Significant Impact of Coffee Consumption on MR-Based Measures of Cardiac Function in a Population-Based Cohort Study without Manifest Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Assessment
2.3. Assessment of Population Characteristics
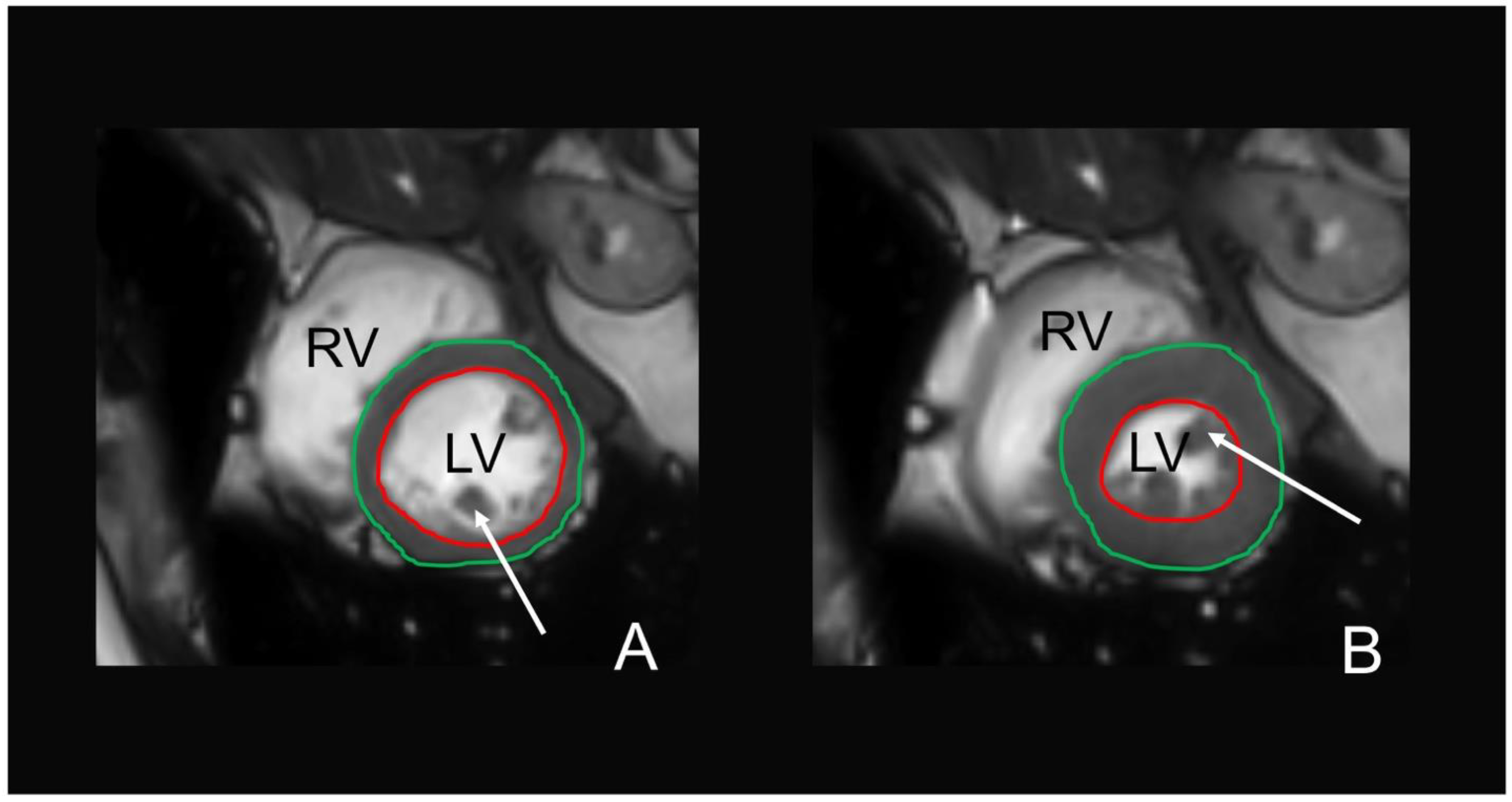
2.4. MR Image Acquisition
2.5. MR Image Analysis
2.6. MRI-Based Intracranial Variables
2.7. MRI-Based Fat Depots
2.8. MRI-Based Cardiac Function
2.9. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Associations between Coffee Consumption and Intracranial MRI Findings
3.3. Associations between Coffee Consumption and MRI-Based Fat Depots
3.4. Associations between Coffee Intake and Cardiac MRI Parameters
3.5. Additional Analysis of VAT and Cardiac Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruusunen, A.; Lehto, S.M.; Tolmunen, T.; Mursu, J.; Kaplan, G.A.; Voutilainen, S. Coffee, tea and caffeine intake and the risk of severe depression in middle-aged Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Public Health Nutr. 2010, 13, 1215–1220. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kuriyama, S.; Akhter, M.; Kakizaki, M.; Nakaya, N.; Ohmori-Matsuda, K.; Shimazu, T.; Nagai, M.; Sugawara, Y.; Hozawa, A.; et al. Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in Japanese women. J. Nutr. 2010, 140, 1007–1013. [Google Scholar] [CrossRef]
- Choi, H.K.; Curhan, G. Coffee consumption and risk of incident gout in women: The Nurses’ Health Study. Am. J. Clin. Nutr. 2010, 92, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Cornelis, M.C. The Impact of Caffeine and Coffee on Human Health. Nutrients 2019, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Prediger, R.D. Effects of caffeine in Parkinson’s disease: From neuroprotection to the management of motor and non-motor symptoms. J. Alzheimer’s Dis. 2010, 20 (Suppl. S1), S205–S220. [Google Scholar] [CrossRef]
- Park, S.Y.; Freedman, N.D.; Haiman, C.A.; Le Marchand, L.; Wilkens, L.R.; Setiawan, V.W. Association of Coffee Consumption With Total and Cause-Specific Mortality Among Nonwhite Populations. Ann. Intern. Med. 2017, 167, 228–235. [Google Scholar] [CrossRef]
- Wang, T.; Huang, T.; Kang, J.H.; Zheng, Y.; Jensen, M.K.; Wiggs, J.L.; Pasquale, L.R.; Fuchs, C.S.; Campos, H.; Rimm, E.B.; et al. Habitual coffee consumption and genetic predisposition to obesity: Gene-diet interaction analyses in three US prospective studies. BMC Med. 2017, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Van Dieren, S.; Uiterwaal, C.S.; van der Schouw, Y.T.; van der, A.D.; Boer, J.M.; Spijkerman, A.; Grobbee, D.E.; Beulens, J.W. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia 2009, 52, 2561–2569. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Dorigatti, F.; Santonastaso, M.; Cozzio, S.; Biasion, T.; Garavelli, G.; Pessina, A.C.; Mos, L. Association between coffee consumption and risk of hypertension. Ann. Med. 2007, 39, 545–553. [Google Scholar] [CrossRef]
- Noordzij, M.; Uiterwaal, C.S.; Arends, L.R.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Blood pressure response to chronic intake of coffee and caffeine: A meta-analysis of randomized controlled trials. J. Hypertens. 2005, 23, 921–928. [Google Scholar] [CrossRef]
