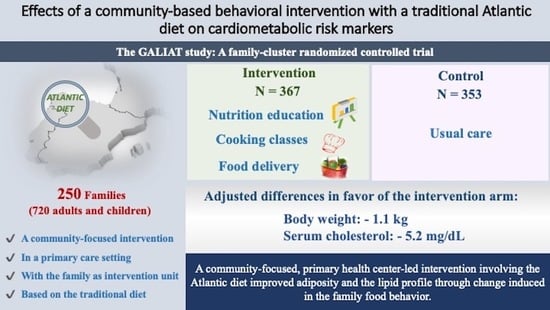
Effects of a Community-Based Behavioral Intervention with a Traditional Atlantic Diet on Cardiometabolic Risk Markers: A Cluster Randomized Controlled Trial (“The GALIAT Study”)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
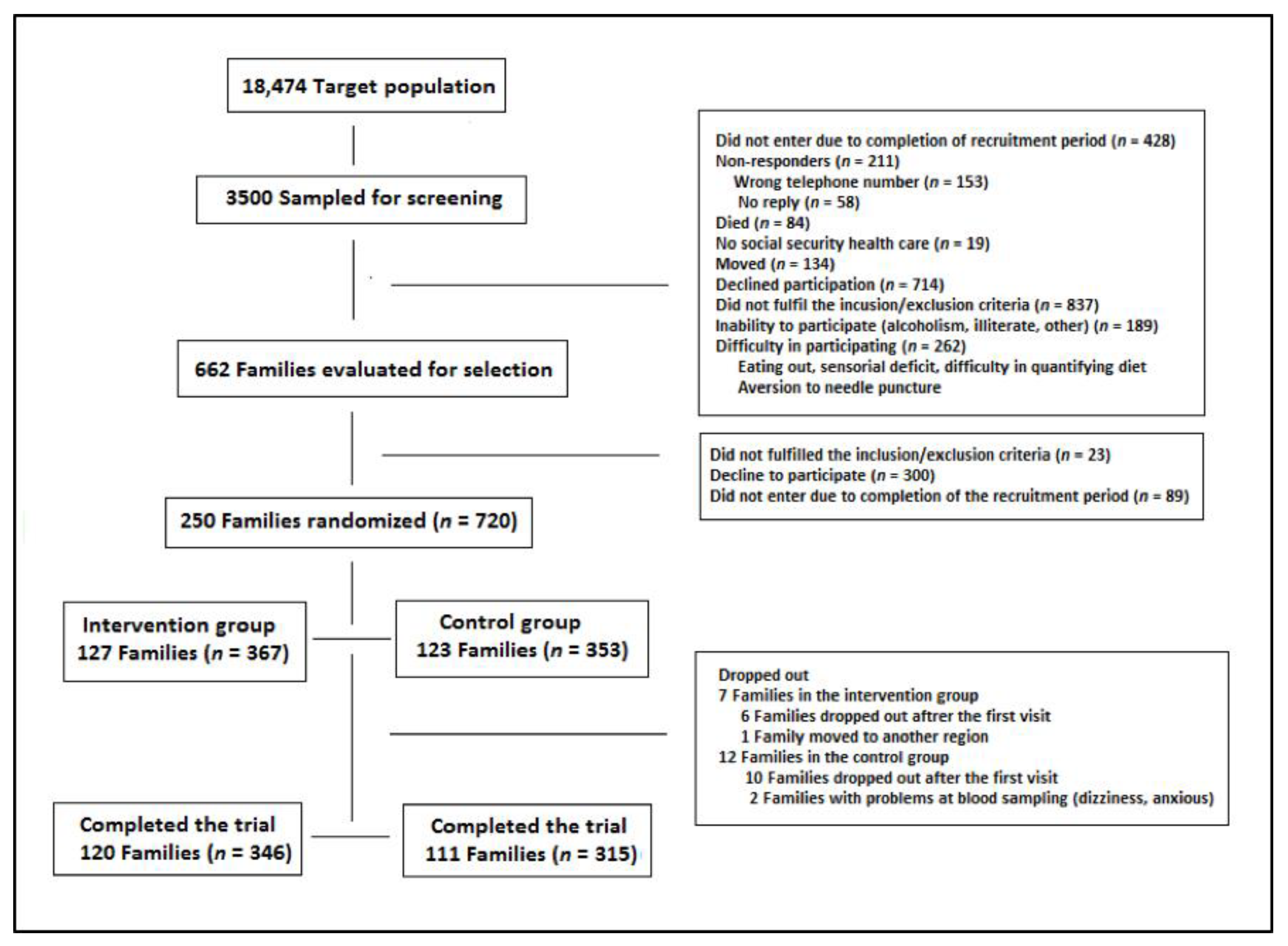
2.2. Study Population
2.3. Intervention Procedures
2.4. Outcomes
2.5. Study Procedures
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Anthropometric Variables
3.3. Metabolic Measurements
3.4. Assessment of Compliance with Recommendations on the Atlantic Diet
3.5. Dietary Intake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- World Health Organization. ‘Best Buys’ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases—Updated (2017) Appendix 3 of the Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/ncds/prevention/be-healthy-be-mobile/WHO_Appendix_BestBuys_Rev1.pdf?ua=1 (accessed on 14 July 2020).
