Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Method and Data Collection
2.3. Definitions of Independent Variables
2.4. Definitions of Dependent Variables
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. General Characteristics of the Cohort and Obese Mothers
3.2. Maternal Obesity and Macrosomia Risk
3.3. Maternal Obesity, and Low Birth Weight (LBW) and Fetal Growth Restriction (FGR)
3.4. Effects of Gestational Weight Gain (GWG) on the Newborn’s Weight
4. Discussion
Advantages and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | Appropriate-for-gestational age |
| AOR | Adjusted odds ratio |
| APGAR | Appearance, pulse, grimace, activity, respiration |
| BMI | Body mass index |
| CI | Confidence intervals |
| CRL | Crown-rump length |
| DOHaD | Developmental Origins of Health and Disease |
| FGR | Fetal growth restriction |
| GDM | Gestational diabetes mellitus |
| GWG | Gestational weight gain |
| IL | Interleukin |
| IOM | Institute of Medicine |
| IQR | Interquartile ranges |
| LBW | Low birth weight |
| LGA | Large-for-gestational age |
| MUAC | Mid-upper arm circumference |
| OGTT | Oral glucose tolerance test |
| OR | Odds ratio |
| PE | Preeclampsia |
| PIH | Pregnancy-induced hypertension |
| PlGF | Placental growth factor |
| sFLT | Soluble FMS-like tyrosine kinase |
| SGA | Small-for-gestational age |
| TNF-α | Tumor necrosis factor-alpha |
| WHO | World Health Organization |
References
- Farpour-Lambert, N.J.; Ells, L.J.; Martinez de Tejada, B.; Scott, C. Obesity and Weight Gain in Pregnancy and Postpartum: An Evidence Review of Lifestyle Interventions to Inform Maternal and Child Health Policies. Front. Endocrinol. 2018, 9, 546. [Google Scholar] [CrossRef]
- Altmäe, S.; Segura, M.T.; Esteban, F.J.; Bartel, S.; Brandi, P.; Irmler, M.; Beckers, J.; Demmelmair, H.; López-Sabater, C.; Koletzko, B.; et al. Maternal Pre-Pregnancy Obesity Is Associated with Altered Placental Transcriptome. PLoS ONE 2017, 12, e0169223. [Google Scholar] [CrossRef]
- Howell, K.R.; Powell, T.L. Effects of Maternal Obesity on Placental Function and Fetal Development. Reprod. Camb. Engl. 2017, 153, R97–R108. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. Pre-Pregnancy Obesity vs. Other Risk Factors in Probability Models of Preeclampsia and Gestational Hypertension. Nutrients 2020, 12, 2681. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.S.; Correa, A.; Reece, E.A. Pre-Pregnancy Obesity as a Modifier of Gestational Diabetes and Birth Defects Associations: A Systematic Review. Matern. Child Health J. 2017, 21, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Więckowska, B.; Sajdak, S. Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus. J. Clin. Med. 2020, 9, 1980. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Lin, Z.; Lin, W.; Liu, Y.; Ou, W.; Zeng, C.; Ke, L. Association of Pre-Pregnancy Body Mass Index with Adverse Pregnancy Outcome among First-Time Mothers. PeerJ 2020, 8, e10123. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Campbell, D.M.; Liston, W.A.; Bhattacharya, S. Effect of Body Mass Index on Pregnancy Outcomes in Nulliparous Women Delivering Singleton Babies. BMC Public Health 2007, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J. Knowledge Synthesis Group Overweight and Obesity in Mothers and Risk of Preterm Birth and Low Birth Weight Infants: Systematic Review and Meta-Analyses. BMJ 2010, 341, c3428. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghchi, M.; Jalali, R.; Mohammadi, M. A Systematic Review and Meta-Analysis on the Prevalence of Low Birth Weight Infants in Iran. J. Pregnancy 2020, 2020, 3686471. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Jones, S.; Paranjothy, S. Reducing Low Birth Weight: Prioritizing Action to Address Modifiable Risk Factors. J. Public Health Oxf. Engl. 2017, 39, 122–131. [Google Scholar] [CrossRef][Green Version]
- Akanmode, A.M.; Mahdy, H. Macrosomia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lewandowska, M. The Role of Maternal Weight in the Hierarchy of Macrosomia Predictors; Overall Effect of Analysis of Three Prediction Indicators. Nutrients 2021, 13, 801. [Google Scholar] [CrossRef]
- Dai, R.-X.; He, X.-J.; Hu, C.-L. Maternal Pre-Pregnancy Obesity and the Risk of Macrosomia: A Meta-Analysis. Arch. Gynecol. Obstet. 2018, 297, 139–145. [Google Scholar] [CrossRef]
- Burkhardt, R.; Hämmerle, C.H.F.; Lang, N.P.; Research Group on Oral Soft Tissue Biology & Wound Healing. Self-Reported Pain Perception of Patients after Mucosal Graft Harvesting in the Palatal Area. J. Clin. Periodontol. 2015, 42, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kim, R.; Vollmer, S.; Subramanian, S.V. Factors Associated With Child Stunting, Wasting, and Underweight in 35 Low- and Middle-Income Countries. JAMA Netw. Open 2020, 3, e203386. [Google Scholar] [CrossRef]
- Ratnasiri, A.W.G.; Lee, H.C.; Lakshminrusimha, S.; Parry, S.S.; Arief, V.N.; DeLacy, I.H.; Yang, J.-S.; DiLibero, R.J.; Logan, J.; Basford, K.E. Trends in Maternal Prepregnancy Body Mass Index (BMI) and Its Association with Birth and Maternal Outcomes in California, 2007-2016: A Retrospective Cohort Study. PLoS ONE 2019, 14, e0222458. [Google Scholar] [CrossRef]