- Steffen, M.; Kuhle, C.; Hensrud, D.; Erwin, P.J.; Murad, M.H. The effect of coffee consumption on blood pressure and the development of hypertension: A systematic review and meta-analysis. J. Hypertens. 2012, 30, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Strandhagen, E.; Thelle, D.S. Filtered coffee raises serum cholesterol: Results from a controlled study. Eur. J. Clin. Nutr. 2003, 57, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ma, D.; Zhang, Y.; Liu, Z.; Wang, P. The effect of coffee consumption on serum lipids: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2012, 66, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, P.; Pasman, W.J.; Van Vliet, T.; Urgert, R.; Katan, M.B. Contribution of caffeine to the homocysteine-raising effect of coffee: A randomized controlled trial in humans. Am. J. Clin. Nutr. 2002, 76, 1244–1248. [Google Scholar] [CrossRef][Green Version]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef]
- Kleemola, P.; Jousilahti, P.; Pietinen, P.; Vartiainen, E.; Tuomilehto, J. Coffee consumption and the risk of coronary heart disease and death. Arch. Intern. Med. 2000, 160, 3393–3400. [Google Scholar] [CrossRef]
- Kokubo, Y.; Iso, H.; Saito, I.; Yamagishi, K.; Yatsuya, H.; Ishihara, J.; Inoue, M.; Tsugane, S. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: The Japan public health center-based study cohort. Stroke 2013, 44, 1369–1374. [Google Scholar] [CrossRef]
- Simon, T.G.; Trejo, M.E.P.; Zeb, I.; Frazier-Wood, A.C.; McClelland, R.L.; Chung, R.T.; Budoff, M.J. Coffee consumption is not associated with prevalent subclinical cardiovascular disease (CVD) or the risk of CVD events, in nonalcoholic fatty liver disease: Results from the multi-ethnic study of atherosclerosis. Metabolism 2017, 75, 1–5. [Google Scholar] [CrossRef]
- Van Dongen, L.H.; Molenberg, F.J.; Soedamah-Muthu, S.S.; Kromhout, D.; Geleijnse, J.M. Coffee consumption after myocardial infarction and risk of cardiovascular mortality: A prospective analysis in the Alpha Omega Cohort. Am. J. Clin. Nutr. 2017, 106, 1113–1120. [Google Scholar] [CrossRef]
- Gunter, M.J.; Murphy, N.; Cross, A.J.; Dossus, L.; Dartois, L.; Fagherazzi, G.; Kaaks, R.; Kuhn, T.; Boeing, H.; Aleksandrova, K.; et al. Coffee Drinking and Mortality in 10 European Countries: A Multinational Cohort Study. Ann. Intern. Med. 2017, 167, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Akoudad, S.; Portegies, M.L.; Koudstaal, P.J.; Hofman, A.; van der Lugt, A.; Ikram, M.A.; Vernooij, M.W. Cerebral Microbleeds Are Associated With an Increased Risk of Stroke: The Rotterdam Study. Circulation 2015, 132, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Zhao, X.; Grove, M.L.; Bressler, J.; Gottesman, R.F.; Guan, W.; Pankow, J.S.; Boerwinkle, E.; Mosley, T.H.; Fornage, M. Cerebral white matter hyperintensities on MRI and acceleration of epigenetic aging: The atherosclerosis risk in communities study. Clin. Epigenet. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hedderich, D.M.; Dieckmeyer, M.; Andrisan, T.; Ortner, M.; Grundl, L.; Schön, S.; Suppa, P.; Finck, T.; Kreiser, K.; Zimmer, C.; et al. Normative brain volume reports may improve differential diagnosis of dementing neurodegenerative diseases in clinical practice. Eur. Radiol. 2020, 30, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T.; et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects With Prediabetes, Subjects With Diabetes, and Normal Control Subjects From the General Population: The KORA-MRI Study. Diabetes 2017, 66, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Freese, J.; Feller, S.; Harttig, U.; Kleiser, C.; Linseisen, J.; Fischer, B.; Leitzmann, M.F.; Six-Merker, J.; Michels, K.B.; Nimptsch, K.; et al. Development and evaluation of a short 24-h food list as part of a blended dietary assessment strategy in large-scale cohort studies. Eur. J. Clin. Nutr. 2014, 68, 324–329. [Google Scholar] [CrossRef]
- Illner, A.K.; Harttig, U.; Tognon, G.; Palli, D.; Salvini, S.; Bower, E.; Amiano, P.; Kassik, T.; Metspalu, A.; Engeset, D.; et al. Feasibility of innovative dietary assessment in epidemiological studies using the approach of combining different assessment instruments. Public Health Nutr. 2011, 14, 1055–1063. [Google Scholar] [CrossRef]
- Mitry, P.; Wawro, N.; Six-Merker, J.; Zoller, D.; Jourdan, C.; Meisinger, C.; Thierry, S.; Nothlings, U.; Knuppel, S.; Boeing, H.; et al. Usual Dietary Intake Estimation Based on a Combination of Repeated 24-H Food Lists and a Food Frequency Questionnaire in the KORA FF4 Cross-Sectional Study. Front. Nutr. 2019, 6, 145. [Google Scholar] [CrossRef]
- Holle, R.; Happich, M.; Lowel, H.; Wichmann, H.E.; Group, M.K.S. KORA—A research platform for population based health research. Gesundheitswesen 2005, 67 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Ruf, E.; Baumert, J.; Meisinger, C.; Doring, A.; Ladwig, K.H.; MONICA/KORA Investigators. Are psychosocial stressors associated with the relationship of alcohol consumption and all-cause mortality? BMC Public Health 2014, 14, 312. [Google Scholar] [CrossRef]
- Wawro, N.; Pestoni, G.; Riedl, A.; Breuninger, T.A.; Peters, A.; Rathmann, W.; Koenig, W.; Huth, C.; Meisinger, C.; Rohrmann, S.; et al. Association of Dietary Patterns and Type-2 Diabetes Mellitus in Metabolically Homogeneous Subgroups in the KORA FF4 Study. Nutrients 2020, 12, 1684. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Beller, E.; Keeser, D.; Wehn, A.; Malchow, B.; Karali, T.; Schmitt, A.; Papazova, I.; Papazov, B.; Schoeppe, F.; de Figueiredo, G.N.; et al. T1-MPRAGE and T2-FLAIR segmentation of cortical and subcortical brain regions-an MRI evaluation study. Neuroradiology 2018, 61, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.I.; Brezova, V.; Eikenes, L.; Haberg, A.; Vangberg, T.R. How Does the Accuracy of Intracranial Volume Measurements Affect Normalized Brain Volumes? Sample Size Estimates Based on 966 Subjects from the HUNT MRI Cohort. AJNR Am. J. Neuroradiol. 