- Smith, A.W.; Borowski, L.A.; Liu, B.; Galuska, D.A.; Signore, C.; Klabunde, C.; Huang, T.T.K.; Krebs-Smith, S.M.; Frank, E.; Pronk, N.; et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am. J. Prev. Med. 2011, 41, 33–42. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Grossman, D.C.; Bibbins-Domingo, K.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; Krist, A.H.; et al. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without cardiovascular risk factors. US Preventive Services Task Force Recommendation Statement. JAMA 2017, 318, 167–174. [Google Scholar]
- World Health Organization. Global Strategy on Diet, Physical Activity and Health. Available online: https://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf (accessed on 14 July 2020).
- Payne, G.H.; James, S.D., Jr.; Hawley, L.; Corrigan, B.; Kramer, R.E.; Overton, S.N.; Farris, R.P.; Wasilewski, Y. CDC’s health equity resource toolkit: Disseminating guidance for state practitioners to address obesity disparities. Health Promot. Pract. 2015, 16, 84–90. [Google Scholar] [CrossRef]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Redeker, N.S.; et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 406–441. [Google Scholar] [CrossRef]
- Sallis, J.F.; Owen, N.; Fisher, E.B. Ecological models of health behavior. In Health Behavior and Health Education. IN Theory, Research, and Practice, 4th ed.; Jossey-Bass: San Francisco, CA, USA, 2008; pp. 465–486. [Google Scholar]
- Romon, M.; Lommez, A.; Tafflet, M.; Basdevant, A.; Oppert, J.M.; Bresson, J.L.; Ducimetière, P.; Charles, M.A.; Borys, J.M. Downward trends in the prevalence of childhood overweight in the setting of 12-year school- and community-based programmes. Public Health Nutr. 2009, 12, 735–742. [Google Scholar] [CrossRef]
- Lombard, C.; Deeks, A.; Jolley, D.; Ball, K.; Teede, H. A low intensity, community based lifestyle programme to prevent weight gain in women with young children: Cluster randomised controlled trial. BMJ 2010, 341, c3215. [Google Scholar] [CrossRef]
- Calvo-Malvar, M.; Leis, R.; Benítez-Estévez, A.J.; Sánchez-Castro, J.; Gude, F. A randomised, family-focused dietary intervention to evaluate the Atlantic diet: The GALIAT study protocol. BMC Public Health 2016, 16, 820. [Google Scholar] [CrossRef]
- González-García, S.; Green, R.F.; Scheelbeeck, P.F.; Harris, F.; Dangour, A.D. Dietary recommendations in Spain–affordability and environmental sustainability? J. Clean. Prod. 2020, 254, 120125. [Google Scholar] [CrossRef]
- Vaz Velho, M.; Pinheiro, R.; Rodrigues, A.S. The Atlantic diet—Origin and features. Int. J. Food Stud. 2016, 5, 106–119. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. International Scientific Symposium. Biodiversity and Sustainable Diets United against Hunger; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/a-i3004e.pdf (accessed on 14 July 2020).
- Esteve-Llorens, X.; Darriba, C.; Moreira, M.T.; Feijoo, G.; González-García, S. Towards an environmentally sustainable and healthy Atlantic dietary pattern: Life cycle carbon footprint and nutritional quality. Sci. Total Environ. 2019, 646, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Llorens, X.; Moreira, M.T.; Feijoo, G.; González-García, S. Linking environmental sustainability and nutritional quality of the Atlantic diet recommendations and real consumption habits in Galicia (NW Spain). Sci. Total Environ. 2019, 683, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. In Techniques for Measuring Body Composition; Brozek, J., Henschel, A., Eds.; National Academy of Sciences: Washington, DC, USA, 1961; pp. 223–244. [Google Scholar]
- Rodríguez-Martín, C.; Garcia-Ortiz, L.; Rodriguez-Sanchez, E.; Martin-Cantera, C.; Soriano-Cano, A.; Arietaleanizbeaskoa, M.S.; Magdalena-Belio, J.F.; Menendez-Suarez, M.; Maderuelo-Fernandez, J.A.; Lugones-Sanchez, C.; et al. The Relationship of the Atlantic Diet with Cardiovascular Risk Factors and Markers of Arterial Stiffness in Adults without Cardiovascular Disease. Nutrients 2019, 11, 742. [Google Scholar] [CrossRef]
- Ortega, R.M.; López, A.M.; Carvajales, P.A.; Requejo, A.M.; Aparicio, A.; Molinero, L.M. Programa Dial v. 3.3.5.0. 2016. Available online: https://www.alceingenieria.net/nutricion/descarga.htm (accessed on 5 April 2021).
