Rat Milk and Plasma Immunological Profile throughout Lactation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Sample Collection
2.3. Immunoglobulin Quantification
2.4. Adipokine and Growth Factor Determination
2.5. Statistical Analysis
3. Results
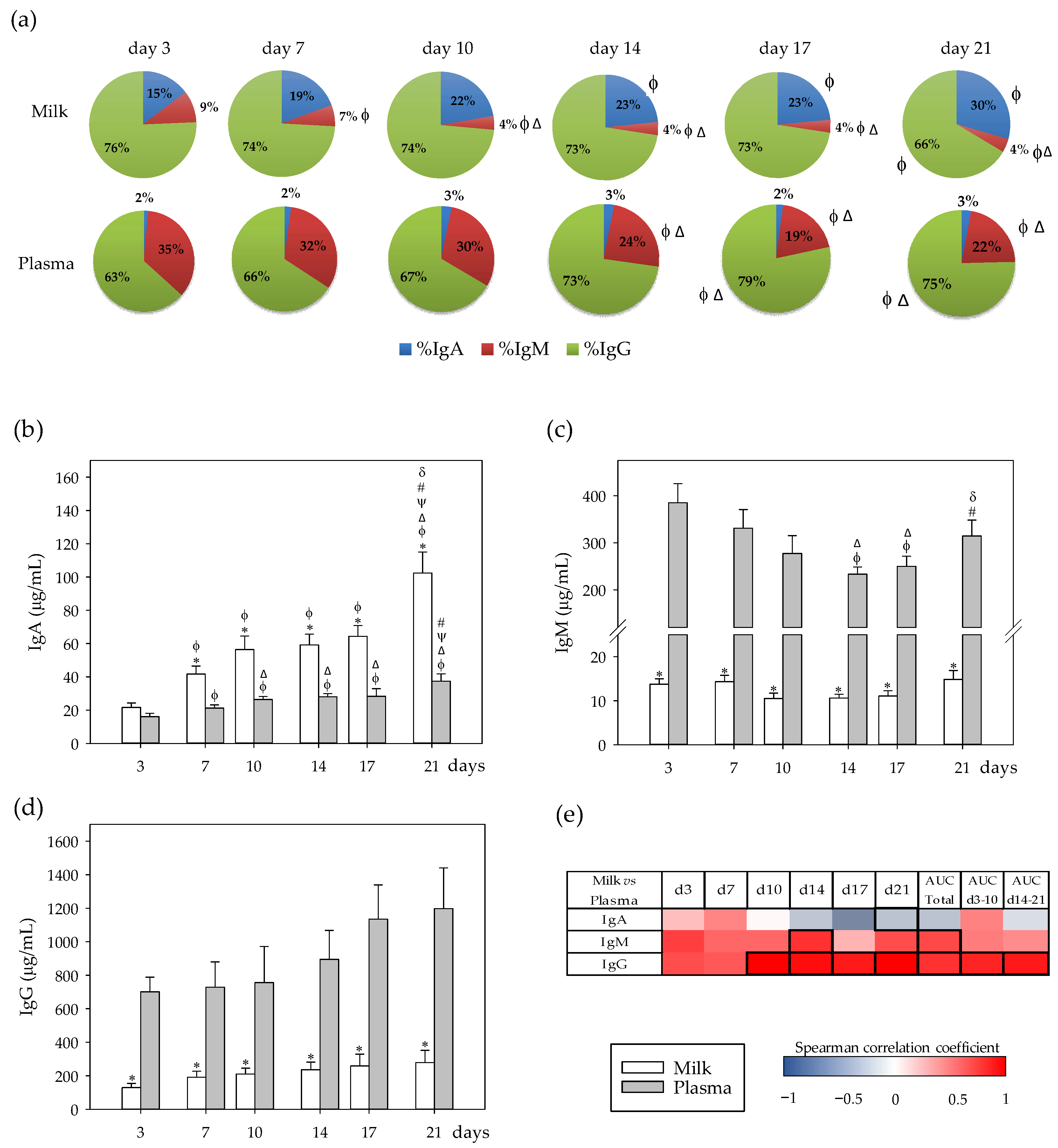
3.1. Immunoglobulin Profiles and Their Correlation between Milk and Plasma
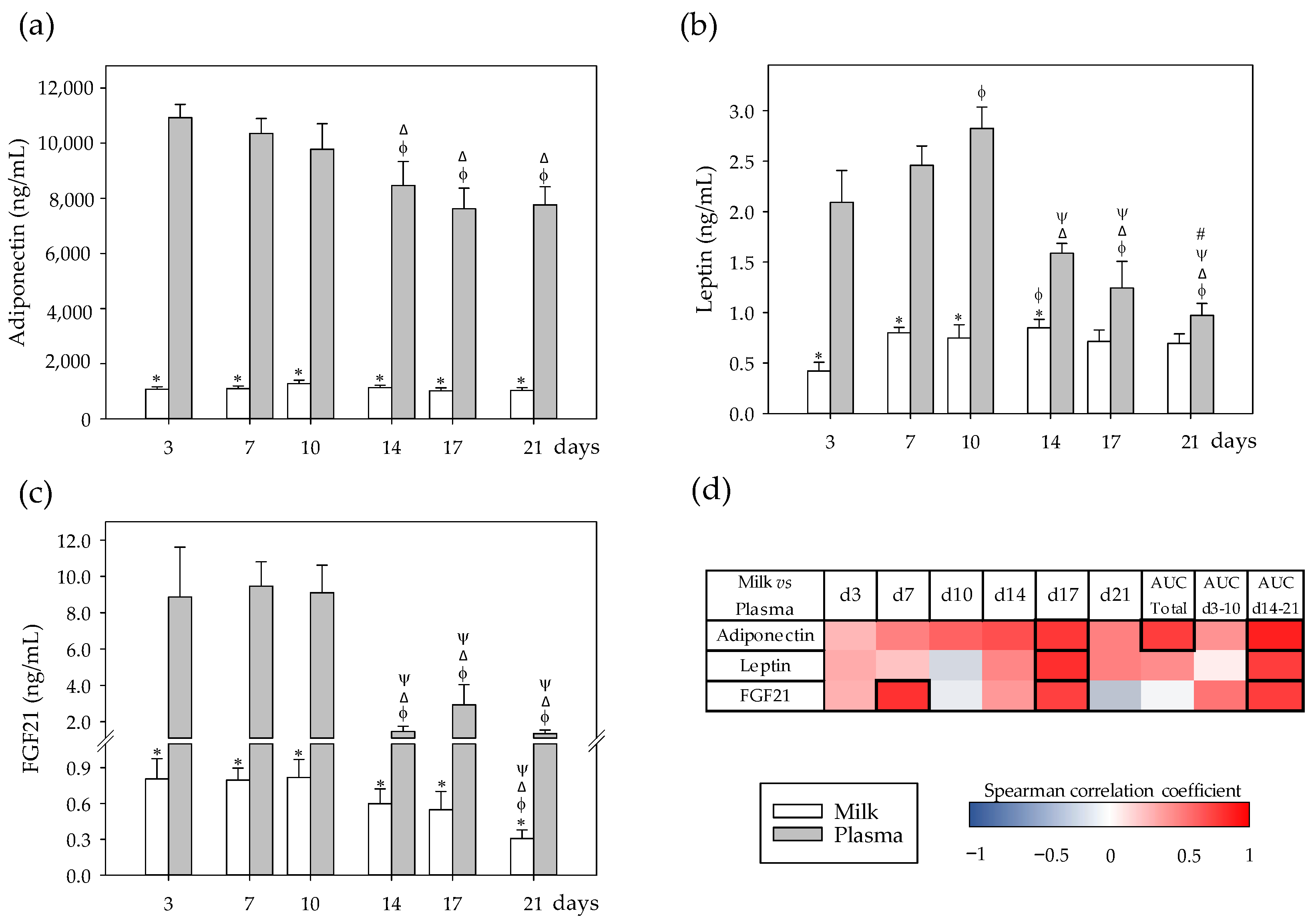
3.2. Adipokine Profiles and Their Correlation between Milk and Plasma
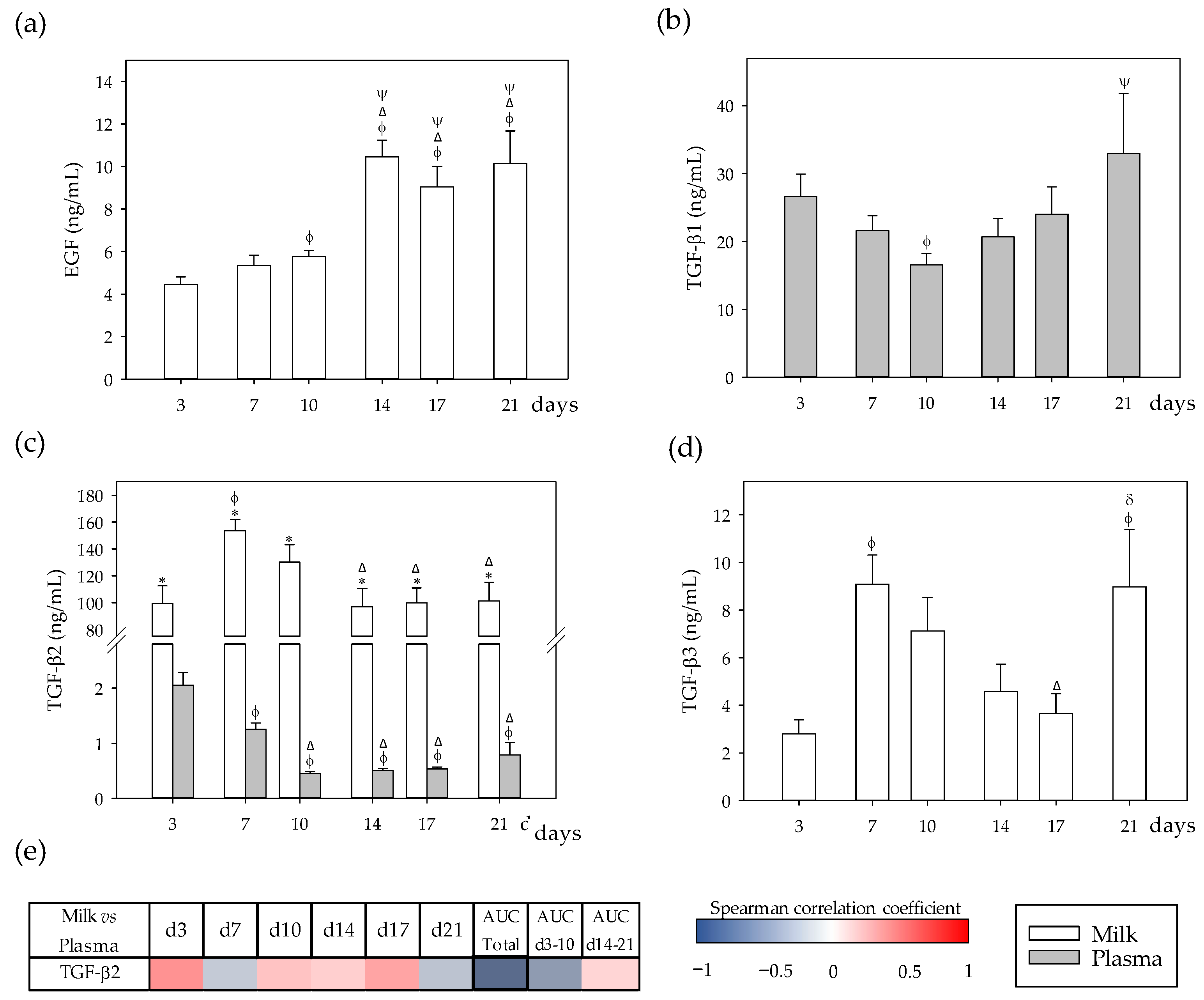
3.3. Growth Factor Profiles and Their Correlation between Milk and Plasma
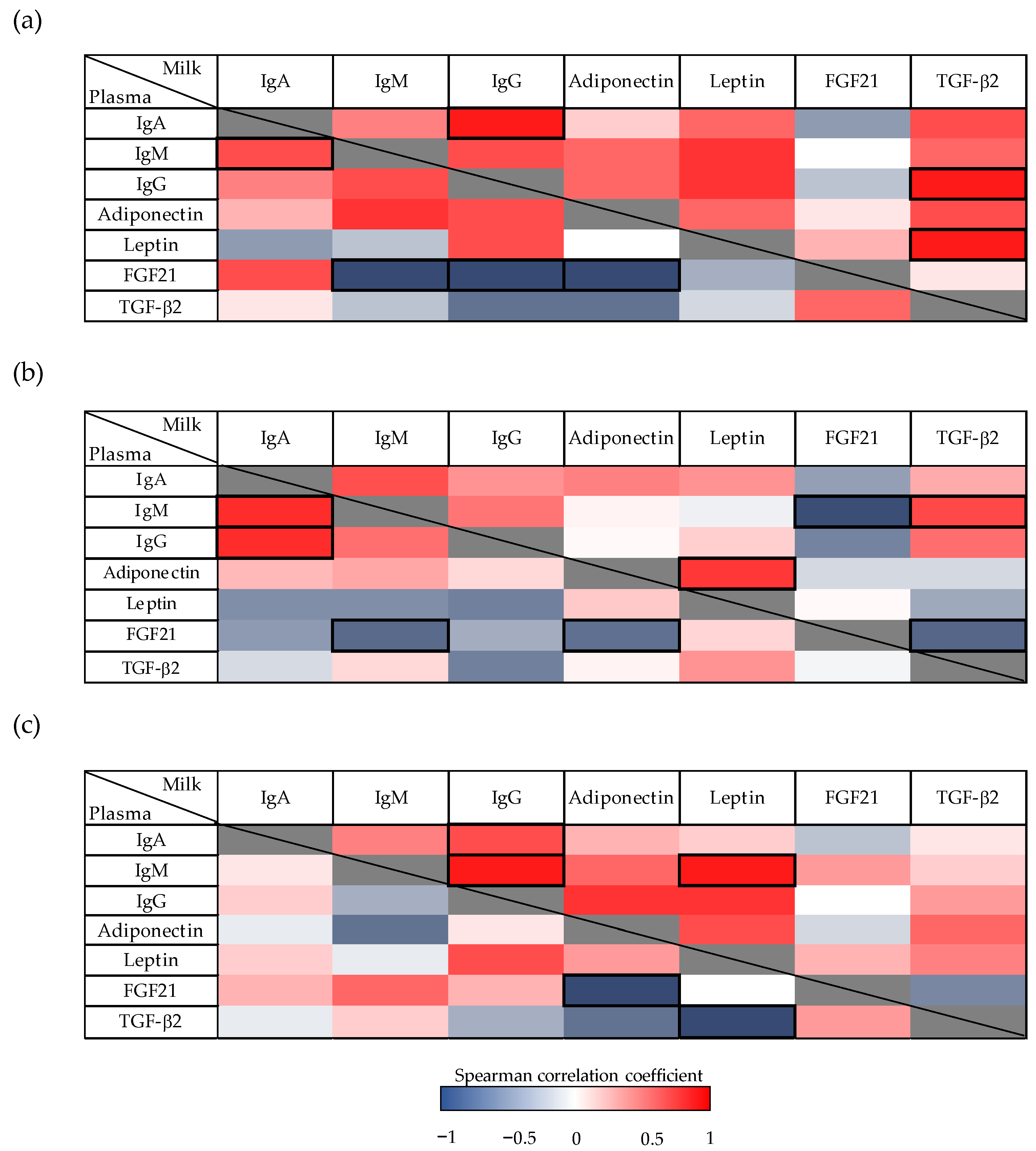
3.4. Correlations between Immunoglobulins and Other Bioactive Factors in Milk and Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, S.A. Vitamin D in preterm and full-term infants. Ann. Nutr. Metab. 2020, 76 (Suppl. 2), 6–14. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Hollis, B.W. Early-life effects of vitamin D: A focus on pregnancy and lactation. Ann. Nutr. Metab. 2020, 76 (Suppl. 2), 16–28. [Google Scholar] [CrossRef]
- Thorisdottir, A.V.; Ramel, A.; Palsson, G.I.; Tomassson, H.; Thorsdottir, I. Iron status of one-year-olds and association with breast milk, cow’s milk or formula in late infancy. Eur. J. Nutr. 2013, 52, 1661–1668. [Google Scholar] [CrossRef]
- Erick, M. Breast milk is conditionally perfect. Med. Hypotheses 2018, 111, 82–89. [Google Scholar] [CrossRef]
- Roed, C.; Skovby, F.; Lund, A.M. Severe vitamin B12 deficiency in infants breastfed by vegans. Ugeskr. Laeger 2009, 171, 3099–3101. [Google Scholar]
- Sebastiani, G.; Barbero, A.H.; Borrás-Novell, C.; Casanova, M.A.; Aldecoa-bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Hondares, E.; Giralt, M.; Mampel, T.; Iglesias, R.; Villarroya, F. Fibroblast growth factor 21 in breast milk controls neonatal intestine function. Sci. Rep. 2015, 5, 13717. [Google Scholar] [CrossRef] [PubMed]
