenas, J. The Role of IL-10 in Microbiome-Associated Immune Modulation and Disease Tolerance. Cytokine 2015, 75, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Mucosal Immunity: Integration between Mother and the Breast-Fed Infant. Vaccine 2003, 21, 3382–3388. [Google Scholar] [CrossRef]
- Chichlowski, M.; De Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria Isolated From Infants and Cultured on Human Milk Oligosaccharides Affect Intestinal Epithelial Function. J. Pediatric Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Jeurink, P.V.; van Bergenhenegouwen, J.; Jiménez, E.; Knippels, L.M.J.; Fernández, L.; Garssen, J.; Knol, J.; Rodríguez, J.M.; Martín, R. Human Milk: A Source of More Life than We Imagine. Benef. Microbes 2013, 4, 17–30. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of Bacterial Diversity in Breast Milk Using Culture-Dependent and Culture-Independent Approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [CrossRef]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal Diet Alters Fecal Microbiota and Metabolome Profiles at Different Ages in Infants Fed Breast Milk or Formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Yun, B.; Maburutse, B.E.; Kang, M.; Park, M.R.; Park, D.J.; Kim, Y.; Oh, S. Short Communication: Dietary Bovine Milk–Derived Exosomes Improve Bone Health in an Osteoporosis-Induced Mouse Model. J. Dairy Sci. 2020, 103, 7752–7760. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus Reuteri Reduces Bone Loss in Older Women with Low Bone Mineral Density: A Randomized, Placebo-Controlled, Double-Blind, Clinical Trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-Blind, Controlled Trial. Null 2017, 36, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation With Probiotic L. Reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Biver, E.; Durosier-Izart, C.; Merminod, F.; Chevalley, T.; van Rietbergen, B.; Ferrari, S.L.; Rizzoli, R. Fermented Dairy Products Consumption Is Associated with Attenuated Cortical Bone Loss Independently of Total Calcium, Protein, and Energy Intakes in Healthy Postmenopausal Women. Osteoporos. Int. 2018, 29, 1771–1782. [Google Scholar] [CrossRef]
- Tu, M.-Y.; Han, K.-Y.; Chang, G.R.-L.; Lai, G.-D.; Chang, K.-Y.; Chen, C.-F.; Lai, J.-C.; Lai, C.-Y.; Chen, H.-L.; Chen, C.-M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients 2020, 12, 3432. [Google Scholar] [CrossRef]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Lee, Y.K.; Salminen, S. (Eds.) Handbook of Probiotics and Prebiotics; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-0-470-13544-0. [Google Scholar]
- Bornet, F.R.J.; Brouns, F.; Tashiro, Y.; Duvillier, V. Nutritional Aspects of Short-Chain Fructooligosaccharides: Natural Occurrence, Chemistry, Physiology and Health Implications. Dig. Liver Dis. 2002, 34, S111–S120. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of Galacto-Oligosaccharide Mixture (B-GOS) on Gut Microbiota, Immune Parameters and Metabonomics in Elderly Persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Aςil, Y.; Glüer, C.-C.; Schrezenmeir, J. Prebiotics, Probiotics, and Synbiotics Affect Mineral Absorption, Bone Mineral Content, and Bone Structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef] [PubMed]
- Timan, P.; Rojanasthien, N.; Manorot, M.; Sangdee, C.; Teekachunhatean, S. Effect of Synbiotic Fermented Milk on Oral Bioavailability of Isoflavones in Postmenopausal Women. Null 2014, 65, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Kode, A.; Mosialou, I.; Silva, B.C.; Rached, M.-T.; Zhou, B.; Wang, J.; Townes, T.M.; Hen, R.; DePinho, R.A.; Guo, X.E.; et al. FOXO1 Orchestrates the Bone-Suppressing Function of Gut-Derived Serotonin. J. Clin. Investig. 2012, 122, 3490–3503. [Google Scholar] [CrossRef]