- Ware, J.E., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction ofscales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Alonso, J.; Regidor, E.; Barrio, G.; Prieto, L.; Rodríguez, C.; de la Fuente, L. Valores poblacionales de referencia de la versión española del Cuestionario de Salud SF-36. Med. Clin. 1998, 111, 410–416. [Google Scholar]
- The-IPAQ-Group International Physical Activity Questionnaire. Available online: http://www.sdp.univ.fvg.it/sites/default/files/IPAQ_English_self-admin_long.pdf (accessed on 14 July 2020).
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obes. Res. 1998, 6 (Suppl. 2), 51S–209S. [Google Scholar]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- Patnode, C.D.; Evans, C.V.; Senger, C.A.; Redmond, N.; Lin, J.S. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017, 318, 175–193. [Google Scholar]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [PubMed]
- Prospective Studies Collaboration; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef]
- Beunza, J.J.; Toledo, E.; Hu, F.B.; Bes-Rastrollo, M.; Serrano-Martínez, M.; Sánchez-Villegas, A.; Alfredo Martínez, J.; Martínez-González, M.A. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: The Seguimiento Universidad de Navarra (SUN) cohort. Am. Clin. Nutr. 2010, 92, 1484–1493. [Google Scholar] [CrossRef]
- Kuehn, B.M. Heritage diets and culturally appropriate dietary advice may help combat chronic diseases. JAMA 2019, 322, 2271–2273. [Google Scholar] [CrossRef]
- Oliveira, A.; Lopes, C.; Rodríguez-Artalejo, F. Adherence to the Southern European Atlantic Diet and occurrence of nonfatal acute myocardial infarction. Am. J. Clin. Nutr. 2010, 92, 211–217. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Oliveira, A.; Lopes, C.; López-García, E.; Rodríguez-Artalejo, F. The Southern European Atlantic Diet is associated with lower concentrations of markers of coronary risk. Atherosclerosis 2013, 226, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- United Nations Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 14 July 2020).
- Smith, L.R.; Chadwick, P.; Radley, D.; Kolotourou, M.; Gammon, C.S.; Rosborough, J.; Sacher, P.M. Assessing the short-term outcomes of a community-based intervention for overweight and obese children: The MEND 5-7 programme. BMJ Open 2013, 3, e002607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwartz, R.P.; Vitolins, M.Z.; Case, L.D.; Armstrong, S.C.; Perrin, E.M.; Cialone, J.; Bell, R.A. The YMCA Healthy, Fit, and Strong Program: A