Alcohol Consumption in Rheumatoid Arthritis: A Path through the Immune System
Abstract
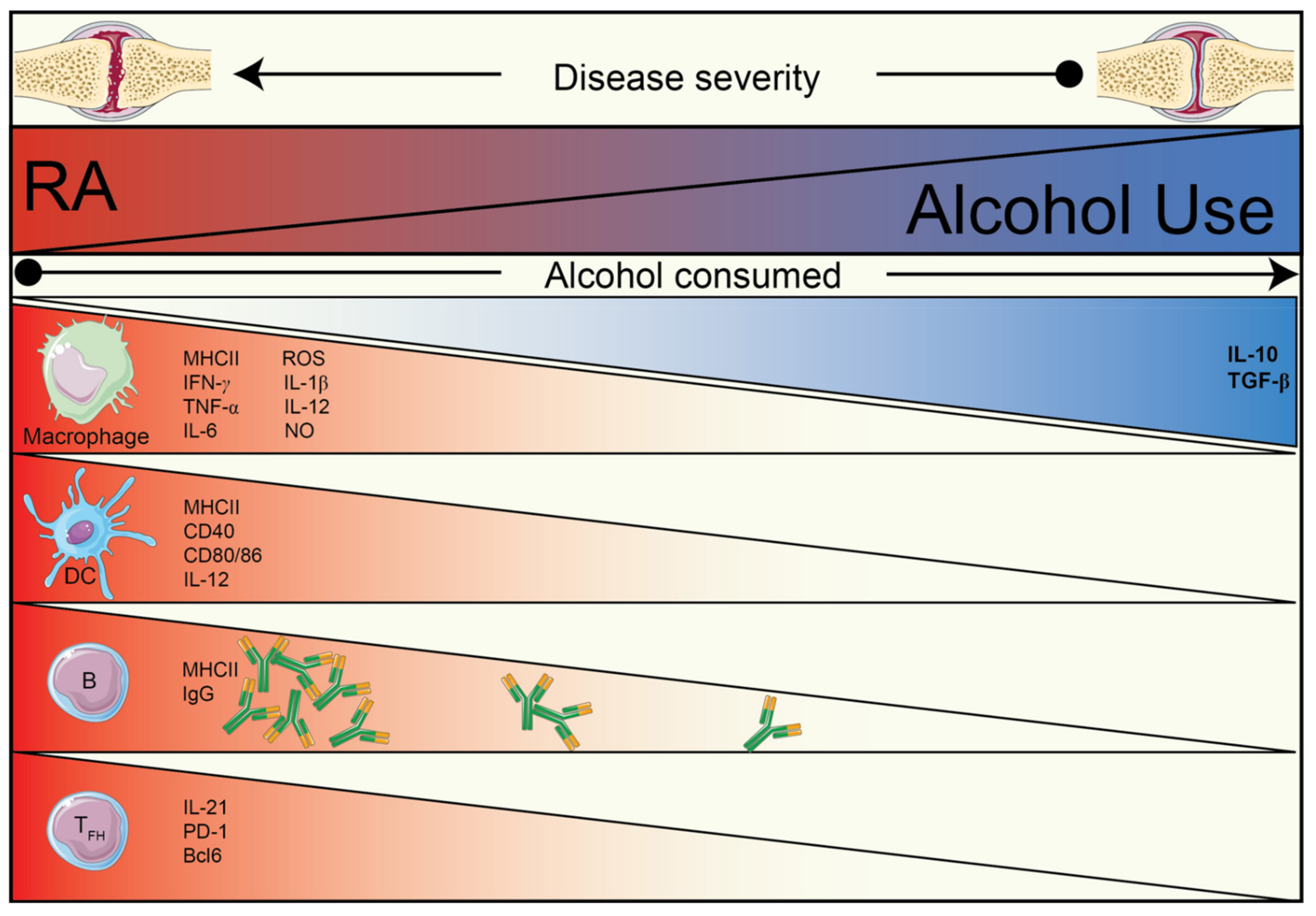
1. Introduction
2. The Effect of Alcohol and Alcohol Metabolites on the Immune System
2.1. The Effect of Alcohol on the Immune System
2.2. The Effects of Acetaldehyde on the Immune System
2.3. The Effects of Acetate on the Immune System
3. Alcohol and Acetate Affect Humoral Autoimmunity
3.1. Alcohol and Its Metabolite, Acetate, Reduce IL-21-Producing TFH Cells
3.2. The Effects of Alcohol/Acetate on T–B Cell Relationship
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bloomfield, K.; Stockwell, T.; Gmel, G.; Rehn, N. International Comparisons of Alcohol Consumption. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2003, 27, 95–109. [Google Scholar]
- Schuckit, M.A. Alcohol-use disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How Is Alcohol Metabolized by the Body? Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 245–254. [Google Scholar]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Nuutinen, H.; Lindros, K.; Hekali, P.; Salaspuro, M. Elevated blood acetate as indicator of fast ethanol elimination in chronic alcoholics. Alcohol 1985, 2, 623–626. [Google Scholar] [CrossRef]
- Korri, U.-M.; Nuutinen, H.; Salaspuro, M. Increased Blood Acetate: A New Laboratory Marker of Alcoholism and Heavy Drinking. Alcohol. Clin. Exp. Res. 1985, 9, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.A.; Husain, K.; Rizvi, S.A.A. Role of Transcription Factors in Steatohepatitis and Hypertension after Ethanol: The Epicenter of Metabolism. Biomolecules 2016, 6, 29. [Google Scholar] [CrossRef]
- Bhattacharya, C. Hepatitis C and Alcohol. J. Clin. Gastroenterol. 2003, 36, 242–252. [Google Scholar] [CrossRef]
- Molina, P.E.; Happel, K.I.; Zhang, P.; Kolls, J.K.; Nelson, S. Focus On: Alcohol and the Immune System. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2010, 33, 97–108. [Google Scholar]
- Szabo, G.; Saha, B. Alcohol’s Effect on Host Defense. Alcohol Res. Curr. Rev. 2015, 37, 159–170. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 16 March 2021).
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nat. Cell Biol. 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Barton, J.C. Autoimmune Conditions in 235 Hemochromatosis Probands withHFEC282Y Homozygosity and Their First-Degree Relatives. J. Immunol. Res. 2015, 2015, 453046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kerlan-Candon, S.; Combe, B.; Vincent, R.; Clot, J.; Pinet, V.; Eliaou, J.-F. HLA-DRB1 gene transcripts in rheumatoid arthritis. Clin. Exp. Immunol. 2001, 124, 142–149. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid Factors: Clinical Applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef]
- Chang, M.H.; Nigrovic, P.A. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Källberg, H.; Jacobsen, S.; Bengtsson, C.; Pedersen, M.; Padyukov, L.; Garred, P.; Frisch, M.; Karlson, E.W.; Klareskog, L.; Alfredsson, L. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: Results from two Scandinavian case–control studies. Ann. Rheum. Dis. 2008, 68, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.R.; Gowers, I.R.; Moore, D.J.; Wilson, A.G. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology 2010, 49, 2140–2146. [Google Scholar] [CrossRef]