- Lawenius, L.; Scheffler, J.M.; Gustafsson, K.L.; Henning, P.; Nilsson, K.H.; Colldén, H.; Islander, U.; Plovier, H.; Cani, P.D.; de Vos, W.M.; et al. Pasteurized Akkermansia Muciniphila Protects from Fat Mass Gain but Not from Bone Loss. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E480–E491. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The Effect of Probiotic Lactobacillus Casei Shirota on Knee Osteoarthritis: A Randomised Double-Blind, Placebo-Controlled Clinical Trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and Bifidobacteria in Human Breast Milk: Influence of Antibiotherapy and Other Host and Clinical Factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The Human Milk Microbiome Changes over Lactation and Is Shaped by Maternal Weight and Mode of Delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Igras, S. Characteristics of milk of various animal and human species. J. NutriLife 2012, 5. [Google Scholar]
- Pisano, M.B.; Deplano, M.; Fadda, M.E.; Cosentino, S. Microbiota of Sardinian Goat’s Milk and Preliminary Characterization of Prevalent LAB Species for Starter or Adjunct Cultures Development. BioMed Res. Int. 2019, 2019, e6131404. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-K.; Liong, M.-T. Angiotensin I-Converting Enzyme Inhibitory Activity and Bioconversion of Isoflavones by Probiotics in Soymilk Supplemented with Prebiotics. Null 2010, 61, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D. Selecting Suitable Bacterial Strains of Lactobacillus and Identifying Soya Drink Fermentation Conditions. Żywność. Nauka. Technologia. Jakość 2005, 2, 189–297. [Google Scholar]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process Optimization for the Development of a Functional Beverage Based on Lactic Acid Fermentation of Oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Gamba, R.R.; Yamamoto, S.; Abdel-Hamid, M.; Sasaki, T.; Michihata, T.; Koyanagi, T.; Enomoto, T. Chemical, Microbiological, and Functional Characterization of Kefir Produced from Cow’s Milk and Soy Milk. Int. J. Microbiol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Brueton, M.; Dupont, C.; Hill, D.; Isolauri, E.; Koletzko, S.; Oranje, A.P.; Staiano, A. Guidelines for the Diagnosis and Management of Cow’s Milk Protein Allergy in Infants. Arch. Dis. Child. 2007, 92, 902–908. [Google Scholar] [CrossRef]
- Hojsak, I.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hulst, J.; Indrio, F.; Lapillonne, A.; Mølgaard, C.; et al. Young Child Formula: A Position Paper by the ESPGHAN Committee on Nutrition. J. Pediatric Gastroenterol. Nutr. 2018, 66, 177–185. [Google Scholar] [CrossRef]
- Services, A.H. Healthy Infants and Young Children. Available online: https://www.albertahealthservices.ca/info/Page8567.aspx (accessed on 15 December 2020).
- 2015–2020 Dietary Guidelines|Health.Gov. Available online: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/#subnav-3 (accessed on 14 December 2020).
- Marangoni, F.; Pellegrino, L.; Verduci, E.; Ghiselli, A.; Bernabei, R.; Calvani, R.; Cetin, I.; Giampietro, M.; Perticone, F.; Piretta, L.; et al. Cow’s Milk Consumption and Health: A Health Professional’s Guide. J. Am. Coll. Nutr. 2019, 38, 197–208. [Google Scholar] [CrossRef]
| Products. | Portion | Calcium Content (mg) |
|---|---|---|
| Whole milk | 200 mL | 236 |
| Semi-skimmed milk | 200 mL | 240 |
| Skimmed milk | 200 mL | 244 |
| Sheep milk | 200 mL | 380 |
| Soy dring (non-enriched) | 200 mL | 26 |
| Soy drink (calcium-enriched) | 200 mL | 240 |
| Rice drink | 200 mL | 22 |
| Almond milk | 200 mL | 90 |
| Flavoured yoghurt | 150 g | 197 |
| Natural yoghurt | 150 g | 207 |
| Hard cheese (e.g., Parmesan, Cheddar) | 30 g | 240 |
| Fresh cheese (e.g., Ricotta, cottage cheese) | 200 | 138 |
| Mozzarella | 60 | 242 |
| Age | RDI (mg) |
|---|---|
| 0–6 months | 200 |