- Çatlı, G.; Dündar, N.O.; Dündar, B.N. Adipokines in breast milk: An update. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Blewett, H.J.H.; Cicalo, M.C.; Holland, C.D.; Field, C.J. The immunological components of human milk. Adv. Food Nutr. Res. 2008, 54, 45–80. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Associations of breast milk microbiota, immune factors, and fatty acids in the rat mother-offspring pair. Nutrients 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation affects breast milk composition. J. Dairy Sci. 2020, 103, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Santana, C.; Pérez-Cano, F.J.; Castellote, C.; Castell, M.; Rivero, M.; Rodríguez-Palmero, M.; Franch, À. Higher immunoglobulin production in conjugated linoleic acid-supplemented rats during gestation and suckling. Br. J. Nutr. 2009, 102, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Nozhenko, Y.; Asnani-Kishnani, M.; Rodríguez, A.M.; Palou, A. Milk leptin surge and biological rhythms of leptin and other regulatory proteins in breastmilk. PLoS ONE 2015, 10, e0145376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Cano, F.J.; Franch, À.; Castellote, C.; Castell, M. The suckling rat as a model for immunonutrition studies in early life. Clin. Dev. Immunol. 2012, 2012, 537310. [Google Scholar] [CrossRef]
- Jakaitis, B.M.; Denning, P.W. Human breast milk and the gastrointestinal innate immune system. Clin. Perinatol. 2014, 41, 423–435. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Hochwallner, H.; Alm, J.; Lupinek, C.; Johansson, C.; Mie, A.; Scheynius, A.; Valenta, R. Transmission of allergen-specific IgG and IgE from maternal blood into breast milk visualized with microarray technology. J. Allergy Clin. Immunol. 2014, 134, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Bioactive proteins in breast milk. J. Paediatr. Child Health 2013, 49 (Suppl. 1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.; He, J.; Diraviyam, T.; Zhang, X. Quantitative investigation on correlation between IgG and FcRn during gestation and lactating periods in rat. Am. J. Reprod. Immunol. 2016, 75, 81–85. [Google Scholar] [CrossRef]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–447. [Google Scholar] [CrossRef]