- Bergman, S.; Symeonidou, S.; Andersson, M.L.; Söderlin, M.K. Alcohol consumption is associated with lower self-reported disease activity and better health-related quality of life in female rheumatoid arthritis patients in Sweden: Data from BARFOT, a multicenter study on early RA. BMC Musculoskelet. Disord. 2013, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.C.; Tan, R.; Stahl, D.; Steer, S.; Lewis, C.M.; Cope, A.P. The protective effect of alcohol on developing rheumatoid arthritis: A systematic review and meta-analysis. Rheumatology 2013, 52, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Solomon, D.H.; Costenbader, K.H.; Karlson, E.W. Alcohol Consumption and Risk of Incident Rheumatoid Arthritis in Women: A Prospective Study. Arthritis Rheumatol. 2014, 66, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiang, C.; Cai, Q.; Wei, X.; He, J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: A dose-response meta-analysis of prospective studies. Ann. Rheum. Dis. 2013, 73, 1962–1967. [Google Scholar] [CrossRef]
- Hedström, A.K.; Hössjer, O.; Klareskog, L.; Alfredsson, L. Interplay between alcohol, smoking and HLA genes in RA aetiology. RMD Open 2019, 5, e000893. [Google Scholar] [CrossRef]
- Volpato, S.; Pahor, M.; Ferrucci, L.; Simonsick, E.M.; Guralnik, J.M.; Kritchevsky, S.B.; Fellin, R.; Harris, T.B. Relationship of Alcohol Intake with Inflammatory Markers and Plasminogen Activator Inhibitior-1 in Well-Functioning Older Adults. Circulation 2004, 109, 607–612. [Google Scholar] [CrossRef]
- Szabo, G.; Mandrekar, P.; Girouard, L.; Catalano, D. Regulation of Human Monocyte Functions by Acute Ethanol Treatment: Decreased Tumor Necrosis Factor-alpha, Interleukin-1 beta and Elevated Interleukin-10, and Transforming Growth Factor-beta Production. Alcohol. Clin. Exp. Res. 1996, 20, 900–907. [Google Scholar] [CrossRef]
- Szabo, G.; Mandrekar, P.; Oak, S.; Mayerle, J. Effect of Ethanol on Inflammatory Responses. Pancreatology 2007, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Jørgensen, T.; Laurberg, P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: A population-based case–control study. Eur. J. Endocrinol. 2012, 167, 483–490. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Lu, B.; Sparks, J.A.; Malspeis, S.; Chang, S.-C.; Karlson, E.W.; Costenbader, K.H. Influence of Alcohol Consumption on the Risk of Systemic Lupus Erythematosus Among Women in the Nurses’ Health Study Cohorts. Arthritis Care Res. 2017, 69, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Jørgensen, T.; Laurberg, P. Graves′ hyperthyroidism and moderate alcohol consumption: Evidence for disease prevention. Clin. Endocrinol. 2013, 79, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Leatherwood, C.; Malspeis, S.; Liu, X.; Lu, B.; Roberts, A.L.; Sparks, J.A.; Karlson, E.W.; Feldman, C.H.; Munroe, M.E.; et al. Associations between daily alcohol consumption and systemic lupus erythematosus-related cytokines and chemokines among US female nurses without SLE. Lupus 2020, 29, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, B.; Ahlbom, A.; Andersson, T.; Grill, V.; Midthjell, K.; Olsson, L.; Carlsson, S. Alcohol consumption is associated with reduced risk of Type 2 diabetes and autoimmune diabetes in adults: Results from the Nord-Trøndelag health study. Diabet. Med. 2012, 30, 56–64. [Google Scholar] [CrossRef]
