Measuring Dietary Botanical Diversity as a Proxy for Phytochemical Exposure
Abstract
1. Introduction
2. Characterizing Dietary Diversity
3. Rethinking the Assessment of the Plant Food Components of Dietary Patterns
3.1. Pharmocognosy
3.2. Phytochemicals
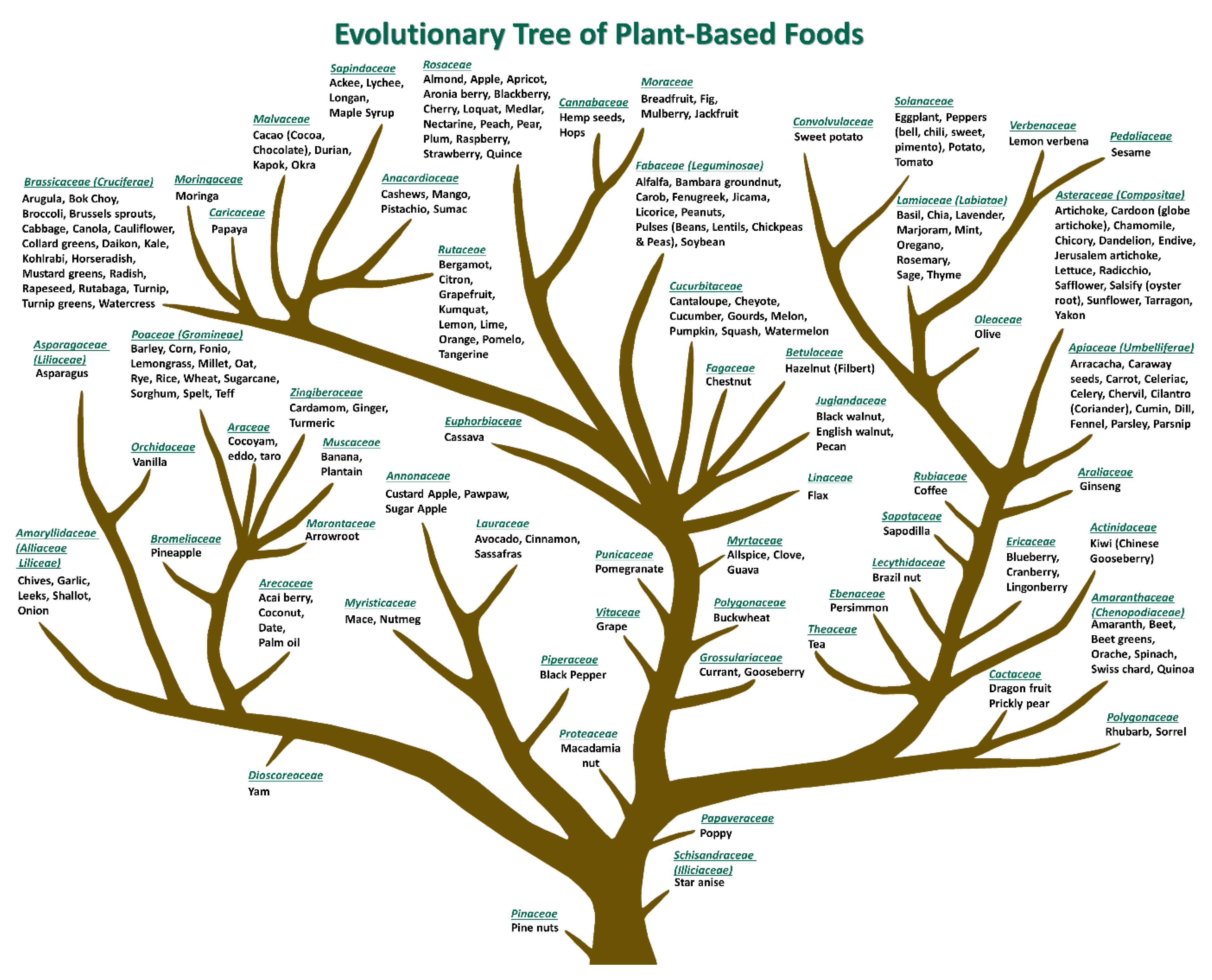
3.3. Botanical Diversity Index (BDI)
4. Food Intake Pattern, the Gut Microbiome, and Human Health and Disease
5. Final Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The global syndemic of obesity, undernutrition, and climate change: The lancet commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Shirley Liu, X.; Struhl, K. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Herforth, A.; Arimond, M.; Alvarez-Sanchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A global review of food-based dietary guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services National Institutes of Health. 2020–2030 Strategic Plan for NIH Nutrition Research. 2020. Available online: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/strategic-plan-nih-nutrition-research (accessed on 1 March 2021).