- Deshmukh, V.L.; Jadhav, M.; Yelikar, K. Impact of HIGH BMI on Pregnancy: Maternal and Foetal Outcome. J. Obstet. Gynaecol. India 2016, 66, 192–197. [Google Scholar] [CrossRef]
- Bhowmik, B.; Siddique, T.; Majumder, A.; Mdala, I.; Hossain, I.A.; Hassan, Z.; Jahan, I.; Moreira, N.C.d.V.; Alim, A.; Basit, A.; et al. Maternal BMI and Nutritional Status in Early Pregnancy and Its Impact on Neonatal Outcomes at Birth in Bangladesh. BMC Pregnancy Childbirth 2019, 19, 413. [Google Scholar] [CrossRef]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef]
- Gizaw, B.; Gebremedhin, S. Factors Associated with Low Birthweight in North Shewa Zone, Central Ethiopia: Case-Control Study. Ital. J. Pediatr. 2018, 44, 76. [Google Scholar] [CrossRef]
- Sebayang, S.K.; Dibley, M.J.; Kelly, P.J.; Shankar, A.V.; Shankar, A.H.; SUMMIT Study Group Determinants of Low Birthweight. Small-for-Gestational-Age and Preterm Birth in Lombok, Indonesia: Analyses of the Birthweight Cohort of the SUMMIT Trial. Trop. Med. Int. Health TM IH 2012, 17, 938–950. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sztorc, L.; Sajdak, S. Smoking and Smoking Cessation in the Risk for Fetal Growth Restriction and Low Birth Weight and Additive Effect of Maternal Obesity. J. Clin. Med. 2020, 9, 3504. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Y.; Ni, Z.-M.; Wang, G.; Liu, S.-Y.; Li, C.; Yu, C.-L.; Wang, Q.; Nie, S.-F. Risk Factors for Low Birth Weight and Preterm Birth: A Population-Based Case-Control Study in Wuhan, China. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 286–292. [Google Scholar] [CrossRef]
- Tanner, L.D.; Brock And, C.; Chauhan, S.P. Severity of Fetal Growth Restriction Stratified According to Maternal Obesity. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2020, 1–5. [Google Scholar] [CrossRef]
- Vinturache, A.E.; Chaput, K.H.; Tough, S.C. Pre-Pregnancy Body Mass Index (BMI) and Macrosomia in a Canadian Birth Cohort. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2017, 30, 109–116. [Google Scholar] [CrossRef]
- Adugna, D.G.; Enyew, E.F.; Jemberie, M.T. Prevalence and Associated Factors of Macrosomia Among Newborns Delivered in University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia: An Institution-Based Cross-Sectional Study. Pediatr. Health Med. Ther. 2020, 11, 495–503. [Google Scholar] [CrossRef]