- Alpízar-Rodríguez, D.; Finckh, A.; Gilbert, B. The Role of Nutritional Factors and Intestinal Microbiota in Rheumatoid Arthritis Development. Nutrients 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Dey, M. Beverages in Rheumatoid Arthritis: What to Prefer or to Avoid. Nutrients 2020, 12, 3155. [Google Scholar] [CrossRef]
- Chen, P.; Schnabl, B. Host-Microbiome Interactions in Alcoholic Liver Disease. Gut Liver 2014, 8, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Hanck, C.; Rossol, S.; Böcker, U.; Tokus, M.; Singer, M.V. Presence of Plasma Endotoxin is Correlated with Tumour Necrosis Factor Receptor Levels and Disease Activity in Alcoholic Cirrhosis. Alcohol Alcohol. 1998, 33, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Banan, A.; Forsyth, C.B.; Fields, J.Z.; Lau, C.K.; Zhang, L.J.; Keshavarzian, A. Effect of Alcohol on miR-212 Expression in Intestinal Epithelial Cells and Its Potential Role in Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2008, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef]
- Drazic, A.; Myklebust, L.M.; Ree, R.; Arnesen, T. The world of protein acetylation. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2016, 1864, 1372–1401. [Google Scholar] [CrossRef]
- Pandey, S.C.; Bohnsack, J.P. Alcohol Makes Its Epigenetic Marks. Cell Metab. 2020, 31, 213–214. [Google Scholar] [CrossRef]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol metabolism contributes to brain histone acetylation. Nat. Cell Biol. 2019, 574, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, M.; Gandhi, N.; Saiyed, Z.; Pichili, V.; Thangavel, S.; Khatavkar, P.; Yndart-Arias, A.; Nair, M. Effects of Alcohol on Histone Deacetylase 2 (HDAC2) and the Neuroprotective Role of Trichostatin A (TSA). Alcohol. Clin. Exp. Res. 2011, 35, 1550–1556. [Google Scholar] [CrossRef]
- Soliman, M.L.; Rosenberger, T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011, 352, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.-F.; Hany, L.; Barat, C.; Ouellet, M.; Tremblay, M.J. Epigenetic Metabolite Acetate Inhibits Class I/II Histone Deacetylases, Promotes Histone Acetylation, and Increases HIV-1 Integration in CD4+ T Cells. J. Virol. 2017, 91, e01943–16. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.J.; Pearce, E.L. Driving immunity: All roads lead to metabolism. Nat. Rev. Immunol. 2018, 18, 81–82. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Ullah, H.; Tovchiga, O.; Daglia, M.; Khan, H. Modulating Gut Microbiota: An Emerging Approach in the Prevention and Treatment of Multiple Sclerosis. Curr. Neuropharmacol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Caslin, B.; Maguire, C.; Karmakar, A.; Mohler, K.; Wylie, D.; Melamed, E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc. Natl. Acad. Sci. USA 2019, 116, 25808–25815. [Google Scholar] [CrossRef]
- Jonsson, I.-M.; Verdrengh, M.; Brisslert, M.; Lindblad, S.; Bokarewa, M.; Islander, U.; Carlsten, H.; Ohlsson, C.; Nandakumar, K.S.; Holmdahl, R.; et al. Ethanol prevents development of destructive arthritis. Proc. Natl. Acad. Sci. USA 2006, 104, 258–263. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Azizov, V.; Dietel, K.; Steffen, F.; Dürholz, K.; Meidenbauer, J.; Lucas, S.; Frech, M.; Omata, Y.; Tajik, N.; Knipfer, L.; et al. Ethanol consumption inhibits TFH cell responses and the development of autoimmune arthritis. Nat. Commun. 2020, 11, 1998. [Google Scholar] [CrossRef]