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2020–2025 Dietary Guidelines for Americans, 9th ed.; US Department of Agriculture: Washington, DC, USA, 2020.
- Frame, L.A.; Costa, E.; Jackson, S.A. Current explorations of nutrition and the gut microbiome: A comprehensive evaluation of the review literature. Nutr. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.I.; Davis, C.; Fraser, C.M.; Schneeman, B.O.; Huttenhower, C.; Verbeke, K.; Walter, J.; Latulippe, M.E. Establishing what constitutes a healthy human gut microbiome: State of the science, regulatory considerations, and future directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef]
- Bilotta, A.J.; Cong, Y. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: Implication in precision medicine. Precis. Clin. Med. 2019, 2, 110–119. [Google Scholar] [CrossRef]
- Rodgers, G.P.; Collins, F.S. Precision nutrition--the answer to “What to eat to stay healthy”. JAMA 2020. [Google Scholar] [CrossRef]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American gut: An open platform for citizen science microbiome research. Msystems 2018, 3. [Google Scholar] [CrossRef]
- Mills, S.; Lane, J.A.; Smith, G.J.; Grimaldi, K.A.; Ross, R.P.; Stanton, C. Precision nutrition and the microbiome part II: Potential opportunities and pathways to commercialisation. Nutrients 2019, 11, 1468. [Google Scholar] [CrossRef]
- Ruel, M.T. Operationalizing dietary diversity: A review of measurement issues and research priorities. J. Nutr. 2003, 133, 3911S–3926S. [Google Scholar] [CrossRef]
- Cano-Ibanez, N.; Gea, A.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Corella, D.; Zomeno, M.D.; Romaguera, D.; Vioque, J.; Aros, F.; Warnberg, J.; et al. Dietary diversity and nutritional adequacy among an older spanish population with metabolic syndrome in the PREDIMED-plus study: A cross-sectional analysis. Nutrients 2019, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Jahangiry, L. Dietary diversity score is associated with cardiovascular risk factors and serum adiponectin concentrations in patients with metabolic syndrome. BMC Cardiovasc. Disord. 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Schatzkin, A.; Harris, T.B.; Ziegler, R.G.; Block, G. Dietary diversity and subsequent mortality in the first national health and nutrition examination survey epidemiologic follow-up study. Am. J. Clin. Nutr. 1993, 57, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Isa, F.; Xie, L.P.; Hu, Z.; Zhong, Z.; Hemelt, M.; Reulen, R.C.; Wong, Y.C.; Tam, P.C.; Yang, K.; Chai, C.; et al. Dietary consumption and diet diversity and risk of developing bladder cancer: Results from the South and East China case-control study. Cancer Causes Control 2013, 24, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Conrad, Z.; Raatz, S.; Jahns, L. Greater vegetable variety and amount are associated with lower prevalence of coronary heart disease: National health and nutrition examination survey, 1999–2014. Nutr. J. 2018, 17, 67. [Google Scholar] [CrossRef]
- Drescher, L.S.; Thiele, S.; Mensink, G.B. A new index to measure healthy food diversity better reflects a healthy diet than traditional measures. J. Nutr. 2007, 137, 647–651. [Google Scholar] [CrossRef]
- Jayawardena, R.; Byrne, N.M.; Soares, M.J.; Katulanda, P.; Yadav, B.; Hills, A.P. High dietary diversity is associated with obesity in Sri Lankan adults: An evaluation of three dietary scores. BMC Public Health 2013, 13, 314. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sharp, S.J.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care 2012, 35, 1293–1300. [Google Scholar] [CrossRef]