- Chew, L.C.; Verma, R.P. Fetal Growth Restriction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nobles, C.J.; Grantz, K.L.; Liu, D.; Williams, A.; Ouidir, M.; Seeni, I.; Sherman, S.; Mendola, P. Ambient Air Pollution and Fetal Growth Restriction: Physician Diagnosis of Fetal Growth Restriction versus Population-Based Small-for-Gestational Age. Sci. Total Environ. 2019, 650, 2641–2647. [Google Scholar] [CrossRef]
- Usta, A.; Usta, C.S.; Yildiz, A.; Ozcaglayan, R.; Dalkiran, E.S.; Savkli, A.; Taskiran, M. Frequency of Fetal Macrosomia and the Associated Risk Factors in Pregnancies without Gestational Diabetes Mellitus. Pan Afr. Med. J. 2017, 26, 62. [Google Scholar] [CrossRef]
- Marshall, N.E.; Biel, F.M.; Boone-Heinonen, J.; Dukhovny, D.; Caughey, A.B.; Snowden, J.M. The Association between Maternal Height, Body Mass Index, and Perinatal Outcomes. Am. J. Perinatol. 2019, 36, 632–640. [Google Scholar] [CrossRef]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between Perinatal Outcomes and Maternal Pre-Pregnancy Body Mass Index. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 1091–1102. [Google Scholar] [CrossRef]
- Nizard, J. [Prevention of IUGR]. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 1008–1017. [Google Scholar] [CrossRef]
- Ferdous, F.; Rashid, M.H.; Ma, E.; Raqib, R.; Hamada, H.; Wagatsuma, Y. Fetal Growth Restriction in Rural Bangladesh: A Prospective Study. Trop. Med. Health 2018, 46, 3. [Google Scholar] [CrossRef]
- Vilar Sánchez, Á.; Fernández Alba, J.J.; González Macías, M.D.C.; Paublete Herrera, M.D.C.; Carnicer Fuentes, C.; Carral San Laureano, F.; Torrejón Cardoso, R.; Moreno Corral, L.J. Maternal underweight and perinatal outcomes: A restrospective cohort study. Nutr. Hosp. 2017, 34, 647–653. [Google Scholar] [CrossRef]
- Staud, F.; Karahoda, R. Trophoblast: The Central Unit of Fetal Growth, Protection and Programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Cassidy, F.C.; Charalambous, M. Genomic Imprinting, Growth and Maternal-Fetal Interactions. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Rosario, F.J.; Powell, T.L.; Jansson, T. Regulation of Placental Amino Acid Transport and Fetal Growth. Prog. Mol. Biol. Transl. Sci. 2017, 145, 217–251. [Google Scholar] [CrossRef]
- Nogues, P.; Dos Santos, E.; Jammes, H.; Berveiller, P.; Arnould, L.; Vialard, F.; Dieudonné, M.-N. Maternal Obesity Influences Expression and DNA Methylation of the Adiponectin and Leptin Systems in Human Third-Trimester Placenta. Clin. Epigenetics 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef] [PubMed]