community-based, family-centered, low-cost obesity prevention/treatment pilot study. Child. Obes. 2012, 8, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.C.; Simmons, A.; Sanigorski, A.M.; Kremer, P.J.; Swinburn, B.A. Preventing childhood obesity: The sentinel site for obesity prevention in Victoria, Australia. Health Promot. Int. 2008, 23, 328–336. [Google Scholar] [CrossRef]
- de Silva-Sanigorski, A.M.; Bell, A.C.; Kremer, P.; Nichols, M.; Crellin, M.; Smith, M.; Sharp, S.; de Groot, F.; Carpenter, L.; Boak, R.; et al. Reducing obesity in early childhood: Results from Romp & Chomp, an Australian community-wide intervention program. Am. J. Clin. Nutr. 2010, 91, 831–840. [Google Scholar] [PubMed]
- Sanigorski, A.M.; Bell, A.C.; Kremer, P.J.; Cuttler, R.; Swinburn, B.A. Reducing unhealthy weight gain in children through community capacity-building: Results of a quasi-experimental intervention program, Be Active Eat Well. Int. J. Obes. 2008, 32, 1060–1067. [Google Scholar] [CrossRef]
- Swinburn, B.; Malakellis, M.; Moodie, M.; Waters, E.; Gibbs, L.; Millar, L.; Herbert, J.; Virgo-Milton, M.; Mavoa, H.; Kremer, P.; et al. Large reductions in child overweight and obesity in intervention and comparison communities 3 years after a community project. Pediatr. Obes. 2014, 9, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Katan, M.B. Weight-loss diets for the prevention and treatment of obesity. N. Engl. J. Med. 2009, 360, 923–925. [Google Scholar] [CrossRef]
| Food Consumption Recommendations | Servings/Frequency |
|---|---|
| Bread, cereals, wholegrain cereals, rice, pasta, and potatoes | 6–8/day |
| Olive oil | 3–4/day |
| Fruit | ≥3/day |
| Vegetables | ≥2/day |
| Dairy products | 3–4/day |
| Nuts, preferably chestnuts and walnuts | 4–6/week |
| Fish and seafood | 3–4/week |
| Eggs | 3–4/week |
| Lean meat | 3–4/week |
| Pulses | 2–3/week |
| Fatty meat, cured sausage, margarine, butter | Sparingly/monthly |
| Sweets, pastries, cakes, ice cream, etc. | Sparingly/monthly |
| Characteristic | Control Arm | Intervention Arm |
|---|---|---|
| Families/study subjects, n | 123/353 | 127/367 |
| Participants per family, mean ± SD | 2.8 ± 1.0 | 2.9 ± 1.0 |
| Male sex, n (%) | 142 (48.1) | 153 (51.9) |
| Age, years, mean ± SD | 38.6 ± 19.8 | 40.2 ± 20.8 |
| Marital status 1, n (%) | ||
| Married/with partner | 194 (67.8) | 210 (73.4) |
| Divorced/separated/widowed | 33 (11.5) | 28 (9.8) |
| Single | 59 (20.6) | 48 (16.8) |
| Educational level 1, n (%) | ||
| None | 29 (10.1) | 30 (10.5) |
