The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations
Abstract
1. Introduction
2. Methodology
3. Low-Carbohydrate Diet: Definition, Rationale and Patient Selection
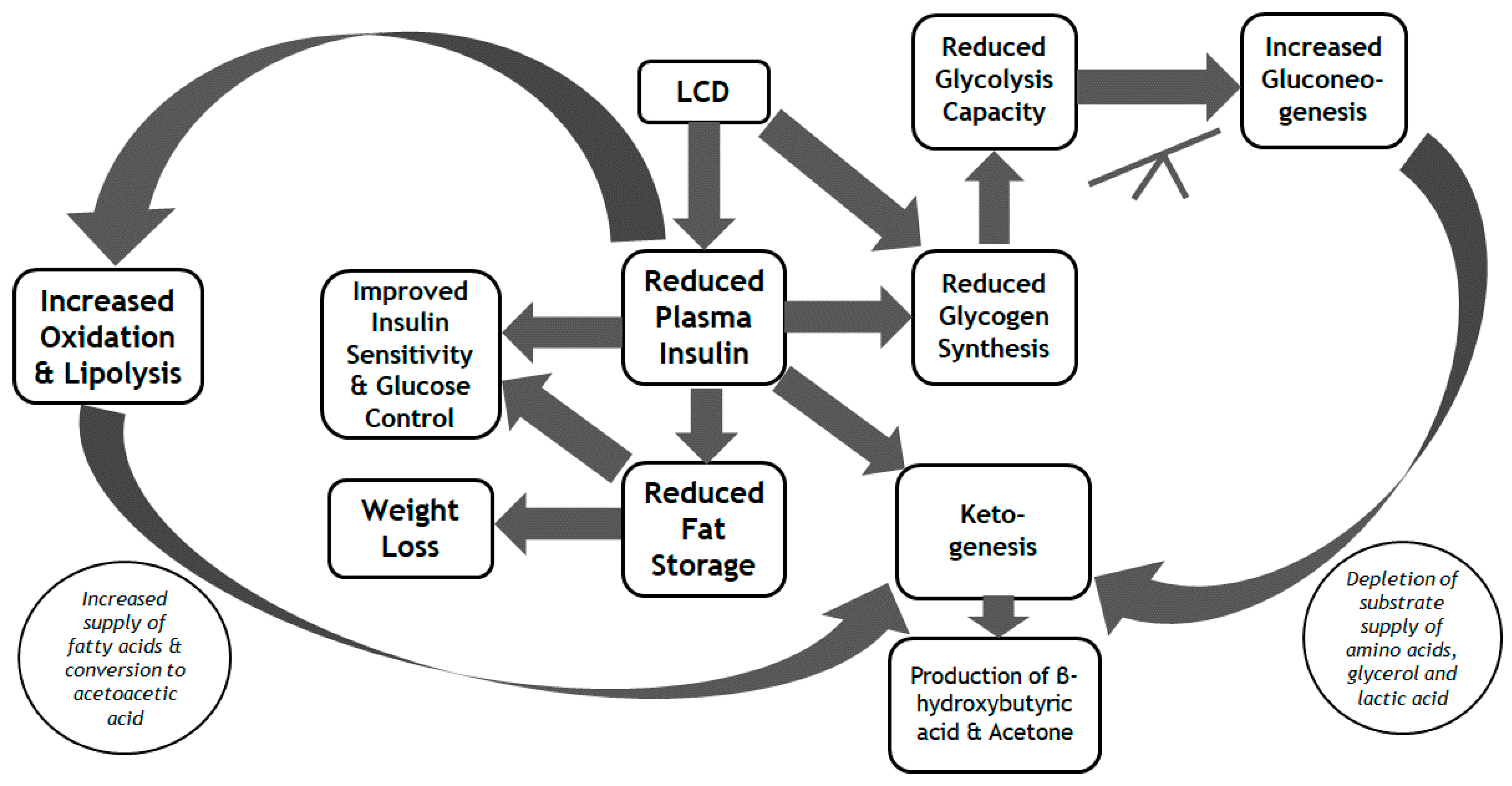
4. Metabolic Efficacy of the LCD
5. Limitations of the LCD
6. Potential Safety Concerns of the LCD
7. Conclusions and Future Directions: LCD in the Context of Healthy Lifestyle
Author Contributions
Funding
Conflicts of Interest
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Saboor Aftab, S.A.; Kumar, S.; Barber, T.M. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clin. Endocrinol. 2013, 78, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Passarello, K.; Kurian, S.; Villanueva, V. Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin. Oncol. Nurs. 2019, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021. [Google Scholar] [CrossRef]
- Wilder, R. The effect on ketonemiaon the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307. [Google Scholar]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: A review of clinical outcomes. Diabet Med. 2019, 36, 326–334. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jonsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Scott, P.M. Which diet is better—Low-fat or low-carb? JAAPA 2006, 19, 49. [Google Scholar] [CrossRef]
- Sampaio, L.P. Ketogenic diet for epilepsy treatment. Arq. Neuropsiquiatr. 2016, 74, 842–848. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52, e7–e11. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.M. How does the ketogenic diet induce anti-seizure effects? Neurosci. Lett. 2017, 637, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.C. Dr. Atkins’ Diet Revolution. Med. Lett. Drugs Ther. 1973, 15, 41–42. [Google Scholar]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate-high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, H.J.; Davis, R.E.; Davies, J.S. A critical review of low-carbohydrate diets in people with Type 2 diabetes. Diabet Med. 2016, 33, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Fukuda, T.; Oyabu, C.; Tanaka, M.; Asano, M.; Yamazaki, M.; Fukui, M. Impact of low-carbohydrate diet on body composition: Meta-analysis of randomized controlled studies. Obes. Rev. 2016, 17, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Meinert Larsen, T.; Harper, A. Atkins and other low-carbohydrate diets: Hoax or an effective tool for weight loss? Lancet 2004, 364, 897–899. [Google Scholar] [CrossRef]
- Hession, M.; Rolland, C.; Kulkarni, U.; Wise, A.; Broom, J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes. Rev. 2009, 10, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014, 312, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S., Jr.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Winwood-Smith, H.S.; Franklin, C.E.; White, C.R. Low-carbohydrate diet induces metabolic depression: A possible mechanism to conserve glycogen. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R347–R356. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Schenk, S.; Barkan, A.L.; Horowitz, J.F. Alterations in carbohydrate metabolism in response to short-term dietary carbohydrate restriction. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E306–E312. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; van de Venne, W.P.V.; Bouten, C.V.; de Graaf, C.; van het Hof, K.H.; Weststrate, J.A. Energy expenditure and physical activity in subjects consuming full-or reduced-fat products as part of their normal diet. Br. J. Nutr. 1996, 76, 785–795. [Google Scholar] [CrossRef]
- Friedman, M.I.; Appel, S. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men: A secondary analysis of energy expenditure and physical activity. PLoS ONE 2019, 14, e0222971. [Google Scholar] [CrossRef] [PubMed]