| 7–12 months | 260 |
| 1–3 years | 700 |
| 4–8 years | 1000 |
| 9–13 years | 1300 |
| 14–18 years | 1300 |
| 19–50 years | 1000 |
| 51–70 years | |
| Women | 1200 |
| Men | 1000 |
| 71 years and older | 1200 |
| Pregnant and breastfeeding | |
| Teenagers | 1300 |
| Adults | 1000 |
| Human [66] | Cow’s [67] | Plant [68] | |
|---|---|---|---|
| Fat (g) | 3.8 | 3.7–3.9 | 0.66–49.2 |
| Proteins (g) | 1.0 | 3.2–3.5 | 0.59–19.00 |
| Casein (g) | 0.3 | 2.8 | 0 |
| Carbohydrates (g) | 7.0 | 7.0 | 27.3–50.0 |
| Lactose (g) | 7.0 | 0.9–4.9 | 0 |
| Calcium (mg) | 34 | 118 | 4.0–180.0 |
| Phosphorus (mg) | 15 | 89.6 | 49.0–1000.0 |
| Sodium (mg) | 15 | 44.5 | 2.2–140.01 |
| Potassium (mg) | 58 | 150 | 65.00–2000.0 |
| Protein (g) | Fat (g) | Carbohydrates (g) | Calcium (mg) | Phosphorus (mg) | Ca:P Ratio | Wit.D (μg) | |
|---|---|---|---|---|---|---|---|
| Milk, 3.5% fat | 3.3 | 3.5 | 4.8 | 118 | 85 | 1.38 | 0.03 |
| Milk, 2% fat | 3.4 | 2.0 | 4.9 | 120 | 86 | 1.40 | 0.02 |
| Cream, 18% | 2.5 | 18.0 | 3.6 | 99 | 71 | 1.39 | 0.14 |
| Natural yoghurt, 2% | 4.3 | 2.0 | 6.2 | 170 | 122 | 1.39 | 0.03 |
| Berries yoghurt | 3.7 | 1.5 | 8.8 | 134 | 96 | 1.40 | 0.02 |
| Cheddar cheese | 27.1 | 31.7 | 0.1 | 703 | 487 | 1.44 | 0.26 |
| Cottage cheese | 12.3 | 4.3 | 3.3 | 80 | 140 | 0.57 | 0.09 |
| Milk | Bacteria Strain | References |
|---|---|---|
| Breast milk | Lactocaseibacillus casei Limosilactobacillus fermentum Lactobacillus gasseri Lactobacillus gastricus Lactiplantibacillus plantarum Lactobacillus reuteri Limosilactobacillus rhamnosus Ligilactobacillus salivarius Lactobacillus vaginalis Bifidobacterium breve Bifidobacterium longum Streptococcus mitis Streptococcus salivarius Streptococcus parasanguinis Enterococcus faecalis Enterococcus gallinarum Staphylococcus epidermidis Staphylococcus lugdunensis Staphylococcus aureus Staphylococcus haemolyticus Staphylococcus pasteuri Veillonella atypical Lactococcus Weissella Serratia Pseudomonas Veillonella Leptotrichia Prevotella | [88,107,108] |
| Cow’s milk | Lactococcus lactis, Streptococcus salivarius ssp. thermophilus, Lactobacillus acidophilus, Lacticaseibacillus casei Limosilactobacillus fermentum. | [109] |
| Goast milk | Lactococcus lactis, Lacticaseibacillus paracasei, Pediococcus pentosaceus, Leuconostoc mesenteroides, Streptococcus salivarius subsp. Thermophilus Enterococcus faecium | [110] |
| Soy milk-added probiotics | Lactococcus acidophilus Lactococcus acidophilus Lactococcus casei Bifidobacterium longum | [111,112] |
| Oats milk-added probiotics | Lactococcus plantarum 1010 | [113] |
| Kefir (from cow’s milk) | Acetobacter orientalis Lactococcus lactis Lactobacillus gallinarum Kazachstania unispora Galactomyces candidum Geotrichum bryndzae Saccharomyces cerevisiae Pichia kudriavzevii | [114] |
| Kefir (from soy milk) | Lactococcus lactis Kazachstania unispora Saccharomyces cerevisiae Lactobacillus nagelii Lactiplantibacillus plantarum | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, A.E.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis. Nutrients 2021, 13, 1329. https://doi.org/10.3390/nu13041329
Ratajczak AE, Zawada A, Rychter AM, Dobrowolska A, Krela-Kaźmierczak I. Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis. Nutrients. 2021; 13(4):1329. https://doi.org/10.3390/nu13041329
Chicago/Turabian StyleRatajczak, Alicja Ewa, Agnieszka Zawada, Anna Maria Rychter, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis" Nutrients 13, no. 4: 1329. https://doi.org/10.3390/nu13041329
APA StyleRatajczak, A. E., Zawada, A., Rychter, A. M., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). Milk and Dairy Products: Good or Bad for Human Bone? Practical Dietary Recommendations for the Prevention and Management of Osteoporosis. Nutrients, 13(4), 1329. https://doi.org/10.3390/nu13041329