- Kratzsch, J.; Bae, Y.J.; Kiess, W. Adipokines in human breast milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 27–38. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.F. Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef]
- Song, J.; Deng, T. The adipocyte and adaptive immunity. Front. Immunol. 2020, 11, 593058. [Google Scholar] [CrossRef]
- Żelechowska, P.; Kozłowska, E.; Pastwińska, J.; Agier, J.; Brzezińska-Błaszczyk, E. Adipocytokine involvement in innate immune mechanisms. J. Interferon Cytokine Res. 2018, 38, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; La Rocca, C.; Carbone, F.; De Rosa, V.; Galgani, M.; Matarese, G. Leptin as immune mediator: Interaction between neuroendocrine and immune system. Dev. Comp. Immunol. 2017, 66, 120–129. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Torres-Castro, P.; Grases-Pintó, B.; Abril-Gil, M.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. Modulation of the systemic immune response in suckling rats by breast milk TGF-β2, EGF and FGF21 supplementation. Nutrients 2020, 12, 1888. [Google Scholar] [CrossRef]
- Torres-Castro, P.; Abril-Gil, M.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J.; Franch, À. TGF-β2, EGF, and FGF21 growth factors present in breast milk promote mesenteric lymph node lymphocytes maturation in suckling rats. Nutrients 2018, 10, 1171. [Google Scholar] [CrossRef]
- Carbone, F.; La Rocca, C.; Matarese, G. Immunological functions of leptin and adiponectin. Biochimie 2012, 94, 2082–2088. [Google Scholar] [CrossRef]
- Bielicki, J.; Huch, R.; von Mandach, U. Time-course of leptin levels in term and preterm human milk. Eur. J. Endocrinol. 2004, 151, 271–276. [Google Scholar] [CrossRef]