- Jeurnink, S.M.; Buchner, F.L.; Bueno-de-Mesquita, H.B.; Siersema, P.D.; Boshuizen, H.C.; Numans, M.E.; Dahm, C.C.; Overvad, K.; Tjonneland, A.; Roswall, N.; et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2012, 131, E963–E973. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Iqbal, R.; Singh, K.; Jaacks, L.M.; Shivashankar, R.; Sudha, V.; Anjana, R.M.; Kadir, M.; Mohan, V.; Ali, M.K.; et al. Association of dietary patterns and dietary diversity with cardiometabolic disease risk factors among adults in South Asia: The CARRS study. Asia Pac. J. Clin. Nutr. 2018, 27, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Truthmann, J.; Richter, A.; Thiele, S.; Drescher, L.; Roosen, J.; Mensink, G.B. Associations of dietary indices with biomarkers of dietary exposure and cardiovascular status among adolescents in Germany. Nutr. Metab. 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.C.; Padhye, N.S.; Bertoni, A.G.; Jacobs, D.R., Jr.; Mozaffarian, D. Everything in moderation--dietary diversity and quality, central obesity and risk of diabetes. PLoS ONE 2015, 10, e0141341. [Google Scholar] [CrossRef]
- Katanoda, K.; Kim, H.S.; Matsumura, Y. New quantitative index for dietary diversity (QUANTIDD) and its annual changes in the Japanese. Nutrition 2006, 22, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Salome, M.; de Gavelle, E.; Dufour, A.; Dubuisson, C.; Volatier, J.L.; Fouillet, H.; Huneau, J.F.; Mariotti, F. Plant-protein diversity is critical to ensuring the nutritional adequacy of diets when replacing animal with plant protein: Observed and modeled diets of french adults (INCA3). J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lachat, C.; Raneri, J.E.; Smith, K.W.; Kolsteren, P.; Van Damme, P.; Verzelen, K.; Penafiel, D.; Vanhove, W.; Kennedy, G.; Hunter, D.; et al. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc. Natl. Acad. Sci. USA 2018, 115, 127–132. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Otto, M.C.; Anderson, C.A.M.; Dearborn, J.L.; Ferranti, E.P.; Mozaffarian, D.; Rao, G.; Wylie-Rosett, J.; Lichtenstein, A.H. Dietary diversity: Implications for obesity prevention in adult populations: A science advisory from the american heart association. Circulation 2018, 138, e160–e168. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Fruits and Vegetables Messages, Tips, Advice and Tools. Available online: https://www.fns.usda.gov/core-nutrition/fruits-and-vegetables (accessed on 16 February 2021).
- Minich, D.M. A review of the science of colorful, plant-based food and practical strategies for “eating the rainbow”. J. Nutr. Metab. 2019, 2019, 2125070. [Google Scholar] [CrossRef]
- Marcoe, K.; Juan, W.; Yamini, S.; Carlson, A.; Britten, P. Development of food group composites and nutrient profiles for the mypyramid food guidance system. J. Nutr. Educ. Behav. 2006, 38, S93–S107. [Google Scholar] [CrossRef]
- Thompson, H.J. Chapter 2-Vegetable and fruit intake and the development of cancer: A brief review and analysis. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 19–36. [Google Scholar] [CrossRef]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1205–1216. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- The Metabolomics Innovation Centre. HMDB: Showing Metabocard for Glucoraphanin (HMDB0038404). Available online: https://hmdb.ca/metabolites/HMDB0038404 (accessed on 4 January 2021).