- Dubova, E.A.; Pavlov, K.A.; Borovkova, E.I.; Bayramova, M.A.; Makarov, I.O.; Shchegolev, A.I. Vascular Endothelial Growth Factor and Its Receptors in the Placenta of Pregnant Women with Obesity. Bull. Exp. Biol. Med. 2011, 151, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bergen, N.E.; Bouwland-Both, M.I.; Steegers-Theunissen, R.P.M.; Hofman, A.; Russcher, H.; Lindemans, J.; Jaddoe, V.W.V.; Steegers, E.A.P. Early Pregnancy Maternal and Fetal Angiogenic Factors and Fetal and Childhood Growth: The Generation R Study. Hum. Reprod. Oxf. Engl. 2015, 30, 1302–1313. [Google Scholar] [CrossRef]
- Catalano, P.; de Mouzon, S.H. Maternal Obesity and Metabolic Risk to the Offspring: Why Lifestyle Interventions May Have Not Achieved the Desired Outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef]
| Variables | Definitions and Categories | Reference Category/ Covariates | Description |
|---|---|---|---|
| Pre-pregnancy BMI | Defined as the quotient of pre-pregnancy weight (in kg) and height (in meters) squared - was assessed in the four following categories: (1) Underweight (<18.5); (2) normal weight (18.5–24.9); (3) overweight (25.0–29.9); (4) obesity (≥30) - was assessed as a continuous variable | Reference: -normal weight | Self-reported |
| GWG | Calculated as the difference between the weight before childbirth and the weight before pregnancy - was assessed in the three following categories (according to the 2009 Institute of Medicine recommendations): (1) above the range; (2) in the range; and (3) below the range. | Reference: -GWG in the range | From medical reports |
| Maternal age | As completed maternal age at conception - was assessed as a continuous variable (years) | The covariate | From medical reports |
| Primiparity | Parity was assessed in the two following categories: (1) primiparity i.e., zero prior delivery; (2) multiparity (≥1 prior deliveries) | The covariate | From medical reports |
| Smoking | -was assessed in the three following categories: (1) Women who had never smoked; (2) smokers (women who had smoked before pregnancy); (3) smokers in the first trimester | The covariate: -smokers in the first trimester | Self-reported |
| Fetal sex | -was assessed in the two following categories: (1) Son; (2) daughter | The covariate | From medical reports |
| Gestational age | The gestational age rating was based on ultrasound examination (crown-rump length (CRL) was assessed between 10th and 13th (+6 days) week) -was assessed as a continuous variable | The covariate | From medical reports |
| Gestational diabetes mellitus (GDM) | In order to diagnose GDM, an oral glucose tolerance test (OGTT) for 75g of glucose on empty stomach (2-h test) was performed in 24−28th gestational week. | The covariate | From medical reports |