- Ozarda, Y.; Gunes, Y.; Tuncer, G.O. The concentration of adiponectin in breast milk is related to maternal hormonal and inflammatory status during 6 months of lactation. Clin. Chem. Lab. Med. 2012, 50, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal growth factor and intestinal barrier function. Mediat. Inflamm 2016, 2016, 1927348. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Shelby, R.D.; Cromeens, B.; Rager, T.M.; Besner, G.E. Influence of growth factors on the development of necrotizing enterocolitis. Clin. Perinatol. 2019, 46, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Grases-Pintó, B.; Torres-Castro, P.; Marín-Morote, L.; Abril-Gil, M.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. Leptin and EGF supplementation enhance the immune system maturation in preterm suckling rats. Nutrients 2019, 11, 2380. [Google Scholar] [CrossRef]
- Sitarik, A.R.; Bobbitt, K.R.; Havstad, S.L.; Fujimura, K.E.; Levin, A.M.; Zoratti, E.M.; Kim, H.; Woodcroft, K.J.; Wegienka, G.; Ownby, D.R.; et al. Breast milk transforming growth factor β is associated with neonatal gut microbial composition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e60–e67. [Google Scholar] [CrossRef]
- Banchereau, J.; Pascual, V.; O’Garra, A. From IL-2 to IL-37: The expanding spectrum of antiinflammatory cytokines. Nat. Immunol. 2012, 13, 925–931. [Google Scholar] [CrossRef]
- Ogawa, J.; Sasahara, A.; Yoshida, T.; Sira, M.M.; Futatani, T.; Kanegane, H.; Miyawaki, T. Role of transforming growth factor-β in breast milk for initiation of IgA production in newborn infants. Early Hum. Dev. 2004, 77, 67–75. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the immune response by TGF-β: From conception to autoimmunity and infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M. Gut microbiota in a rat oral sensitization model: Effect of a cocoa-enriched diet. Oxid. Med. Cell. Longev. 2017, 2017, 7417505. [Google Scholar] [CrossRef] [PubMed]
- Grases-Pintó, B.; Abril-Gil, M.; Castell, M.; Pérez-Cano, F.J.; Franch, À. Enhancement of immune maturation in suckling rats by leptin and adiponectin supplementation. Sci. Rep. 2019, 9, 1786. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Sakamoto, K.; Zeng, M.Y.; Wang, Y.; Hakim, J.; Matus-Acuña, V.; Inohara, N.; Núñez, G. Maternal immunization confers protection to the offspring against an attaching and effacing pathogen through delivery of IgG in breast milk. Cell Host Microbe 2019, 25, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Marnila, P.; Gill, H.S. Milk immunoglobulins and complement factors. Br. J. Nutr. 2000, 84 (Suppl. 1), S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Kehrli, M.E., Jr. Immunoglobulins and Immunocytes in the Mammary Gland and its Secretions. In Mucosal Immunology, 3rd ed.; Mestecky, J.F., Beinenstock, J., Lamm, M.E., Mayer, L., McGhee, J.R., Strober, W., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2005; Volume 3, pp. 1763–1793. [Google Scholar]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Woo, J.G.; Morrow, A.L. Characteristics and potential functions of human milk adiponectin. J. Pediatr. 2010, 156 (Suppl. 2), S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Woo, J.G.; Geraghty, S.R.; Altaye, M.; Davidson, B.S.; Banach, W.; Dolan, L.M.; Ruiz-Palacios, G.M.; Morrow, A.L. Adiponectin is present in human milk and is associated with maternal factors. Am. J. Clin. Nutr. 2006, 83, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Bronsky, J.; Mitrova, K.; Karpisek, M.; Mazoch, J.; Durilova, M.; Fisarkova, B.; Stechova, K.; Prusa, R.; Nevoral, J. Adiponectin, AFABP, and leptin in human breast milk during 12 months of lactation. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 474–477. [Google Scholar] [CrossRef]
- Weyermann, M.; Beermann, C.; Brenner, H.; Rothenbacher, D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin. Chem. 2006, 52, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Gnacińska, M.; Małgorzewicz, S.; Stojek, M.; Łysiak-Szydłowska, W.; Sworczak, K. Role of adipokines in complications related to obesity: A review. Adv. Med. Sci. 2009, 54, 150–157. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Flier, J.S. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Invest 1998, 101, 1020–1027. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Mehrad-Majd, H.; Vahid, F.; Safarian, M. Association of maternal breast milk and serum levels of macronutrients, hormones, and maternal body composition with infant’s body weight. Eur. J. Clin. Nutr. 2018, 72, 394–400. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; McGuire, M.K.; Portocarrero, C.P.; McGuire, M.A.; Beerman, K. Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochem. Biophys. Res. Commun. 1997, 240, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Lupica, M.M.; Benetti, S.; Petrucci, E.; Liguori, S.A.; Cordero Di Montezemolo, L. Adiponectin in breast milk: Relation to serum adiponectin concentration in lactating mothers and their infants. Acta Paediatr. 2012, 101, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Smith-Kirwin, S.M.; O’Connor, D.M.; De Johnston, J.; Lancey, E.D.; Hassink, S.G.; Funanage, V.L. Leptin expression in human mammary epithelial cells and breast milk. J. Clin. Endocrinol. Metab. 1998, 83, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, Y.; Yonezawa, T.; Song, S.H.; Takahashi, T.; Ardiyanti, A.; Sato, K.; Hagino, A.; Roh, S.G.; Katoh, K. Gene expression and hormonal regulation of adiponectin and its receptors in bovine mammary gland and mammary epithelial cells. Anim. Sci. J. 2011, 82, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, B.; Fituch, C.C.; Williams, C.S.; Hurst, N.M.; Schanler, R.J. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr. Res. 2003, 54, 15–19. [Google Scholar] [CrossRef]
- Beardmore, J.M.; Richards, R.C. Concentrations of epidermal growth factor in mouse milk throughout lactation. J. Endocrinol. 1983, 96, 287–292. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, Y.; Lönnerdal, B. Milk growth factors and expression of small intestinal growth factor receptors during the perinatal period in mice. Pediatr. Res. 2016, 80, 759–765. [Google Scholar] [CrossRef]
- Schaudies, R.P.; Grimes, J.; Wray, H.L.; Koldovský, O. Identification and partial characterization of multiple forms of biologically active EGF in rat milk. Am. J. Physiol. 1990, 259, G1056–G1061. [Google Scholar] [CrossRef]