| Elementary | 120 (41.8) | 102 (35.7) |
| Secondary | 91 (31.7) | 102 (35.7) |
| University or higher | 47 (16.4) | 52 (18.2) |
| Employment status 1, n (%) | ||
| Employed | 147 (52.1) | 137 (48.6) |
| Retired | 40 (14.2) | 56 (19.9) |
| Other | 95 (33.7) | 89 (31.6) |
| Smoking status 1, n (%) | ||
| Never smoker | 126 (44.5) | 120 (42.0) |
| Ex-smoker | 50 (17.7) | 71 (24.8) |
| Current smoker | 107 (37.8) | 95 (33.2) |
| Alcohol intake 1, n (%) | ||
| Abstainers | 123 (43.5) | 124 (43.2) |
| Light drinkers (1–140 g/week) | 132 (46.6) | 130 (45.3) |
| Heavy drinkers (>140 g/week) | 28 (9.9) | 33 (11.5) |
| Comorbidities 1, n (%) | ||
| Cardiovascular disease | 42 (16.0) | 49 (18.3) |
| Cerebrovascular accident | 3 (1.1) | 3 (1.1) |
| Diabetes | 16 (5.9) | 16 (5.9) |
| Current medications 1, n (%) | ||
| Cholesterol-lowering | 23 (8.7) | 32 (12.5) |
| Anti-hypertensives | 44 (18.1) | 56 (24.2) |
| Health-related quality of life (SF-12v1) 1, n (%) | ||
| Physical component summary | 48.6 (9.3) | 47.3 (10.1) |
| Mental component summary | 51.1 (10.1) | 52.1 (8.8) |
| International Physical Activity Questionnaire 1, n (%) | ||
| Inactive | 56 (19.5) | 44 (15.4) |
| Minimally active | 68 (23.7) | 85 (29.7) |
| Active | 163 (56.8) | 157 (54.9) |
| Intervention | Control | Adjusted Mean Differences (95% CI) | p Value | ICC | |||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | ||||
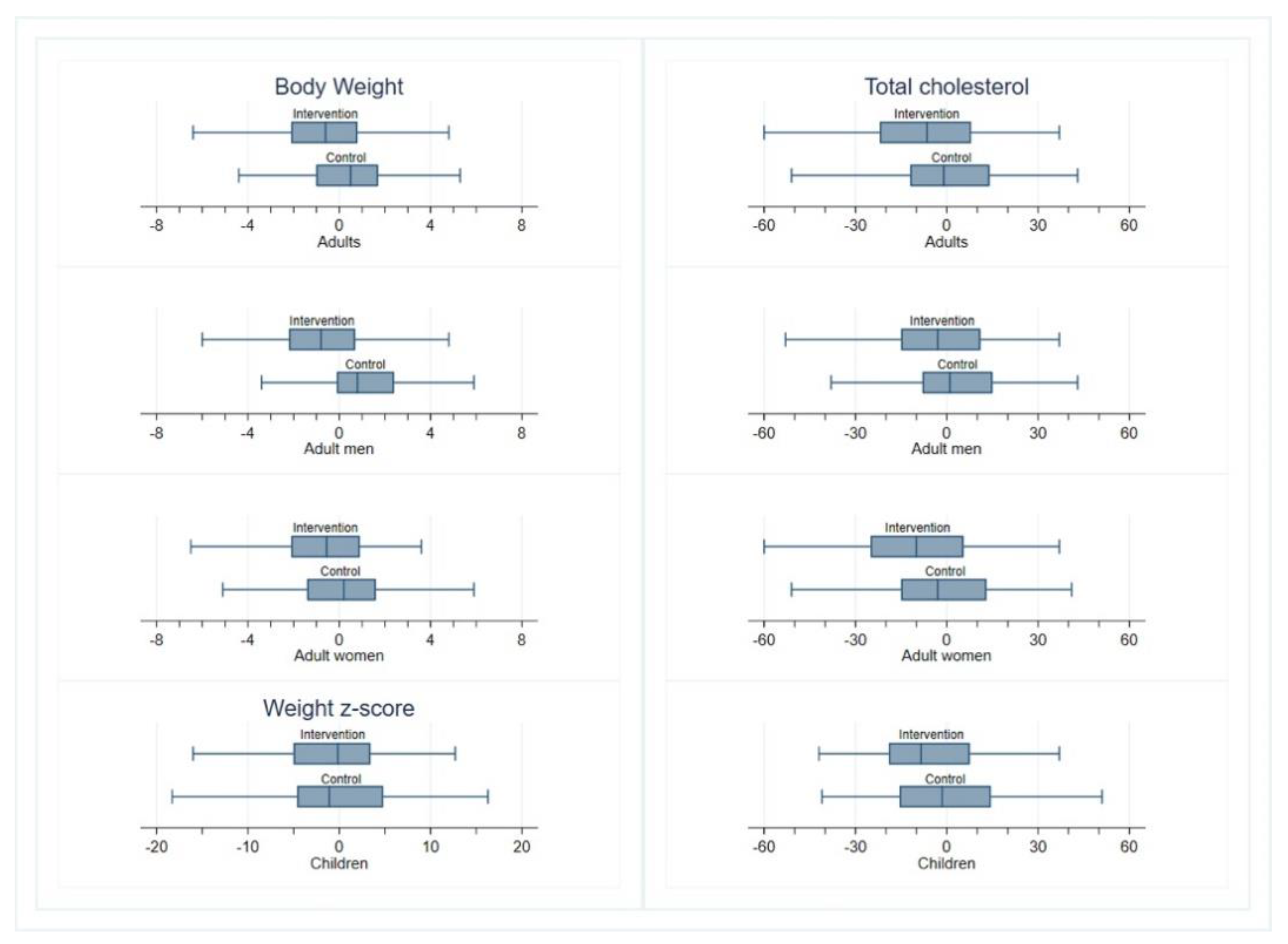
| Weight, kg | 70.2 ± 22.0 | 69.8 ± 21.4 | 67.9 ± 21.2 | 68.5 ± 21.0 | −0.9 (−1.3, −0.5) | <0.001 | 0.215 |
| Only adults 1 | 77.3 ± 16.9 | 76.5 ± 16.9 | 74.8 ± 15.1 | 75.2 ± 15.2 | −1.1 (−1.6, −0.7) | <0.001 | - |
| Women 1 | 71.3 ± 15.8 | 70.5 ± 15.8 | 69.2 ± 14.3 | 69.3 ± 14.3 | −0.8 (−1.4, −0.2) | 0.012 | - |
| Men 1 | 86.2 ± 14.5 | 85.3 ± 14.5 | 83.3 ± 11.9 | 84.2 ± 11.7 | −1.7 (−2.4, −1.0) | <0.001 | - |
| Children 2, Z-score | 18.0 ± 32.2 | 17.4 ± 30.4 | 17.5 ± 30.5 | 16.5 ± 32.1 | 0.47 (−2.91, 3.86) | 0.783 | - |
| BMI 1, kg/m2 | 28.4 ± 5.2 | 28.1 ± 5.2 | 27.6 ± 5.1 | 27.7 ± 5.1 | −0.44 (−0.62, −0.25) | <0.001 | - |
| Hip-to-waist ratio | 0.91 ± 0.09 | 0.89 ± 0.10 | 0.89 ± 0.09 | 0.89 ± 0.10 | −0.012 (−0.019, −0.005) | 0.001 | 0.242 |
| Body fat 1, % | 35.3 ± 6.9 | 34.1 ± 7.0 | 34.3 ± 7.1 | 33.9 ± 7.1 | −0.85 (−1.20, −0.50) | <0.001 | - |
| TC, mg/dL | 196 ± 39 | 189 ± 39 | 189 ± 36 | 188 ± 38 | −5.2 (−8.8, −1.6) | 0.004 | 0.222 |
| Only adults 1 | 202 ± 39 | 195 ± 39 | 196 ± 35 | 195 ± 36 | −4.9 (−9.0, −0.8) | 0.020 | - |