- Kolber, M.A.; Walls, R.M.; Hinners, M.L.; Singer, D.S. Evidence of Increased Class I MHC Expression on Human Peripheral Blood Lymphocytes during Acute Ethanol Intoxication. Alcohol. Clin. Exp. Res. 1988, 12, 820–823. [Google Scholar] [CrossRef]
- Mikszta, J.A.; Waltenbaugh, C.; Kim, B.S. Impaired antigen presention by splenocytes of ethanol-consuming C57BL/6 mice. Alcohol 1995, 12, 265–271. [Google Scholar] [CrossRef]
- Mandrekar, P.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Szabo, G. Inhibition of Myeloid Dendritic Cell Accessory Cell Function and Induction of T Cell Anergy by Alcohol Correlates with Decreased IL-12 Production. J. Immunol. 2004, 173, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.H.; Abe, M.; Thomson, A.W. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J. Leukoc. Biol. 2006, 79, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Edsen-Moore, M.R.; Turner, L.E.; Cook, R.T.; Legge, K.L.; Waldschmidt, T.J.; Schlueter, A.J. Mechanisms by Which Chronic Ethanol Feeding Limits the Ability of Dendritic Cells to Stimulate T-Cell Proliferation. Alcohol. Clin. Exp. Res. 2010, 35, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.J.; Fan, J.; Wilke, W.W.; Coleman, R.A.; Cook, R.T.; Schlueter, A.J. Chronic Ethanol Consumption Decreases Murine Langerhans Cell Numbers and Delays Migration of Langerhans Cells as Well as Dermal Dendritic Cells. Alcohol. Clin. Exp. Res. 2008, 32, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-S. Ethanol Metabolism and Effects: Nitric Oxide and its Interaction. Curr. Clin. Pharmacol. 2007, 2, 145–153. [Google Scholar] [CrossRef]
- Norkina, O.; Dolganiuc, A.; Catalano, D.; Kodys, K.; Mandrekar, P.; Syed, A.; Efros, M.; Szabo, G. Acute Alcohol Intake Induces SOCS1 and SOCS3 and Inhibits Cytokine-Induced STAT1 and STAT3 Signaling in Human Monocytes. Alcohol. Clin. Exp. Res. 2008, 32, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Jeliazkova, V.; Catalano, D.; Szabo, G. Acute Alcohol Exposure Exerts Anti-Inflammatory Effects by Inhibiting IκB Kinase Activity and p65 Phosphorylation in Human Monocytes. J. Immunol. 2007, 178, 7686–7693. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, L.R.; Randall, M.J.; Ather, J.L.; DePuccio, D.P.; Landry, C.C.; Qian, X.; Janssen-Heininger, Y.M.; van der Vliet, A.; Dixon, A.E.; Amiel, E.; et al. Mitochondrial ROS induced by chronic ethanol exposure promote hyper-activation of the NLRP3 inflammasome. Redox Biol. 2017, 12, 883–896. [Google Scholar] [CrossRef]
- Kim, S.-K.; Choe, J.-Y.; Park, K.-Y. Ethanol Augments Monosodium Urate-Induced NLRP3 Inflammasome Activation via Regulation of AhR and TXNIP in Human Macrophages. Yonsei Med. J. 2020, 61, 533–541. [Google Scholar] [CrossRef] [PubMed]