- Klurfeld, D.M.; Davis, C.D.; Karp, R.W.; Allen-Vercoe, E.; Chang, E.B.; Chassaing, B.; Fahey, G.C., Jr.; Hamaker, B.R.; Holscher, H.D.; Lampe, J.W.; et al. Considerations for best practices in studies of fiber or other dietary components and the intestinal microbiome. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1087–E1097. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision nutrition and the microbiome, Part I: Current state of the science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.H. Corporate growth and diversification. J. Law Econ. 1971, 14. [Google Scholar] [CrossRef]
- Bowman, S.A.; Clemens, J.C.; Friday, J.E.; Moshfegh, A.J.; Food Surveys Research Group; Beltsville Human Nutrition Research Center; Agricultural Research Service; U.S. Department of Agriculture. Food Patterns Equivalents Database 2017–2018: Methodology and User Guide. 2020. Available online: http://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 30 March 2021).
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The national cancer institute’s dietary assessment primer: A resource for diet research. J. Acad. Nutr. Diet 2015, 115, 1986–1995. [Google Scholar] [CrossRef]
- Thompson, H.J.; Heimendinger, J.; Diker, A.; O’Neill, C.; Haegele, A.; Meinecke, B.; Wolfe, P.; Sedlacek, S.; Zhu, Z.; Jiang, W. Dietary botanical diversity affects the reduction of oxidative biomarkers in women due to high vegetable and fruit intake. J. Nutr. 2006, 136, 2207–2212. [Google Scholar] [CrossRef]
- National Cancer Institute. Dietary Questionnaire (DQX) Datasets. National Cancer Institute Cancer Data Access System Website. Available online: https://cdas.cancer.gov/datasets/plco/97/ (accessed on 29 March 2021).
- Zinocker, M.K.; Lindseth, I.A. The western diet-microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Human Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; Le Roy, C.I. The complexities of the diet-microbiome relationship: Advances and perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the human gut microbiome: An international review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Vipperla, K.; O’Keefe, S.J. Diet, microbiota, and dysbiosis: A ‘recipe’ for colorectal cancer. Food Funct. 2016, 7, 1731–1740. [Google Scholar] [CrossRef]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef]
- Maskarinec, G.; Hullar, M.A.J.; Monroe, K.R.; Shepherd, J.A.; Hunt, J.; Randolph, T.W.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; Lim, U.; et al. Fecal microbial diversity and structure are associated with diet quality in the multiethnic cohort adiposity phenotype study. J. Nutr. 2019, 149, 1575–1584. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Personalized Microbiome Class Students; et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802.e785. [Google Scholar] [CrossRef]