| Preeclampsia/ Pregnancy-induced hypertension (PIH) | PIH was defined as blood pressure (systolic and diastolic) ≥ 140/90 mmHg, developing de novo after the 20th gestational week (obtained in at least two measurements four hours apart, and measured with an oscillometric device in a sitting position). Preeclampsia (PE) was diagnosed when this arterial hypertension was accompanied by the following organ disorders (de novo development): renal dysfunction and/or hepatic disorders and/or thrombocytopenia, visual and/or cerebral disorders, or pulmonary edema. | The covariate: -preeclampsia -prior PIH | From medical reports |
| Pre-Pregnancy Normal BMI (n = 593) | Pre-Pregnancy Obesity (n = 98) | ||
|---|---|---|---|
| Characteristics | Median (IQR) or n (%) | Median (IQR) or n (%) | p * |
| Pre−pregnancy weight (kg) | 60 (55−65) | 90 (87−97) | <0.0001 |
| Pre−pregnancy BMI (kg/m2) | 21.7 (20.3−23.2) | 32.7 (31.1−35.3) | <0.0001 |
| GWG (kg) | 14 (11−17) | 11 (7−16) | <0.0001 |
| GWG categories | <0.0001 | ||
| GWG above the range | 170 (28.7%) | 54 (55.1%) | |
| GWG in the range | 246 (41.5%) | 25 (25.5%) | |
| GWG below the range | 177 (29.8%) | 19 (19.4%) | |
| Primiparous women | 250 (42.2%) | 39 (39.8%) | 0.661 |
| Maternal age | 35 (30−37) | 36 (33−38) | 0.004 |
| Smoking | 90 (15.2%) | 25 (25.5%) | 0.011 |
| Education <12 years ** | 30 (5.8%) | 19 (22.4%) | <0.0001 |
| Lower financial status ** | 58 (19.2%) | 30 (50.8%) | <0.0001 |
| Pregnancy outcomes | |||
| Fetal sex, daughter | 287 (48.4%) | 46 (46.9%) | 0.789 |
| Gestational age (weeks) | 39 (38−40) | 39 (38−40) | 0.513 |
| Birth <37th week | 37 (6.2%) | 15 (15.3%) | 0.002 |
| Birth weight (grams) | 3390 (3090−3670) | 3620 (2960−3980) | 0.021 |
| Birth weight categories | <0.0001 | ||
| <2500g | 32 (5.4%) | 13 (13.3%) | |
| 2500−4000g | 517 (87.2%) | 62 (63.3%) | |
| >4000g | 44 (7.4%) | 23 (23.5%) | |
| Birth weight categories | <0.0001 | ||
| <10th percentile | 42 (7.1%) | 10 (10.2%) | |
| 10−90th percentile | 503 (84.8%) | 64 (65.3%) | |
| >90th percentile | 48 (8.1%) | 24 (24.5%) | |
| Fetal growth restriction (FGR) | 12 (2.0%) | 5 (5.2%) | 0.065 |
| Preeclampsia (PE) | 9 (1.7%) | 9 (13.4%) | <0.0001 |
| GDM | 79 (13.3%) | 32 (32.7%) | <0.0001 |
| Cesarean section | 239 (40.3%) | 57 (58.2%) | 0.001 |
| APGAR in 5th minute <7 | 2 (0.3%) | (0%) | 1 |
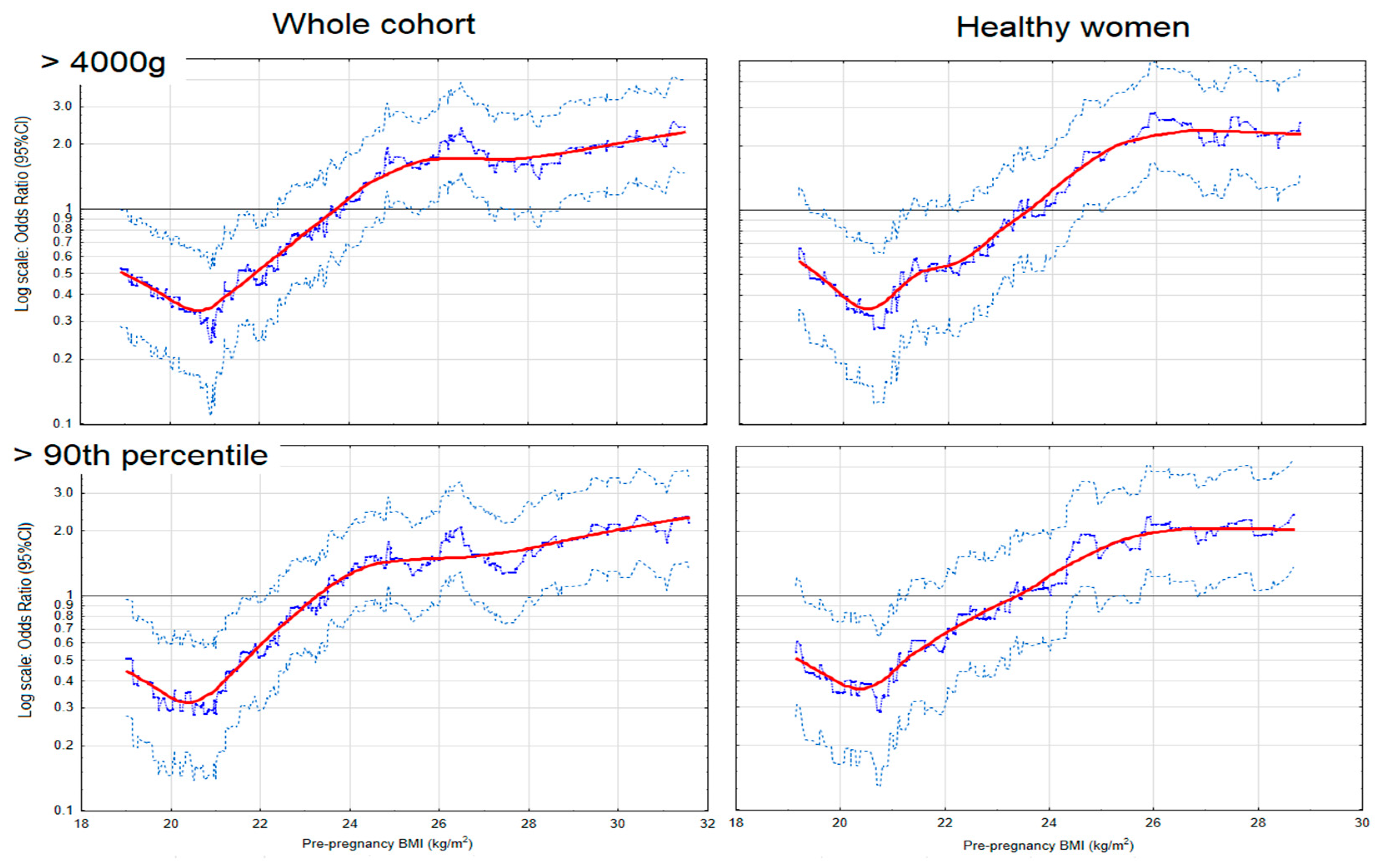
| Odds Ratios of Excessive Birth Weight for BMI Categories | |||
|---|---|---|---|
| Birth Weight | Cases/ Controls | OR (95% CI:); p | AOR * (95% CI:); p |