| Women 1 | 204 ± 37 | 194 ± 35 | 195 ± 33 | 193 ± 35 | −5.6 (−10.6, −0.6) | 0.029 | - |
| Men 1 | 199 ± 41 | 197 ± 44 | 196 ± 38 | 198 ± 39 | −3.6 (−10.3, 3.1) | 0.297 | - |
| Children 2 | 174 ± 32 | 166 ± 32 | 158 ± 24 | 159 ± 27 | −3.7 (−10.7, 3.3) | 0.305 | - |
| LDL-C, mg/dL | 118 ± 34 | 114 ± 34 | 112 ± 31 | 112 ± 32 | −3.4 (−6.5, −0.3) | 0.034 | 0.263 |
| HDL-C, mg/dL | 55 (47, 66) | 55 (46, 65) | 55 (46, 65) | 54 (45, 64) | −0.9 (−2.2, 0.3) | 0.142 | 0.269 |
| TG, mg/dL | 84 (63, 114) | 82 (57, 110) | 79 (58, 117) | 81 (57, 121) | −3.9 (−8.0, 0.5) | 0.079 | 0.282 |
| FPG, mg/dL | 86 (79, 93) | 83 (77, 90) | 84 (79, 93) | 82 (76, 90) | 0.4 (−1.0, 1.8) | 0.563 | 0.344 |
| HbA1c, % | 5.4 (5.2, 5.5) | 5.3 (5.2, 5.6) | 5.3 (5.2, 5.5) | 5.4 (5.2, 5.6) | −0.02 (−0.05, 0.02) | 0.343 | 0.290 |
| Insulin, mIU/L | 9.0 (6.1, 13.2) | 10.4 (7.4, 14.8) | 8.7 (6.0, 12.5) | 10.7 (7.4, 14.8) | −0.46 (−1.12, 0.24) | 0.189 | 0.262 |
| HOMA-IR, units | 1.89 (1.27, 2.88) | 2.16 (1.44, 3.28) | 1.86 (1.22, 2.84) | 2.18 (1.42, 3.28) | −0.09 (−0.24, 0.08) | 0.297 | 0.297 |
| CPR, mg/L | 0.15 (0.06, 0.37) | 0.17 (0.08, 0.41) | 0.16 (0.06, 0.40) | 0.15 (0.06, 0.38) | 0.10 (−0.22, 0.42) | 0.525 | - |
| TNF-α, mg/dL | 7.7 (6.2, 9.8) | 6.9 (5.4, 8.7) | 8.2 (6.2, 9.9) | 7.2 (5.6, 9.0) | −0.18 (−0.52, 0.19) | 0.333 | 0.331 |
| IL-6, pg/mL | 2.8 (1.9, 3.9) | 2.1 (1.9, 3.0) | 2.7 (1.9, 3.7) | 2.0 (1.9, 3.1) | −0.01 (−0.17, 0.17) | 0.926 | 0.248 |
| Leptin, ng/mL | 7.4 (2.9, 15.7) | 5.5 (2.0, 12.0) | 6.3 (3.0, 14.0) | 5.1 (2.2, 11.4) | −0.29 (−1.22, 0.80) | 0.585 | 0.347 |
| SBP, mmHg | 123 ± 18 | 123 ± 18 | 122 ± 17 | 122 ± 17 | −0.5 (−2.3, 1.3) | 0.590 | 0.233 |
| DBP, mmHg | 71 ± 11 | 70 ± 10 | 70 ± 10 | 69 ± 11 | −0.6 (−1.7, 0.6) | 0.347 | 0.146 |
| Components | Target | Intervention (n = 328) | Control (n = 305) | ||
|---|---|---|---|---|---|
| Baseline (%) (95% CI) | 6 Months (%) (95% CI) | Baseline (%) (95% CI) | 6 Months (%) (95% CI) | ||
| Bread, cereals, wholegrain cereals, rice, pasta and potatoes | ≥6 servings/day | 1.93 (0.92, 3.99) | 1.21 (0.46, 3.19) | 1.44 (0.60, 3.42) | 0.98 (0.32, 3.01) |
| Olive oil | ≥3 servings/day | 48.76 (43.64, 53.90) | 47.88 (42.53, 53.28) | 49.57 (44.33, 54.82) | 36.07 (30.86, 41.62) |
| Fresh fruit | ≥3 servings/day | 43.80 (38.78, 48.96) | 50.91 (45.52, 56.28) | 42.65 (37.54. 47.92) | 36.72 (31.49, 42.28) |
| Vegetables | ≥2 servings/day | 7.16 (4.92, 10.32) | 6.36 (4.18, 9.57) | 6.63 (4.44, 9.78) | 3.28 (1.77, 5.99) |
| Dairy products | ≥3 servings/day | 70.25 (65.34, 74.73) | 75.15 (70.20, 79.52) | 67.44 (62.32, 72.17) | 64.59 (59.05, 69.76) |