- Laitinen, K.; Mokkala, K. Overall dietary quality relates to gut microbiota diversity and abundance. Int. J. Mol. Sci. 2019, 20, 1835. [Google Scholar] [CrossRef]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Djoumbou-Feunang, Y.; Dorrestein, P.C.; Dragsted, L.O.; Draper, J.; et al. Perspective: Dietary biomarkers of intake and exposure-exploration with omics approaches. Adv. Nutr. 2020, 11, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-modulated metabolites at the interface of host immunity. J. Immunol. 2017, 198, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Hardy, T.; Stewart, C.; Errington, L.; Day, C.P.; Trenell, M.I.; Avery, L. Systematic review assessing the effectiveness of dietary intervention on gut microbiota in adults with type 2 diabetes. Diabetologia 2018, 61, 1700–1711. [Google Scholar] [CrossRef]
- Cresci, G.A.M.; Lampe, J.W.; Gibson, G. Targeted approaches for in situ gut microbiome manipulation. J. Parenter. Enteral Nutr. 2020. [Google Scholar] [CrossRef]
| Chemical Classes | Examples of Bioactive Compounds |
|---|---|
| Alkaloids | 7-Acetylintermedine, 7-Acetyllycopsamine, Anabasine, Anatabine, Atropine, Berberine, Brucine, Caffeine, Capsaicin, Catuabine, Codeine, Coniine, Cytisine, Ecgonine, Emetine, Ephedrine, Ergine, Hydrastine, Hygrine, Morphine, Narceine, Narcotine, Nicotine, Nornicotine, Papaverine, Pelletierine, Pilocarpine, Piperine, Quinine, Sanguinarine, Scopolamine, Seratonin, Sparteine, Strychnine, Symphytine, Thebaine, Theobromine, Trigonelline, Vinblastine, Vincristine |
| Amines | Piperazine, Piperidine, Pyrrolidine (Tetrahdyropyrrole) |
| Cyanogenic glycosides | Dhurrin, Laetrile (Amygdalin), Linamarin, Lotaustralin, Prunasin, Sambunigrin, Taxiphyllin, Vicianin |
| Diterpenes | Dihydrogrindelaldehyde, Dihydrogrindelic Acid, Erythrofordin, Hedychilactone, Hedychinone, labd-13E-en-15-oate, Norerythrofordin, Phytol, Retinoids, Retinol, Taxol |
| Flavonoids | Apigenin, Baicalein, Biochanin A, Catechin, Coumestrol, Cyanidin, Daidzein, Deguelin, Delphinidin, Epicatechin, Epicatechin, Epigallocatechin, Eriodictyol, Fisetin, Galangin, gallate, gallate, Gallocatechin, Genistein, Glycitein, Hesperidin, Isorhamnetin, Kaempferol, Luteolin, Malvidin, Myricetin, Naringenin, Naringin, Pachypodol, Pelargonidin, Peonidin, Petunidin, Quercetin, Rhamnazin, Rotenone, Rutin, Silymarin, Tangeritin, Wogonin |
| Glucosinolates | Glucoberteroin, Glucobrassicanapin, Glucobrassicin, Glucocheirolin, Glucoerucin, Glucoiberin, Gluconapin, Gluconapoleiferin, Gluconasturtiin, Progoitrin, Sinigrin |
| Monoterpenes | Borneol, Camphor, Carene, Carveol, Carvone, Citral, Citronellal, Citronellol, Eucalyptol, Eucalyptol, Geraniol, Limonene, Linalool, Myrcene, α-Pinene, β-Pinene, Terpineol |
| Non-protein amino acids | Alliin, Butiin, Canavanine, S-Allyl Cysteine, Djenkolic Acid, Ethionine, Etiin, Isoalliin, Methiin, Propiin |
| Phenylpropanes | Caffeic Acid, Piceatannol, Pterostilbene, Resveratrol, Rosavins, Sesamol, Theaflavin, Thearubigin |
| Polyacetylenes | Capillin, Dihydropanaxacol, Falcarindiol, Falcarinone, Panaxacol, Panaxydol, Panaxynol (Falcarinol), Panaxytriol |
| Polyketides | Acetogenins (Annonacin Uvaricin), Aflatoxin, Aloenin, Aloesin, Amphotericin, Anthraquinones, Azithromycin, Barbaloin, Bullatacin, Clarithromycin, Discodermolide, Erythromycin A, Pikromycin, Tetracyclines |
| Sesquiterpenes | Artemisinin, Bisabolol, Cadinene, Caryophyllene, Copaene, Farnesene, Farnesol, Guaiazulene, Lactucin, Longifolene, Parthenolide, Vetivazulene |
| Tetraterpenes | Annatto, α-Carotene, β-Carotene, and β-Cryptoxanthin, Crocetin, Crocin, Cryptoxanthine, Lutein, Lycopene, Phytoene, Phytofluene, Sporopollenin, Zeaxanthine |