| Macrosomia (>4000 g) (n = 97) ** | |||
| Whole cohort | |||
| Obesity | 23/62 | 4.37 (2.47−7.7); <0.001 | 3.21 (1.69−6.1); <0.001 |
| Overweight | 26/135 | 2.27 (1.35−3.82); 0.002 | 1.42 (0.8−2.51); 0.234 |
| Normal BMI | 44/518 | 1 | 1 |
| Underweight | 4/40 | 1.18 (0.40−3.44); 0.766 | 1.53 (0.50−4.71); 0.461 |
| ‘Healthy’ subgroup | |||
| Obesity | 9/26 | 4.93 (2.12−11.49); <0.001 | 4.33 (1.71−10.96); 0.002 |
| Overweight | 18/96 | 2.67 (1.42−5.01); 0.002 | 1.65 (0.82−3.34); 0.164 |
| Normal BMI | 29/413 | 1 | 1 |
| Underweight | 4/30 | 1.90 (0.63−5.76); 0.257 | 2.56 (0.79−8.29); 0.117 |
| GWG in the range | |||
| Obesity | 6/13 | 5.37 (1.83−15.7); 0.002 | 3.94 (1.2−12.95); 0.024 |
| Overweight | 4/35 | 1.33 (0.43−4.14); 0.623 | 1.06 (0.32−3.52); 0.927 |
| Normal BMI | 19/221 | 1 | 1 |
| Underweight | 3/14 | 2.49 (0.66−9.44); 0.179 | 3.43 (0.83−14.26); 0.090 |
| LGA (>90th percentile) (n = 99) *** | |||
| Whole cohort | |||
| Obesity | 24/64 | 3.94 (2.26−6.9); <0.001 | 3.05 (1.65−5.6); <0.001 |
| Overweight | 23/137 | 1.76 (1.04−3.00); 0.037 | 1.23 (0.7−2.17); 0.478 |
| Normal BMI | 48/504 | 1 | 1 |
| Underweight | 4/36 | 1.17 (0.40−3.42); 0.779 | 1.43 (0.47−4.33); 0.531 |
| ‘Healthy’ subgroup | |||
| Obesity | 8/26 | 4.19 (1.75−10.04); 0.001 | 3.66 (1.45−9.28); 0.006 |
| Overweight | 16/98 | 2.22 (1.16−4.23); 0.015 | 1.49 (0.74−3.01); 0.264 |
| Normal BMI | 30/408 | 1 | 1 |
| Underweight | 4/27 | 2.02 (0.66−6.14); 0.218 | 2.50 (0.78−7.99); 0.123 |
| GWG in the range | |||
| Obesity | 4/18 | 2.64 (0.81−8.6); 0.108 | 1.97 (0.56−6.89); 0.289 |
| Overweight | 4/39 | 1.22 (0.39−3.8); 0.732 | 0.99 (0.30−3.3); 0.993 |
| Normal BMI | 18/214 | 1 | 1 |
| Underweight | 3/13 | 2.74 (0.72−10.52); 0.141 | 3.12 (0.74−13.14); 0.121 |
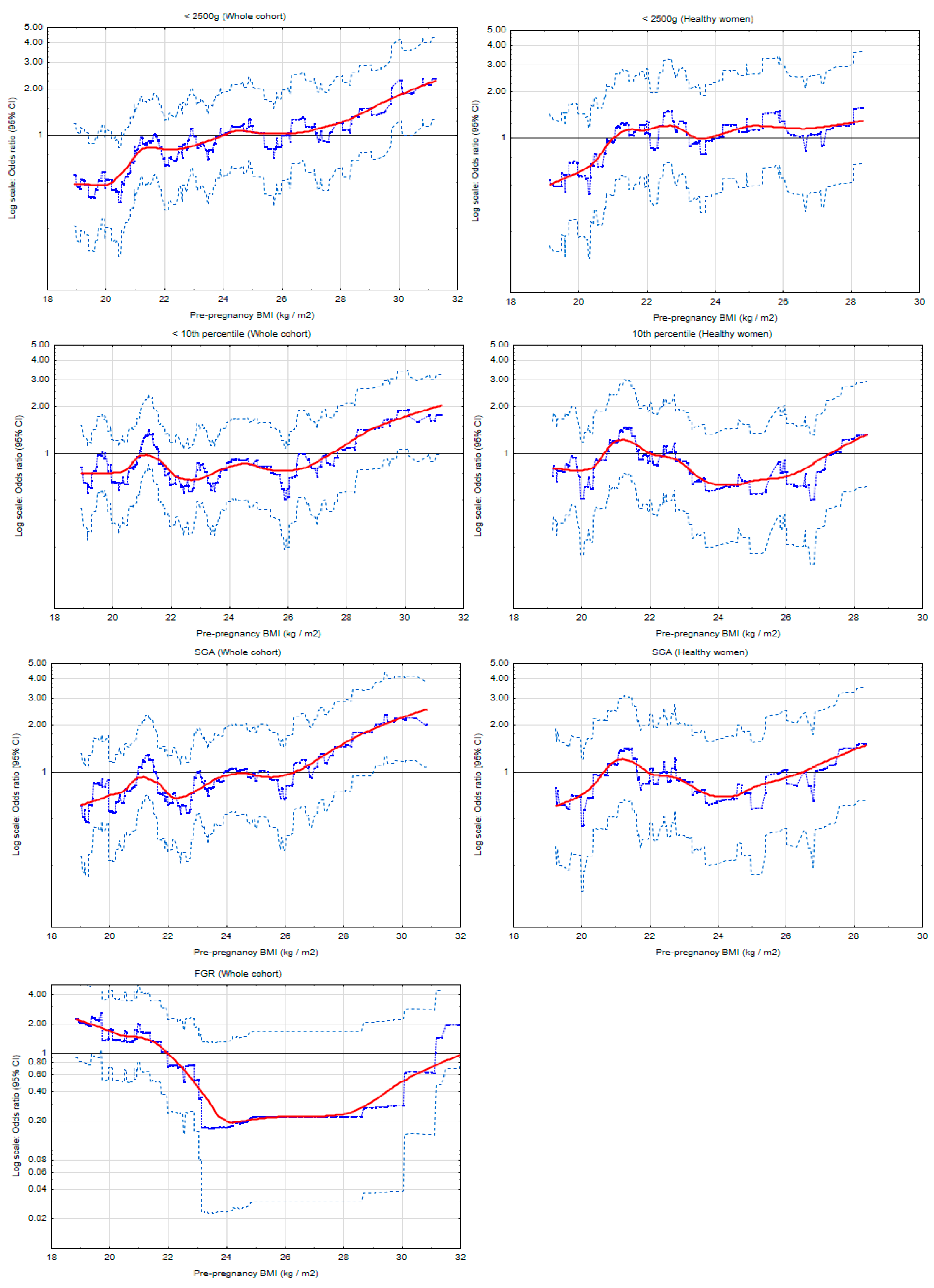
| Odds Ratios of Lower Birth Weight for BMI Categories | |||
|---|---|---|---|
| Birth Weight | Cases/ Controls | OR (95% CI:); p | AOR * (95% CI:); p |
| LBW (<2500 g) ** (n = 60) | |||
| Whole cohort | |||
| Obesity | 13/62 | 3.39 (1.69−6.81); 0.001 | 1.76 (0.54−5.72); 0.349 |
| Overweight | 12/135 | 1.44 (0.72−2.87); 0.301 | 1.51 (0.52−4.41); 0.454 |
| Normal BMI | 32/518 | 1 | 1 |
| Underweight | 3/40 | 1.21 (0.36−4.14); 0.757 | 0.42 (0.05−3.57); 0.428 |
| ‘Healthy’ subgroup | |||
| Obesity | 2/26 | 1.59 (0.35−7.17); 0.547 | 1.10 (0.16−7.43); 0.925 |
| Overweight | 6/96 | 1.29 (0.51−3.3); 0.594 | 0.92 (0.21−4.01); 0.914 |
| Normal BMI | 20/413 | 1 | 1 |
| Underweight | 1/30 | 0.69 (0.09−5.31); 0.720 | 0.49 (0.05−5.12); 0.547 |
| GWG in the range | |||