2015, 36, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oglio, E.; Ceccarelli, A.; Glanz, B.I.; Healy, B.C.; Tauhid, S.; Arora, A.; Saravanan, N.; Bruha, M.J.; Vartanian, A.V.; Dupuy, S.L.; et al. Quantification of global cerebral atrophy in multiple sclerosis from 3T MRI using SPM: The role of misclassification errors. J. Neuroimaging 2015, 25, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, L.; Barkhof, F.; Fazekas, F.; Bronge, L.; Augustin, M.; Sjögren, M.; Wallin, A.; Ader, H.; Leys, D.; Pantoni, L. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001, 32, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Wurslin, C.; Machann, J.; Rempp, H.; Claussen, C.; Yang, B.; Schick, F. Topography mapping of whole body adipose tissue using A fully automated and standardized procedure. J. Magn. Reson. Imaging 2010, 31, 430–439. [Google Scholar] [CrossRef]
- Beller, E.; Lorbeer, R.; Keeser, D.; Schoeppe, F.; Sellner, S.; Hetterich, H.; Bamberg, F.; Schlett, C.L.; Peters, A.; Ertl-Wagner, B.; et al. Hepatic fat is superior to BMI, visceral and pancreatic fat as a potential risk biomarker for neurodegenerative disease. Eur. Radiol. 2019, 29, 6662–6670. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J. Cardiovasc. Magn. Reson. 2013, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Caudron, J.; Fares, J.; Bauer, F.; Dacher, J.N. Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics 2011, 31, 239–259. [Google Scholar] [CrossRef]
- Brown, O.I.; Allgar, V.; Wong, K.Y. Coffee reduces the risk of death after acute myocardial infarction: A meta-analysis. Coron. Artery Dis. 2016, 27, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Gorjanovic, S.; Komes, D.; Lalicic-Petronijevic, J.; Pastor, F.T.; Belscak-Cvitanovic, A.; Veljovic, M.; Pezo, L.; Suznjevic, D.Z. Antioxidant efficiency of polyphenols from coffee and coffee substitutes-electrochemical versus spectrophotometric approach. J. Food Sci. Technol. 2017, 54, 2324–2331. [Google Scholar] [CrossRef]
- Araujo, L.F.; Mirza, S.S.; Bos, D.; Niessen, W.J.; Barreto, S.M.; van der Lugt, A.; Vernooij, M.W.; Hofman, A.; Tiemeier, H.; Ikram, M.A. Association of Coffee Consumption with MRI Markers and Cognitive Function: A Population-Based Study. J. Alzheimer’s Dis. 2016, 53, 451–461. [Google Scholar] [CrossRef]
- Shinoda, M.; Fujii, M.; Takahashi, O.; Kawatsu, A.; Uemura, A.; Niimi, Y. Inverse Relationship between Coffee Consumption and Cerebral Microbleeds in Men, but Not Women. J. Stroke Cerebrovasc. Dis. 2015, 24, 2196–2199. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Buechler, C.; Wanninger, J.; Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 2011, 17, 2801–2811. [Google Scholar]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism 2015, 64, 60–78. [Google Scholar] [CrossRef]
- Ruttgers, D.; Fischer, K.; Koch, M.; Lieb, W.; Muller, H.P.; Jacobs, G.; Kassubek, J.; Nothlings, U. Association of food consumption with total volumes of visceral and subcutaneous abdominal adipose tissue in a Northern German population. Br. J. Nutr. 2015, 114, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yatsuya, H.; Muramatsu, T.; Toyoshima, H.; Murohara, T.; Tamakoshi, K. Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: A cross-sectional study. Nutr. Diabetes 2012, 2, e33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.T.; Solares, G.J.; Kim, K.; Ding, Z.; Ivy, J.L. Caffeinated nitric oxide-releasing lozenge improves cycling time trial performance. Int. J. Sports Med. 2015, 36, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Vongpatanasin, W.; Ayers, C.; Turer, A.; Chandra, A.; Carnethon, M.R.; Greenland, P.; de Lemos, J.A.; Neeland, I.J. Regional Fat Distribution and Blood Pressure Level and Variability: The Dallas Heart Study. Hypertension 2016, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Puccio, E.M.; McPhillips, J.B.; Barrett-Connor, E.; Ganiats, T.G. Clustering of atherogenic behaviors in coffee drinkers. Am. J. Public Health 1990, 80, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Torres-Collado, L.; Garcia-de la Hera, M.; Navarrete-Munoz, E.M.; Compan-Gabucio, L.M.; Gonzalez-Palacios, S.; Vioque, J. Coffee Drinking and Associated Factors in an Elderly Population in Spain. Int. J. Environ. Res. Public Health 2018, 15, 1661. [Google Scholar] [CrossRef]
- Caldeira, D.; Martins, C.; Alves, L.B.; Pereira, H.; Ferreira, J.J.; Costa, J. Caffeine does not increase the risk of atrial fibrillation: A systematic review and meta-analysis of observational studies. Heart 2013, 99, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Artalejo, F.; Lopez-Garcia, E. Coffee Consumption and Cardiovascular Disease: A Condensed Review of Epidemiological Evidence and Mechanisms. J. Agric. Food Chem. 2018, 66, 5257–5263. [Google Scholar] [CrossRef]
- Leopold, J.A. Antioxidants and coronary artery disease: From pathophysiology to preventive therapy. Coron. Artery Dis. 2015, 26, 176–183. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef]
- Nwabuo, C.C.; Betoko, A.S.; Reis, J.P.; Moreira, H.T.; Vasconcellos, H.D.; Guallar, E.; Cox, C.; Sidney, S.; Ambale-Venkatesh, B.; Lewis, C.E.; et al. Coffee and tea consumption in the early adult lifespan and left ventricular function in middle age: The CARDIA study. ESC Heart Fail. 2020, 7, 1510–1519. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010. [Google Scholar] [CrossRef]
- Giacomin, E.; Palmerini, E.; Ballo, P.; Zaca, V.; Bova, G.; Mondillo, S. Acute effects of caffeine and cigarette smoking on ventricular long-axis function in healthy subjects. Cardiovasc. Ultrasound 2008, 6, 9. [Google Scholar] [CrossRef]
- Pincomb, G.A.; Lovallo, W.R.; Passey, R.B.; Whitsett, T.L.; Silverstein, S.M.; Wilson, M.F. Effects of caffeine on vascular resistance, cardiac output and myocardial contractility in young men. Am. J. Cardiol. 1985, 56, 119–122. [Google Scholar] [CrossRef]
- Farag, N.H.; Vincent, A.S.; McKey, B.S.; Whitsett, T.L.; Lovallo, W.R. Hemodynamic mechanisms underlying the incomplete tolerance to caffeine’s pressor effects. Am. J. Cardiol. 2005, 95, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Miller, M.G.; Chu, Y.F.; Lyle, B.J.; Joseph, J.A. Coffee, but not caffeine, has positive effects on cognition and psychomotor behavior in aging. Age 2013, 35, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; El-Sohemy, A. Coffee, caffeine, and coronary heart disease. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 745–751. [Google Scholar] [CrossRef]