| Triterpenes, saponins, steroids | Betulinic Acid, Ginsenosides, Glabrolide, Glycyrrhizin, Lanosterol, Lantadene, Lantanolic Acid, Lantic Acid, Licorice Acid, Liquiritic Acid, Lupeol, Oleanolic Acid, β-Sitosterol, Squalene, SU1, Ursolic Acid |
| Botanical Family | Example Foods | Mean ± SD Servings */day |
|---|---|---|
| Poaceae (Gramineae) | Cereals/grains, corn, rice | 4.29 ± 2.26 |
| Rubiaceae | Coffee | 3.15 ± 4.13 |
| Theaceae | Tea | 1.29 ± 2.79 |
| Solanaceae | Potatoes, tomatoes, peppers | 2.32 ± 3.5 |
| Rosaceae | Other fruits (apples, pears, apricots, strawberries) | 1.74 ± 1.56 |
| Fabaceae (Leguminosae) | Dried beans and peas, peanuts | 1.52 ± 2.00 |
| Rutaceae | Citrus fruits | 1.36 ± 1.46 |
| Vitaceae | Grapes, raisins | 0.78 ± 1.56 |
| Cucurbitaceae | Cantaloupe, cucumber, squash, watermelon | 0.76 ± 0.60 |
| Musaceae | Banana | 0.58 ± 0.48 |
| Brassicaceae (Cruciferae) | Broccoli, Brussels sprouts, cabbage, kale | 0.56 ± 0.44 |
| Asteraceae (Compositae) | Lettuce | 0.56 ± 0.42 |
| Apiaceae (Umbelliferae) | Celery, carrots, cauliflower | 0.32 ± 0.26 |
| Amaranthaceae (Chenopodiaceae) | Spinach, Swiss chard, beet greens | 0.20 ± 0.30 |
| Amaryllidaceae (Alliaceae, Liliaceae) | Garlic, onion | 0.14 ± 0.12 |
| Convolvulaceae | Sweet potato | 0.06 ± 0.12 |
| Bromeliaceae | Pineapple | 0.04 ± 0.08 |
| N | Mean ± SD | ANOVA p-Value | |
|---|---|---|---|
| Daily energy intake (kcal) | |||
| <1500 | 119 | 0.76 ± 0.10 | 0.80 |
| 1500–<2000 | 119 | 0.75 ± 0.08 | |
| 2000–<2500 | 66 | 0.76 ± 0.09 | |
| ≥2500 | 50 | 0.75 ± 0.09 | |
| Hours spent in vigorous physical activity per week | |||
| None | 60 | 0.72 ± 0.09 | 0.002 |
| <1 | 64 | 0.75 ± 0.08 | |
| 1 | 45 | 0.76 ± 0.09 | |
| 2 | 55 | 0.76 ± 0.09 | |
| 3 | 52 | 0.78 ± 0.06 | |
| 4+ | 75 | 0.76 ± 0.09 | |
| Body mass index (kg/m2) | |||
| <25 | 88 | 0.76 ± 0.09 | 0.44 |
| 25–<30 | 121 | 0.76 ± 0.09 | |
| 30+ | 145 | 0.75 ± 0.08 | |
| Age (years) | |||
| <55 | 34 | 0.77 ± 0.09 | 0.90 |
| 55–<60 | 83 | 0.76 ± 0.09 | |
| 60–<65 | 114 | 0.75 ± 0.09 | |
| 65–<70 | 92 | 0.76 ± 0.08 | |
| 70+ | 31 | 0.75 ± 0.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, H.J.; Levitt, J.O.; McGinley, J.N.; Chandler, P.; Guenther, P.M.; Huybrechts, I.; Playdon, M.C. Measuring Dietary Botanical Diversity as a Proxy for Phytochemical Exposure. Nutrients 2021, 13, 1295. https://doi.org/10.3390/nu13041295
Thompson HJ, Levitt JO, McGinley JN, Chandler P, Guenther PM, Huybrechts I, Playdon MC. Measuring Dietary Botanical Diversity as a Proxy for Phytochemical Exposure. Nutrients. 2021; 13(4):1295. https://doi.org/10.3390/nu13041295
Chicago/Turabian StyleThompson, Henry J., Jack O. Levitt, John N. McGinley, Paulette Chandler, Patricia M. Guenther, Inge Huybrechts, and Mary C. Playdon. 2021. "Measuring Dietary Botanical Diversity as a Proxy for Phytochemical Exposure" Nutrients 13, no. 4: 1295. https://doi.org/10.3390/nu13041295
APA StyleThompson, H. J., Levitt, J. O., McGinley, J. N., Chandler, P., Guenther, P. M., Huybrechts, I., & Playdon, M. C. (2021). Measuring Dietary Botanical Diversity as a Proxy for Phytochemical Exposure. Nutrients, 13(4), 1295. https://doi.org/10.3390/nu13041295