- De Castro, L.F.; de Araújo Mathias, K.; Nunes, J.V.; Galastri, A.L.B.; da Silva, D.H.L.; Longhi, L.N.A.; de Souza Lima Blotta, M.H.; Mamoni, R.L. Ethanol modulates the effector functions of human monocyte-derived macrophages in response to Paracoccidioides brasiliensis yeast cells. Med. Mycol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yamin, R.; Berhani, O.; Peleg, H.; Aamar, S.; Stein, N.; Gamliel, M.; Hindi, I.; Scheiman-Elazary, A.; Gur, C. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Cook, R.T.; Li, F.; Vandersteen, D.; Ballas, Z.K.; Cook, B.L.; Labrecque, D.R. Ethanol and Natural Killer Cells. I. Activity and Immunophenotype in Alcoholic Humans. Alcohol. Clin. Exp. Res. 1997, 21, 974–980. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Zhu, Z.; Luong, D.; Meadows, G.G. Chronic alcohol consumption enhances iNKT cell maturation and activation. Toxicol. Appl. Pharmacol. 2015, 282, 139–150. [Google Scholar] [CrossRef]
- Gu, M.; Samuelson, D.R.; Taylor, C.M.; Molina, P.E.; Luo, M.; Siggins, R.W.; Shellito, J.E.; Welsh, D.A. Alcohol-Associated Intestinal Dysbiosis Alters Mucosal-Associated Invariant T-Cell Phenotype and Function. Alcohol. Clin. Exp. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Melvan, J.N.; Siggins, R.W.; Stanford, W.L.; Porretta, C.; Nelson, S.; Bagby, G.J.; Zhang, P. Alcohol Impairs the Myeloid Proliferative Response to Bacteremia in Mice by Inhibiting the Stem Cell Antigen-1/ERK Pathway. J. Immunol. 2012, 188, 1961–1969. [Google Scholar] [CrossRef]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. Curr. Rev. 2015, 37, 185–197. [Google Scholar]
- Lopez, M.C.; Huang, D.S.; Borgs, P.; Wang, Y.; Watson, R.R. Modification of Lymphocyte Subsets in the Intestinal-Associated Immune System and Thymus by Chronic Ethanol Consumption. Alcohol. Clin. Exp. Res. 1994, 18, 8–11. [Google Scholar] [CrossRef]
- Wagner, F.; Fink, R.; Hart, R.; Lersch, C.; Dancygier, H.; Classen, M. Ethanol inhibits interferon-gamma secretion by human peripheral lymphocytes. J. Stud. Alcohol 1992, 53, 277–280. [Google Scholar] [CrossRef]
- Starkenburg, S.; Munroe, M.E.; Waltenbaugh, C. Early Alteration in Leukocyte Populations and Th1/Th2 Function in Ethanol-Consuming Mice. Alcohol. Clin. Exp. Res. 2001, 25, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Santalla, M.J.; Vidal, C.; Vinuela, J.; Perez, L.F.; Gonzalez-Quintela, A. Increased Serum IgE in Alcoholics: Relationship with Th1/Th2 Cytokine Production by Stimulated Blood Mononuclear Cells. Alcohol. Clin. Exp. Res. 2001, 25, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Zisman, D.A.; Strieter, R.M.; Kunkel, S.L.; Tsai, W.C.; Wilkowski, J.M.; Bucknell, K.A.; Standiford, T.J. Ethanol Feeding Impairs Innate Immunity and Alters the Expression of Th1- and Th2-Phenotype Cytokines in Murine Klebsiella Pneumonia. Alcohol. Clin. Exp. Res. 1998, 22, 621–627. [Google Scholar] [CrossRef]
- Nelson, S.; Bagby, G.J.; Bainton, B.G.; Summer, W.R. The Effects of Acute and Chronic Alcoholism on Tumor Necrosis Factor and the Inflammatory Response. J. Infect. Dis. 1989, 160, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, Z.; Ren, T.; Kim, S.-J.; He, Y.; Seo, W.; Guillot, A.; Ding, Y.; Wu, R.; Shao, S.; et al. Alcohol inhibits T-cell glucose metabolism and hepatitis in ALDH2-deficient mice and humans: Roles of acetaldehyde and glucocorticoids. Gut 2019, 68, 1311–1322. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Edsen-Moore, M.; McGill, J.; Coleman, R.A.; Cook, R.T.; Legge, K.L. Chronic Alcohol Consumption Increases the Severity of Murine Influenza Virus Infections. J. Immunol. 2008, 181, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 2007, 30, 5–13. [Google Scholar] [PubMed]