- Raaberg, L.; Nexø, E.; Tollund, L.; Poulsen, S.S.; Christensen, S.B.; Christensen, M.S. Epidermal growth factor reactivity in rat milk. Regul. Pept. 1990, 30, 149–157. [Google Scholar] [CrossRef]
- Oddy, W.H.; Rosales, F. A systematic review of the importance of milk TGF-β on immunological outcomes in the infant and young child. Pediatr. Allergy Immunol. 2010, 21, 47–59. [Google Scholar] [CrossRef]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune components in human milk are associated with early infant immunological health outcomes: A prospective three-country analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.L.; Jilling, T.; Lapin, B.; Maheshwari, A.; Caplan, M.S. Maternal breast milk transforming growth factor-beta and feeding intolerance in preterm infants. Pediatr. Res. 2014, 76, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Penttila, I.A.; van Spriel, A.B.; Zhang, M.F.; Xian, C.J.; Steeb, C.B.; Cummins, A.G.; Zola, H.; Read, L.C. Transforming growth factor-beta levels in maternal milk and expression in postnatal rat duodenum and ileum. Pediatr. Res. 1998, 44, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Abrol, P.; Sheth, S.; Pampura, A.; Boner, A.L.; et al. Colostrum and mature human milk of women from London, Moscow, and Verona: Determinants of immune composition. Nutrients 2016, 8, 695. [Google Scholar] [CrossRef]
- Byyny, R.L.; Orth, D.N.; Cohen, S.; Doyne, E.S. Epidermal growth factor: Effects of androgens and adrenergic agents. Endocrinology 1974, 95, 776–782. [Google Scholar] [CrossRef]
- Hawkes, J.S.; Bryan, D.L.; Gibson, R.A. Variations in transforming growth factor beta in human milk are not related to levels in plasma. Cytokine 2002, 17, 182–186. [Google Scholar] [CrossRef]
- Bruder, E.D.; Van Hoof, J.; Young, J.B.; Raff, H. Epidermal growth factor and parathyroid hormone-related peptide mRNA in the mammary gland and their concentrations in milk: Effects of postpartum hypoxia in lactating rats. Horm. Metab. Res. 2008, 40, 446–453. [Google Scholar] [CrossRef]