- Van Dijk, R.; Kuijpers, D.; Kaandorp, T.A.M.; van Dijkman, P.R.M.; Vliegenthart, R.; van der Harst, P.; Oudkerk, M. Effects of caffeine intake prior to stress cardiac magnetic resonance perfusion imaging on regadenoson- versus adenosine-induced hyperemia as measured by T1 mapping. Int. J. Cardiovasc. Imaging 2017, 33, 1753–1759. [Google Scholar] [CrossRef]
- Seitz, A.; Kaesemann, P.; Chatzitofi, M.; Lobig, S.; Tauscher, G.; Bekeredjian, R.; Sechtem, U.; Mahrholdt, H.; Greulich, S. Impact of caffeine on myocardial perfusion reserve assessed by semiquantitative adenosine stress perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2019, 21, 33. [Google Scholar] [CrossRef]
- van Dijk, R.; Ties, D.; Kuijpers, D.; van der Harst, P.; Oudkerk, M. Effects of Caffeine on Myocardial Blood Flow: A Systematic Review. Nutrients 2018, 10, 1083. [Google Scholar] [CrossRef]
- Greulich, S.; Kaesemann, P.; Seitz, A.; Birkmeier, S.; Abu-Zaid, E.; Vecchio, F.; Sechtem, U.; Mahrholdt, H. Effects of caffeine on the detection of ischemia in patients undergoing adenosine stress cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2017, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Doerner, J.M.; Kuetting, D.L.; Luetkens, J.A.; Naehle, C.P.; Dabir, D.; Homsi, R.; Nadal, J.; Schild, H.H.; Thomas, D.K. Caffeine and taurine containing energy drink increases left ventricular contractility in healthy volunteers. Int. J. Cardiovasc. Imaging 2015, 31, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, R.; Hetterich, H.; Strobl, R.; Schafnitzel, A.; Patscheider, H.; Schindler, A.; Muller-Peltzer, K.; Sommer, W.; Peters, A.; Meisinger, C.; et al. Lack of association of MRI determined subclinical cardiovascular disease with dizziness and vertigo in a cross-sectional population-based study. PLoS ONE 2017, 12, e0184858. [Google Scholar] [CrossRef]

| Total n = 300 | Women n = 132 | Men n = 168 | p-Value * | |
|---|---|---|---|---|
| Age (years) | 56.3 ± 9.1 | 56.3 ± 8.8 | 56.2 ± 9.3 | 0.967 |
| Coffee intake (g/day) | 392.5 ± 131.7 | 392.6 ± 118.8 | 392.5 ± 141.4 | 0.994 |
| LDL (mg/dl) | 138.9 ± 33.4 | 135.8 ± 33.1 | 141.4 ± 33.6 | 0.152 |
| Triglycerides (mg/dl) | 126.0 ± 78.7 | 101.6 ± 43.5 | 145.1 ± 93.6 | <0.001 |
| Smoking status | 0.103 | |||
| Never-smoker | 109 (36.3%) | 55 (41.7%) | 54 (32.1%) | |
| Ex-smoker | 134 (44.7%) | 50 (37.9%) | 84 (50.0%) | |
| Current smoker | 57 (19.0%) | 27 (20.5%) | 30 (17.9%) | |
| Alcohol consumption (g/day) | 18.5 ± 24.1 | 7.8 ± 13.7 | 26.9 ± 27.0 | <0.001 |
| Diabetes mellitus | 33 (11%) | 11 (8.3%) | 22 (13.1%) | 0.198 |
| Systolic BP (mmHg) | 119.8 ± 16.4 | 112.8 ± 14.4 | 125.4 ± 15.8 | <0.001 |
| Diastolic BP (mmHg) | 74.8 ± 9.9 | 71.7 ± 8.7 | 77.3 ± 10.1 | <0.001 |
| Coffee Intake (g/day) | Model A | Model B | Model C |
|---|---|---|---|
| Per 1 SD increment | β (95%CI) | β (95%CI) | β (95%CI) |
| Gray matter volume | −0.0006 (−0.0022; 0.001) | −0.0006 (−0.0022; 0.001) | −0.0006 (−0.0022; 0.001) |
| White matter hyperintensities | −0.0012 (−0.0029; 0.0006) | −0.0012 (−0.0029; 0.0006) | −0.0011 (−0.0029; 0.0006) |
| WMH volume | 403 (−237.4; 1043.4) | 448.4 (−205.9; 1102.6) | 381.4 (−275.7; 1038.6) |