- Latvala, J.; Parkkila, S.; Melkko, J.; Niemelä, O. Acetaldehyde Adducts in Blood and Bone Marrow of Patients with Ethanol-Induced Erythrocyte Abnormalities. Mol. Med. 2001, 7, 401–405. [Google Scholar] [CrossRef]
- Ganesan, M.; Krutik, V.M.; Makarov, E.; Mathews, S.; Kharbanda, K.K.; Poluektova, L.Y.; Casey, C.A.; Osna, N.A. Acetaldehyde suppresses the display of HBV-MHC class I complexes on HBV-expressing hepatocytes. Am. J. Physiol. Liver Physiol. 2019, 317, G127–G140. [Google Scholar] [CrossRef]
- Mikuls, T.R.; Duryee, M.J.; Rahman, R.; Anderson, D.R.; Sayles, H.R.; Hollins, A.; Michaud, K.; Wolfe, F.; Thiele, G.E.; Sokolove, J.; et al. Enrichment of malondialdehyde–acetaldehyde antibody in the rheumatoid arthritis joint. Rheumatology 2017, 56, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Grönwall, C.; Amara, K.; Hardt, U.; Krishnamurthy, A.; Steen, J.; Engström, M.; Sun, M.; Ytterberg, A.J.; Zubarev, R.A.; Scheel-Toellner, D.; et al. Autoreactivity to malondialdehyde-modifications in rheumatoid arthritis is linked to disease activity and synovial pathogenesis. J. Autoimmun. 2017, 84, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Edison, J.; Meeshaw, E.; Sayles, H.; England, B.R.; Duryee, M.J.; Hunter, C.D.; Kelmenson, L.B.; Moss, L.K.; Feser, M.L.; et al. Autoantibodies to Malondialdehyde—Acetaldehyde Are Detected Prior to Rheumatoid Arthritis Diagnosis and After Other Disease Specific Autoantibodies. Arthritis Rheumatol. 2020, 72, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, K.J.; Rao, R.K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. Liver Physiol. 2001, 280, G1280–G1288. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K. Acetaldehyde-Induced Increase in Paracellular Permeability in Caco-2 Cell Monolayer. Alcohol. Clin. Exp. Res. 1998, 22, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019, 29, 695–703. [Google Scholar] [CrossRef]
- Li, Y.; Gruber, J.J.; Litzenburger, U.M.; Zhou, Y.; Miao, Y.R.; LaGory, E.L.; Li, A.M.; Hu, Z.; Yip, M.; Hart, L.S.; et al. Acetate supplementation restores chromatin accessibility and promotes tumor cell differentiation under hypoxia. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Lee, J.V.; Berry, C.T.; Kim, K.; Sen, P.; Kim, T.; Carrer, A.; Trefely, S.; Zhao, S.; Fernandez, S.; Barney, L.E.; et al. Acetyl-CoA promotes glioblastoma cell adhesion and migration through Ca2+–NFAT signaling. Genes Dev. 2018, 32, 497–511. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Ohbuchi, A.; Kono, M.; Takenokuchi, M.; Imoto, S.; Saigo, K. Acetate moderately attenuates the generation of neutrophil extracellular traps. Blood Res. 2018, 53, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Yin, N.; Chhangawala, S.; Xu, K.; Leslie, C.S.; Li, M.O. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 2016, 354, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Renner, K.; Singer, K.; Koehl, G.E.; Geissler, E.K.; Peter, K.; Siska, P.J.; Kreutz, M. Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Cham, C.M.; Gajewski, T.F. Glucose Availability Regulates IFN-γ Production and p70S6 Kinase Activation in CD8+ Effector T Cells. J. Immunol. 2005, 174, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Villa, M.; Sanin, D.E.; Buck, M.D.; O’Sullivan, D.; Ching, R.; Matsushita, M.; Grzes, K.M.; Winkler, F.; Chang, C.-H.; et al. Acetate Promotes T Cell Effector Function during Glucose Restriction. Cell Rep. 2019, 27, 2063–2074.e5. [Google Scholar] [CrossRef]