- Brown, K.D.; Blakeley, D.M.; Fleet, I.R.; Hamon, M.; Heap, R.B. Kinetics of transfer of 125I-labelled epidermal growth factor from blood into mammary secretions of goats. J. Endocrinol. 1986, 109, 325–332. [Google Scholar] [CrossRef]
- Externest, D.; Meckelein, B.; Schmidt, M.A.; Frey, A. Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage. Infect. Immun. 2000, 68, 3830–3839. [Google Scholar] [CrossRef]
- Lebman, D.A.; Edmiston, J.S. The role of TGF-β in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 1999, 1, 1297–1304. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, X.Y.; Wu, X.P.; Fu, X.H.; Du, X.Y.; Xie, H.; Peng, Y.Q.; Luo, X.H.; Liao, E.Y. Relationship between age-related serum concentrations of TGF-β1 and TGF-β2 and those of osteoprotegerin and leptin in native Chinese women. Clin. Chim. Acta 2009, 403, 63–69. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grases-Pintó, B.; Abril-Gil, M.; Torres-Castro, P.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. Rat Milk and Plasma Immunological Profile throughout Lactation. Nutrients 2021, 13, 1257. https://doi.org/10.3390/nu13041257
Grases-Pintó B, Abril-Gil M, Torres-Castro P, Castell M, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Franch À. Rat Milk and Plasma Immunological Profile throughout Lactation. Nutrients. 2021; 13(4):1257. https://doi.org/10.3390/nu13041257
Chicago/Turabian StyleGrases-Pintó, Blanca, Mar Abril-Gil, Paulina Torres-Castro, Margarida Castell, María J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, and Àngels Franch. 2021. "Rat Milk and Plasma Immunological Profile throughout Lactation" Nutrients 13, no. 4: 1257. https://doi.org/10.3390/nu13041257
APA StyleGrases-Pintó, B., Abril-Gil, M., Torres-Castro, P., Castell, M., Rodríguez-Lagunas, M. J., Pérez-Cano, F. J., & Franch, À. (2021). Rat Milk and Plasma Immunological Profile throughout Lactation. Nutrients, 13(4), 1257. https://doi.org/10.3390/nu13041257