| Obesity | 6/13 | 17.00 (4.8−60.1); <0.001 | 17.42 (1.5−202.6); 0.022 |
| Overweight | 8/35 | 8.42 (2.76−25.7); <0.001 | 9.07 (1.29−63.70); 0.027 |
| Normal BMI | 6/221 | 1 | 1 |
| Underweight | 3/14 | 7.89 (1.78−34.93); 0.006 | 2.51 (0.07−96.7); 0.622 |
| SGA *** (n = 56) | |||
| Whole cohort | |||
| Obesity | 6/63 | 1.40 (0.57−3.47); 0.466 | 0.91 (0.33−2.52); 0.861 |
| Overweight | 13/137 | 1.40 (0.72−2.72); 0.327 | 1.33 (0.64−2.74); 0.443 |
| Normal BMI | 34/500 | 1 | 1 |
| Underweight | 3/36 | 1.23 (0.36−4.18); 0.746 | 1.27 (0.36−4.51); 0.707 |
| ‘Healthy’ subgroup | |||
| Obesity | 2/26 | 1.64 (0.36−7.42); 0.521 | 1.46 (0.31−6.93); 0.638 |
| Overweight | 6/98 | 1.31 (0.51−3.35); 0.580 | 1.19 (0.43−3.32); 0.738 |
| Normal BMI | 19/405 | 1 | 1 |
| Underweight | 2/27 | 1.58 (0.35−7.14); 0.553 | 1.46 (0.31−6.88); 0.630 |
| GWG in the range | |||
| Obesity | 2/18 | 1.81 (0.38−8.66); 0.457 | 1.75 (0.29−10.5); 0.543 |
| Overweight | 4/39 | NA | 1.28 (0.33−5.03); 0.722 |
| Normal BMI | 13/212 | 1 | 1 |
| Underweight | 1/13 | 1.25 (0.15−10.34); 0.833 | 1.89 (0.21−17.1); 0.572 |
| Odds Ratios of FGR for BMI Categories | |||
|---|---|---|---|
| Birth Weight | Cases/ Controls | OR (95% CI:); p | AOR * (95% CI:); p |
| FGR (n = 21) ** | |||
| Whole cohort | |||
| Obesity | 5/91 | 2.64 (0.91−7.7); 0.075 | 3.12 (1.02−9.54); 0.045 |
| Overweight | 0/170 | NC | NC |
| Normal BMI | 12/576 | 1 | 1 |
| Underweight | 4/43 | 4.47 (1.38−14.4); 0.012 | 3.84 (1.13−13.0); 0.031 |
| ‘Healthy’ subgroup | |||
| Obesity | 1/36 | 1.57 (0.19−12.9); 0.675 | 2.37 (0.27−20.84); 0.438 |
| Overweight | 0/118 | NC | NC |
| Normal BMI | 8/452 | 1 | 1 |
| Underweight | 2/33 | 3.42 (0.7−16.78); 0.129 | 2.52 (0.49−12.97); 0.268 |
| GWG in the range | |||
| Obesity | 1/24 | 3.33 (0.33−33.3); 0.305 | 4.06 (0.38−43.07); 0.245 |
| Overweight | 0/47 | NC | NC |
| Normal BMI | 3/240 | 1 | 1 |
| Underweight | 3/17 | 14.12 (2.65−75.3); 0.002 | 11.82 (1.95−71.6); 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, M. Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses. Nutrients 2021, 13, 1213. https://doi.org/10.3390/nu13041213
Lewandowska M. Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses. Nutrients. 2021; 13(4):1213. https://doi.org/10.3390/nu13041213
Chicago/Turabian StyleLewandowska, Małgorzata. 2021. "Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses" Nutrients 13, no. 4: 1213. https://doi.org/10.3390/nu13041213
APA StyleLewandowska, M. (2021). Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses. Nutrients, 13(4), 1213. https://doi.org/10.3390/nu13041213