- Balmer, M.L.; Ma, E.H.; Bantug, G.R.; Grählert, J.; Pfister, S.; Glatter, T.; Jauch, A.; Dimeloe, S.; Slack, E.; Dehio, P.; et al. Memory CD8 + T Cells Require Increased Concentrations of Acetate Induced by Stress for Optimal Function. Immunity 2016, 44, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.L.; Ma, E.H.; Thompson, A.J.; Epple, R.; Unterstab, G.; Lötscher, J.; Dehio, P.; Schürch, C.M.; Warncke, J.D.; Perrin, G.; et al. Memory CD8+ T Cells Balance Pro- and Anti-inflammatory Activity by Reprogramming Cellular Acetate Handling at Sites of Infection. Cell Metab. 2020, 32, 457–467.e5. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Pedro, J.M.B.-S.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Choi, J.; Crotty, S. Bcl6-Mediated Transcriptional Regulation of Follicular Helper T cells (TFH). Trends Immunol. 2021, 42, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Rigby, R.J.; Wong, R.K.; Yu, D.; Brink, R.; Cannons, J.L.; Schwartzberg, P.L.; Cook, M.C.; Walters, G.D.; Vinuesa, C.G. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009, 206, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, Q.; Su, D.; Che, N.; Chen, H.; Geng, L.; Chen, J.; Chen, W.; Li, X.; Sun, L. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R255. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Lv, T.-T.; Yin, Z.-J.; Wang, X.-B. Elevated circulating Th17 and follicular helper CD4+T cells in patients with rheumatoid arthritis. APMIS 2015, 123, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, P.; Rasool, M. Multifaceted role of IL-21 in rheumatoid arthritis: Current understanding and future perspectives. J. Cell. Physiol. 2017, 233, 3918–3928. [Google Scholar] [CrossRef]
- Young, D.A.; Hegen, M.; Ma, H.L.M.; Whitters, M.J.; Albert, L.M.; Lowe, L.; Senices, M.; Wu, P.W.; Sibley, B.; Leathurby, Y.; et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007, 56, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shan, Y.; Jiang, Z.; Feng, J.; Li, C.; Ma, L.; Jiang, Y. High frequencies of activated B cells and follicular helper T cells are correlated with disease activity in patients with new onset rheumatoid arthritis. Clin. Exp. Immunol. 2013, 174, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, K.S.C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nat. Cell Biol. 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Arroyo-Villa, I.; Bautista-Caro, M.-B.; Balsa, A.; Aguado-Acín, P.; Bonilla-Hernán, M.-G.; Plasencia, C.; Villalba, A.; Nuño, L.; Puig-Kröger, A.; Martín-Mola, E.; et al. Constitutively altered frequencies of circulating follicullar helper T cell counterparts and their subsets in rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, F.; Zhou, M.; Chen, L.; Lu, Y. A preliminary study on the characterization of follicular helper T (Tfh) cells in rheumatoid arthritis synovium. Acta Histochem. 2014, 116, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Spolski, R.; Feng, C.G.; Qi, C.