| Fish and seafood | ≥3 servings/week | 84.57 (80.48, 87.94) | 88.79 (84.90, 91.77) | 80.98 (76.50, 84.77) | 77.70 (72.68, 82.03) |
| Lean meat | ≥3 servings/week | 98.35 (96.37, 99.26) | 96.97 (94.46, 98.36) | 99.42 (97.7, 99.86) | 97.05 (94.42, 98.46) |
| Eggs | ≥3 servings/week | 69.70 (64.77, 74.21) | 67.58 (62.33, 72.41) | 70.03 (64.99, 74.62) | 63.93 (58.38, 69.14) |
| Pulses | ≥2 servings/week | 22.59 (18.58, 27.18) | 44.24 (38.97, 49.65) | 21.04 (17.06, 25.65) | 32.13 (27.12, 37.58) |
| Nuts, preferably chestnuts, walnuts, almonds and hazelnuts | ≥4 servings/week | 8.26 (5.84, 11.58) | 9.70 (6.94, 13.40) | 4.32 (2.62, 7.05) | 5.57 (3.49, 8.79) |
| Fatty meat, cured sausage, margarine, butter | ≤4 servings/month | 19.01 (15.29, 23.38) | 28.79 (24.15, 33.91) | 16.43 (12.89, 20.71) | 21.31 (17.07, 26.27) |
| Sweets, pastries, cakes, candies, ice cream | ≤4 servings/month | 33.06 (28.41, 38.07) | 48.18 (42.83, 53.58) | 33.72 (28.93, 38.86) | 44.59 (39.10, 50.22) |
| Sugar-sweetened beverages | ≤4 servings/month | 54.82 (49.66, 59.88) | 67.58 (62.33, 72.41) | 48.99 (43.76, 54.25) | 53.11 (47.49, 58.66) |
| Moderate and vigorous physical activity | ≥60 min/day | 61.71 (56.60, 66.57) | 45.45 (40.15, 50.86) | 60.52 (55.27, 65.53) | 57.70 (52.08, 63.14) |
| Intervention (n = 300) | Controls (n = 294) | Adjusted Mean Difference (95% CI) | p Value | ICC | |||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | ||||
| Total energy, kcal/day | 1945 ± 534 | 1730 ± 478 | 2026 ± 563 | 1907 ± 528 | −152.7 (−242.0, −63.4) | 0.001 | 0.374 |
| Protein, % E | 16.2 ±2.8 | 17.6 ± 3.0 | 16.0 ± 2.6 | 16.9 ± 3.0 | 0.62 (−0.02, 1.26) | 0.060 | 0.471 |
| Total fat, % E | 41.2 ± 6.5 | 39.5 ± 7.2 | 41.3 ± 6.8 | 41.4 ± 7.2 | −1.95 (−3.52, −0.37) | 0.016 | 0.537 |
| Monounsaturated fatty acids, g/day | 43.1 ± 14.9 | 36.6 ± 13.0 | 45.1 ± 14.6 | 41.9 ± 15.4 | −5.10 (−7.87, −2.33) | <0.001 | 0.417 |
| Polyunsaturated fatty acids, g/day | 10.8 ± 4.1 | 9.7 ± 4.5 | 11.3 ± 4.6 | 10.6 ± 4.5 | −0.89 (−1.81, 0.03) | 0.058 | 0.423 |
| Saturated fatty acids, g/day | 27.9 ± 11.4 | 23.1 ± 8.9 | 29.1 ± 11.4 | 28.1 ± 10.6 | −4.47 (−6.22, −2.72) | <0.001 | 0.353 |
| Cholesterol, g/day | 307 ± 132 | 269 ± 104 | 325 ± 151 | 301 ± 115 | −32.4 (−54.4, −10.3) | <0.001 | 0.401 |
| Total carbohydrates, % E | 48.9 ± 8.6 | 50.2 ± 9.6 | 50.0 ± 9.4 | 49.7 ± 9.3 | 0.96 (−1.07, 3.00) | 0.354 | 0.516 |
| Solvable fiber, g/day | 4.3 ± 1.6 | 4.1 ± 1.8 | 4.2 ± 2.0 | 3.9 ± 1.7 | 0.19 (−0.14, 0.52) | 0.260 | 0.474 |