- Bestard, M.A.; Rothschild, J.A.; Crocker, G.H. Effect of low- and high-carbohydrate diets on swimming economy: A crossover study. J. Int. Soc. Sports Nutr. 2020, 17, 64. [Google Scholar] [CrossRef]
- McSwiney, F.T.; Doyle, L.; Plews, D.J.; Zinn, C. Impact Of Ketogenic Diet On Athletes: Current Insights. Open Access J. Sports Med. 2019, 10, 171–183. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Sitko, S.; Cirer-Sastre, R.; Corbi, F.; Lopez Laval, I. Effects of a low-carbohydrate diet on body composition and performance in road cycling: A randomized, controlled trial. Nutr. Hosp. 2020, 37, 1022–1027. [Google Scholar]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Accurso, A.; Bernstein, R.K.; Dahlqvist, A.; Draznin, B.; Feinman, R.D.; Fine, E.J.; Gleed, A.; Jacobs, D.B.; Larson, G.; Lustig, R.H.; et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: Time for a critical appraisal. Nutr. Metab. 2008, 5, 9. [Google Scholar] [CrossRef]
- Meng, Y.; Bai, H.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2017, 131, 124–131. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Schugar, R.C.; Crawford, P.A. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 374–380. [Google Scholar] [CrossRef]
- Korsmo-Haugen, H.K.; Brurberg, K.G.; Mann, J.; Aas, A.M. Carbohydrate quantity in the dietary management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Okawa, S.; Takahashi, M. The effects on weight loss and gene expression in adipose and hepatic tissues of very-low carbohydrate and low-fat isoenergetic diets in diet-induced obese mice. Nutr. Metab. 2016, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Schlienger, J.L.; Colette, C.; Bonnet, F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2020, 2020, 101192. [Google Scholar] [CrossRef] [PubMed]
- Drummond, S. Obesity: A diet that is acceptable is more likely to succeed. J. Fam. Health Care 2007, 17, 219–221. [Google Scholar] [PubMed]
- Turton, J.L.; Raab, R.; Rooney, K.B. Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS ONE 2018, 13, e0194987. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef]
- Van Elswyk, M.E.; Weatherford, C.A.; McNeill, S.H. A Systematic Review of Renal Health in Healthy Individuals Associated with Protein Intake above the US Recommended Daily Allowance in Randomized Controlled Trials and Observational Studies. Adv. Nutr. 2018, 9, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.F.H.; Pedersen, E.; Schwab, U.; Riserus, U.; Aas, A.M.; Uusitupa, M.; Thanopoulou, A.; Kendall, C.; Sievenpiper, J.L.; Kahleova, H.; et al. The Effects of Different Quantities and Qualities of Protein Intake in People with Diabetes Mellitus. Nutrients 2020, 12, 365. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Micha, R.; Penalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar]
- Hannan, M.T.; Tucker, K.L.; Dawson-Hughes, B.; Cupples, L.A.; Felson, D.T.; Kiel, D.P. Effect of dietary protein on bone loss in elderly men and women: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2000, 15, 2504–2512. [Google Scholar] [CrossRef]
- Shah, P.; Isley, W.L. Ketoacidosis during a low-carbohydrate diet. N. Engl. J. Med. 2006, 354, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O. What dietary modification best improves insulin sensitivity and why? Clin. Endocrinol. 2012, 77, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; van der, A.D.; Spijkerman, A.M.; Grobbee, D.E.; van der Schouw, Y.T. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gogebakan, O.; Bumke-Vogt, C.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am. J. Clin. Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Markova, M.; Seebeck, N.; Loft, A.; Hornemann, S.; Gantert, T.; Kabisch, S.; Herz, K.; Loske, J.; Ost, M.; et al. High-protein diet more effectively reduces hepatic fat than low-protein diet despite lower autophagy and FGF21 levels. Liver Int. 2020, 40, 2982–2997. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e578. [Google Scholar] [CrossRef]