-F.; Cheng, J.; Sher, A.; Iii, H.C.M.; Liu, C.; Schwartzberg, P.L.; Leonard, W.J. A Critical Role for IL-21 in Regulating Immunoglobulin Production. Science 2002, 298, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Tanaka, Y.; Isaacs, J.D. Why remission is not enough: Underlying disease mechanisms in RA that prevent cure. Nat. Rev. Rheumatol. 2021, 17, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Van Delft, M.A.; Huizinga, T.W. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Bas, S.; Perneger, T.V.; Seitz, M.; Tiercy, J.; Roux-Lombard, P.; Guerne, P.A. Diagnostic tests for rheumatoid arthritis: Comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology 2002, 41, 809–814. [Google Scholar] [CrossRef]
- Mendenhall, C.; Roselle, G.A.; Lybecker, L.A.; Marshall, L.E.; Grossman, C.J.; Myre, S.A.; Weesner, R.E.; Morgan, D.D. Hepatitis B vaccination. Dig. Dis. Sci. 1988, 33, 263–269. [Google Scholar] [CrossRef]
- Yanaba, K.; Hamaguchi, Y.; Venturi, G.M.; Steeber, D.A.; Clair, E.W.S.; Tedder, T.F. B Cell Depletion Delays Collagen-Induced Arthritis in Mice: Arthritis Induction Requires Synergy between Humoral and Cell-Mediated Immunity. J. Immunol. 2007, 179, 1369–1380. [Google Scholar] [CrossRef]
- Renato, G.M. B Cell Depletion in Early Rheumatoid Arthritis: A New Concept in Therapeutics. Ann. N. Y. Acad. Sci. 2009, 1173, 729–735. [Google Scholar] [CrossRef]
- Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 6th ed.; Garland Science Publishing: New York, NY, USA, 2005. [Google Scholar]
- Nandakumar, K.S.; Bäcklund, J.; Vestberg, M.; Holmdahl, R. Collagen type II (CII)-specific antibodies induce arthritis in the absence of T or B cells but the arthritis progression is enhanced by CII-reactive T cells. Arthritis Res. 2004, 6, R544–R550. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pearson, J.A.; Peng, J.; Hu, Y.; Sha, S.; Xing, Y.; Huang, G.; Li, X.; Hu, F.; Xie, Z.; et al. Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Dietel, K. Der Einfluss von Ethanol auf die Autoimmunantwort an Einem Mausmodell der Rheumatoiden Arthritis. Ph.D. Thesis, University of Erlangen-Nuremberg, Erlangen, Germany, 2004. [Google Scholar]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274.e5. [Google Scholar] [CrossRef] [PubMed]
- Kwun, J.; Manook, M.; Page, E.; Burghuber, C.; Hong, J.; Knechtle, S.J. Crosstalk between T and B Cells in the Germinal Center After Transplantation. Transplantation 2017, 101, 704–712. [Google Scholar] [CrossRef] [PubMed]
| Moderate Consumption | Heavy Consumption | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Alcohol in grams/day | <28 | <14 | >56 # | >42 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizov, V.; Zaiss, M.M. Alcohol Consumption in Rheumatoid Arthritis: A Path through the Immune System. Nutrients 2021, 13, 1324. https://doi.org/10.3390/nu13041324
Azizov V, Zaiss MM. Alcohol Consumption in Rheumatoid Arthritis: A Path through the Immune System. Nutrients. 2021; 13(4):1324. https://doi.org/10.3390/nu13041324
Chicago/Turabian StyleAzizov, Vugar, and Mario M. Zaiss. 2021. "Alcohol Consumption in Rheumatoid Arthritis: A Path through the Immune System" Nutrients 13, no. 4: 1324. https://doi.org/10.3390/nu13041324
APA StyleAzizov, V., & Zaiss, M. M. (2021). Alcohol Consumption in Rheumatoid Arthritis: A Path through the Immune System. Nutrients, 13(4), 1324. https://doi.org/10.3390/nu13041324