| Presence of WMH (yes/no) | OR: 0.97 (0.75; 1.26) | OR: 0.99 (0.75; 1.30) | OR: 0.99 (0.75; 1.31) |
| ARWMC score | IRR: 1.05 (0.92; 1.20) | IRR: 1.07 (0.93; 1.22) | IRR: 1.06 (0.92; 1.22) |
| Cerebral microbleeds | OR: 1.08 (0.76; 1.55) | OR: 1.10 (0.75; 1.60) | OR: 1.10 (0.75;1.61) |
| Coffee Intake (g/day) | Model A | Model B | Model C |
|---|---|---|---|
| Per 1 SD increment | β (95%CI) | β (95%CI) | β (95%CI) |
| TAT | −0.07 (−0.71; 0.56) | 0.09 (−0.48; 0.67) | 0.08 (−0.50; 0.66) |
| VAT | −0.32 (−0.57; −0.06) * | −0.23 (−0.45; −0.01) * | −0.20 (−0.43; 0.02) |
| PDFFhepatic | −0.62 (−1.52; 0.29) | −0.33 (−1.15; 0.48) | −0.26 (−1.09; 0.56) |
| Coffee Intake (g/day) | Model A | Model B | Model C |
|---|---|---|---|
| Per 1 SD increment | β (95%CI) | β (95%CI) | β (95%CI) |
| Early diastolic filling rate (ml/s) | 7.03 (−5.27; 19.32) | 5.61 (−6.51; 17.72) | 4.47 (−7.73; 16.67) |
| Late diastolic filling rate (ml/s) | 22.69 (7.73; 37.65) ** | 18.93 (4; 33.87) * | 18.19 (3.12; 33.27) * |
| End-diastolic volume (ml/m2) | 1.29 (−0.35; 2.94) | 1.17 (−0.43; 2.76) | 1.08 (−0.54; 2.69) |
| End-systolic volume (ml/m2) | −0.26 (−1.16; 0.64) | −0.32 (−1.22; 0.58) | −0.35 (−1.26; 0.56) |
| Stroke volume (ml/m2) | 1.56 (0.48; 2.64) ** | 1.49 (0.45; 2.53) ** | 1.44 (0.39; 2.48) ** |
| Ejection fraction (%) | 0.94 (0.08; 1.80) * | 0.98 (0.10; 1.85) * | 0.97 (0.09; 1.86) * |
| Peak ejection rate (ml/s) | −9.78 (−24.52; 4.96) | −6.97 (−21.58; 7.64) | −5.33 (−20.03; 9.36) |
| Myocardial mass (g/m2) | 0.44 (−0.9; 1.77) | 0.84 (0.47; 1.51) | 0.84 (0.47; 1.52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beller, E.; Lorbeer, R.; Keeser, D.; Galiè, F.; Meinel, F.G.; Grosu, S.; Bamberg, F.; Storz, C.; Schlett, C.L.; Peters, A.; et al. Significant Impact of Coffee Consumption on MR-Based Measures of Cardiac Function in a Population-Based Cohort Study without Manifest Cardiovascular Disease. Nutrients 2021, 13, 1275. https://doi.org/10.3390/nu13041275
Beller E, Lorbeer R, Keeser D, Galiè F, Meinel FG, Grosu S, Bamberg F, Storz C, Schlett CL, Peters A, et al. Significant Impact of Coffee Consumption on MR-Based Measures of Cardiac Function in a Population-Based Cohort Study without Manifest Cardiovascular Disease. Nutrients. 2021; 13(4):1275. https://doi.org/10.3390/nu13041275
Chicago/Turabian StyleBeller, Ebba, Roberto Lorbeer, Daniel Keeser, Franziska Galiè, Felix G. Meinel, Sergio Grosu, Fabian Bamberg, Corinna Storz, Christopher L. Schlett, Annette Peters, and et al. 2021. "Significant Impact of Coffee Consumption on MR-Based Measures of Cardiac Function in a Population-Based Cohort Study without Manifest Cardiovascular Disease" Nutrients 13, no. 4: 1275. https://doi.org/10.3390/nu13041275
APA StyleBeller, E., Lorbeer, R., Keeser, D., Galiè, F., Meinel, F. G., Grosu, S., Bamberg, F., Storz, C., Schlett, C. L., Peters, A., Schneider, A., Linseisen, J., Meisinger, C., Rathmann, W., Ertl-Wagner, B., & Stoecklein, S. (2021). Significant Impact of Coffee Consumption on MR-Based Measures of Cardiac Function in a Population-Based Cohort Study without Manifest Cardiovascular Disease. Nutrients, 13(4), 1275. https://doi.org/10.3390/nu13041275