- Koelman, L.; Markova, M.; Seebeck, N.; Hornemann, S.; Rosenthal, A.; Lange, V.; Pivovarova-Ramich, O.; Aleksandrova, K. Effects of High and Low Protein Diets on Inflammatory Profiles in People with Morbid Obesity: A 3-Week Intervention Study. Nutrients 2020, 12, 3636. [Google Scholar] [CrossRef]
- Pivovarova-Ramich, O.; Markova, M.; Weber, D.; Sucher, S.; Hornemann, S.; Rudovich, N.; Raila, J.; Sunaga-Franze, D.; Sauer, S.; Rohn, S.; et al. Effects of diets high in animal or plant protein on oxidative stress in individuals with type 2 diabetes: A randomized clinical trial. Redox Biol. 2020, 29, 101397. [Google Scholar] [CrossRef]
- Clifton, P.M.; Condo, D.; Keogh, J.B. Long term weight maintenance after advice to consume low carbohydrate, higher protein diets--a systematic review and meta analysis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 224–235. [Google Scholar] [CrossRef]
- St Jeor, S.T.; Howard, B.V.; Prewitt, T.E.; Bovee, V.; Bazzarre, T.; Eckel, R.H.; Nutrition Committee of the Council on Nutrition, P.A.; Metabolism of the American Heart, A. Dietary protein and weight reduction: A statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation 2001, 104, 1869–1874. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef]
- Cipryan, L.; Maffetone, P.B.; Plews, D.J.; Laursen, P.B. Effects of a four-week very low-carbohydrate high-fat diet on biomarkers of inflammation: Non-randomised parallel-group study. Nutr. Health 2020, 26, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Myette-Cote, E.; Durrer, C.; Neudorf, H.; Bammert, T.D.; Botezelli, J.D.; Johnson, J.D.; DeSouza, C.A.; Little, J.P. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: A randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1210–R1219. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kani, A.H.; Alavian, S.M.; Esmaillzadeh, A.; Adibi, P.; Haghighatdoost, F.; Azadbakht, L. Effects of a Low-Calorie, Low-Carbohydrate Soy Containing Diet on Systemic Inflammation Among Patients with Nonalcoholic Fatty Liver Disease: A Parallel Randomized Clinical Trial. Horm. Metab. Res. 2017, 49, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Diamond, D.M.; O’Neill, B.J.; Volek, J.S. Low carbohydrate diet: Are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 291–300. [Google Scholar] [CrossRef]
- Weickert, M.O.; Arafat, A.M.; Blaut, M.; Alpert, C.; Becker, N.; Leupelt, V.; Rudovich, N.; Mohlig, M.; Pfeiffer, A.F. Changes in dominant groups of the gut microbiota do not explain cereal-fiber induced improvement of whole-body insulin sensitivity. Nutr. Metab. 2011, 8, 90. [Google Scholar] [CrossRef]
- Weickert, M.O. High fiber intake, dietary protein, and prevention of type 2 diabetes. Expert Rev. Endocrinol. Metab. 2018, 13, 223–224. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Cuomo, R.; Andreozzi, P.; Zito, F.P.; Passananti, V.; De Carlo, G.; Sarnelli, G. Irritable bowel syndrome and food interaction. World J. Gastroenterol. 2014, 20, 8837–8845. [Google Scholar] [PubMed]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Hjorth, M.F.; et al. Fasting Glucose State Determines Metabolic Response to Supplementation with Insoluble Cereal Fibre: A Secondary Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2385. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Meyer, N.M.T.; Honsek, C.; Gerbracht, C.; Dambeck, U.; Kemper, M.; Osterhoff, M.A.; Birkenfeld, A.L.; Arafat, A.M.; Weickert, M.O.; et al. Obesity Does Not Modulate the Glycometabolic Benefit of Insoluble Cereal Fibre in Subjects with Prediabetes-A Stratified Post Hoc Analysis of the Optimal Fibre Trial (OptiFiT). Nutrients 2019, 11, 2726. [Google Scholar] [CrossRef] [PubMed]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef]