| Unsolvable fiber, g/day | 7.2 ± 3.0 | 7.4 ± 3.8 | 6.9 ± 3.6 | 6.5 ± 3.4 | 0.65 (−0.00, 1.31) | 0.050 | 0.477 |
| Starch, g/day | 96 ± 38 | 87 ± 32 | 106 ± 42 | 99 ± 38 | −9.0 (−15.6, −2.3) | 0.009 | 0.430 |
| Calcium, mg/day | 840 ± 313 | 812 ± 302 | 806 ± 308 | 788 ± 296 | 1.30 (−50.1, 47.5) | 0.958 | 0.331 |
| Iron, mg/day | 13.6 7.0 | 13.5 ± 6.1 | 13.6 ± 6.1 | 13.0 ± 5.7 | 0.48 (−0.51, 1.46) | 0.346 | 0.260 |
| Ascorbic acid, mg/day | 118 59 | 124 ± 62 | 114 ± 61 | 105 ± 62 | 15.9 (3.2, 28.7) | 0.014 | 0.517 |
| Vitamin E, mg/day | 7.4 ± 3.0 | 7.3 ± 3.5 | 7.4 ± 3.6 | 6.9 ± 3.4 | 0.26 (−0.46, 0.98) | 0.472 | 0.489 |
| Beta carotene, µg/day | 1856 (1115, 2899) | 2457 (1490, 4169) | 1794 (1011, 2726) | 1426 (637, 2389) | 1053 (626, 1581) | <0.001 | 0.538 |
| Folic acid, µg/day | 218 (81) | 235 (89) | 207 (75) | 206 (75) | 23.8 (6.9, 40.7) | 0.006 | 0.536 |
| Vitamin B12, µg/day | 4.7 (3.7, 6.6) | 4.6 (3.3, 6.3) | 4.7 (3.6, 7.0) | 4.4 (3.4, 5.9) | −0.03 (−0.53, 0.52) | 0.899 | 0.466 |
| Vitamin B6, g/day | 2.0 ± 0.8 | 2.1 ± 0.7 | 2.1 ± 0.8 | 2.1 ± 0.8 | −0.01 (−0.16, 0.14) | 0.896 | 0.370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Malvar, M.; Benítez-Estévez, A.J.; Sánchez-Castro, J.; Leis, R.; Gude, F. Effects of a Community-Based Behavioral Intervention with a Traditional Atlantic Diet on Cardiometabolic Risk Markers: A Cluster Randomized Controlled Trial (“The GALIAT Study”). Nutrients 2021, 13, 1211. https://doi.org/10.3390/nu13041211
Calvo-Malvar M, Benítez-Estévez AJ, Sánchez-Castro J, Leis R, Gude F. Effects of a Community-Based Behavioral Intervention with a Traditional Atlantic Diet on Cardiometabolic Risk Markers: A Cluster Randomized Controlled Trial (“The GALIAT Study”). Nutrients. 2021; 13(4):1211. https://doi.org/10.3390/nu13041211
Chicago/Turabian StyleCalvo-Malvar, Mar, Alfonso J. Benítez-Estévez, Juan Sánchez-Castro, Rosaura Leis, and Francisco Gude. 2021. "Effects of a Community-Based Behavioral Intervention with a Traditional Atlantic Diet on Cardiometabolic Risk Markers: A Cluster Randomized Controlled Trial (“The GALIAT Study”)" Nutrients 13, no. 4: 1211. https://doi.org/10.3390/nu13041211
APA StyleCalvo-Malvar, M., Benítez-Estévez, A. J., Sánchez-Castro, J., Leis, R., & Gude, F. (2021). Effects of a Community-Based Behavioral Intervention with a Traditional Atlantic Diet on Cardiometabolic Risk Markers: A Cluster Randomized Controlled Trial (“The GALIAT Study”). Nutrients, 13(4), 1211. https://doi.org/10.3390/nu13041211