- Raffensperger, J.F. The least-cost low-carbohydrate diet is expensive. Nutr. Res. 2008, 28, 6–12. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Oduro-Donkor, D.; Turner, M.C.; Farnaud, S.; Renshaw, D.; Kyrou, I.; Hanson, P.; Hattersley, J.; Weickert, M.O.; Menon, V.; Randeva, H.S.; et al. Modification of fecal microbiota as a mediator of effective weight loss and metabolic benefits following bariatric surgery. Expert Rev. Endocrinol. Metab. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Estruch, R.; Corella, D.; Fito, M.; Ros, E.; Predimed, I. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Look, A.R.G. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity (Silver Spring) 2014, 22, 5–13. [Google Scholar]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
| Safety Concern | Nature of the Problem | Clinical Sequelae |
|---|---|---|
| Nutritional Deficiencies | Reduced dietary intake of fibre, minerals, vitamins, trace elements and PUFA [12]; Depleted glycogen stores from restricted carbohydrate intake [43] | Increased mortality from restricted fibre, essential micronutrients and PUFA [48,49,50]; Dyslipidaemia [12]; Bone health and renal calculi [12]; Hypoglycaemia (T1D) |
| Ketosis | Short-term gastro-intestinal symptoms [12]; Longer-term effects incompletely understood [11] Released Calcium Stores [11] | Vomiting, Diarrhoea and Obstipation; Gastrointestinal reflux [12]; Theoretical Nephrolithiasis and Osteoporosis [11]; Ketoacidosis (rare) [52] |
| High-Protein Diet | Epidemiological studies show association with dysglycaemia and unfavourable metabolic effects [53,54]; Interventional studies show metabolic benefits [55,56,57,58,59] | Impaired GFR in women with mild renal impairment [11,45,46,47]; Metabolic benefits likely mediated by associated lifestyle changes in interventional studies [60] |
| Hyperuricaemia | Theoretical risk of excessive conversion of purines from animal proteins [11,61] | Gouty arthritis Uric acid nephrolithiasis |
| Inflammatory Status | Theoretical promotion of inflammatory effects from a relative increase in dietary saturated fat intake | Evidence is contradictory Data show either no appreciable effect [63] or improvement of inflammatory status [64,65,66,67] in response to the LCD |
| Mental and Emotional Status | Central role of food and eating within our society | Potential lowering of mood and negative impact on relationships |
| Ecological and Ethical Concerns | Environmental effects of soy bean and meat production and deforestation | Health implications of climate change |
| Financial Implications | Increased expense of the LCD [77], and disproportionate financial effects on lower socio-economic groups (reduced affordability and feasibility) | Health implications of financially restricted diet to those groups most likely to benefit from dietary change |
| Dysbiosis | Relative increase in dietary intake of fats and protein, with a deficiency in dietary fibre intake [78] | Appetitive, immunomodulatory, inflammatory and dysmetabolic sequelae; Impact on mental health and wellbeing [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, T.M.; Hanson, P.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations. Nutrients 2021, 13, 1187. https://doi.org/10.3390/nu13041187
Barber TM, Hanson P, Kabisch S, Pfeiffer AFH, Weickert MO. The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations. Nutrients. 2021; 13(4):1187. https://doi.org/10.3390/nu13041187
Chicago/Turabian StyleBarber, Thomas M., Petra Hanson, Stefan Kabisch, Andreas F. H. Pfeiffer, and Martin O. Weickert. 2021. "The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations" Nutrients 13, no. 4: 1187. https://doi.org/10.3390/nu13041187
APA StyleBarber, T. M., Hanson, P., Kabisch, S., Pfeiffer, A. F. H., & Weickert, M. O. (2021). The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy Versus Longer-Term Limitations. Nutrients, 13(4), 1187. https://doi.org/10.3390/nu13041187







