Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia
Abstract
1. Introduction
2. Functional Dyspepsia Presentation and Shared Care
2.1. Presenting Symptoms and Medical History Related to Dietary Management of Functional Dyspepsia
2.2. Availability, Access and Referral to Specialist FD Dietetic Services
3. Relationship between FD and Eating
3.1. Does Eating Induce or Relieve FD Symptoms?
3.2. Are Symptoms or Test Results Consistent with Delaying Gastric Emptying or Impaired Gastric Accommodation?
4. Nutrient-Specific Dietary Management of FD
4.1. Are Epigastric Symptoms Attributable to Specific Macronutrient/s?
4.2. Do Specific Carbohydrates Induce FD Symptoms or Does Removal of Specific Carbohydrates Alleviate Symptoms?
4.2.1. Dietary Carbohydrate Modification Based on Diagnostic Tests
4.2.2. Carbohydrates and Small Intestinal Bacterial Overgrowth
4.2.3. Carbohydrate and Fibre Considerations in Dietetic management of FD
4.3. Do Specific Food Proteins Induce FD Symptoms or Does Removal of Specific Proteins Alleviate Symptoms?
4.4. Do Specific Dietary Fats or Total Intake of Fats Induce or Alleviate FD Symptoms
4.5. Anti-inflammatory Approach to FD Dietary Management
5. Micronutrients and Additives in FD
5.1. Natural Food Chemicals
5.2. Food Additives in FD Aetiology and Symptom Induction
6. Dietary Influences on Microbiota in FD
7. Complementary Therapies and Micronutrient Supplementation in Dietary Management of FD
8. FD that is Unresponsive to Dietary Management
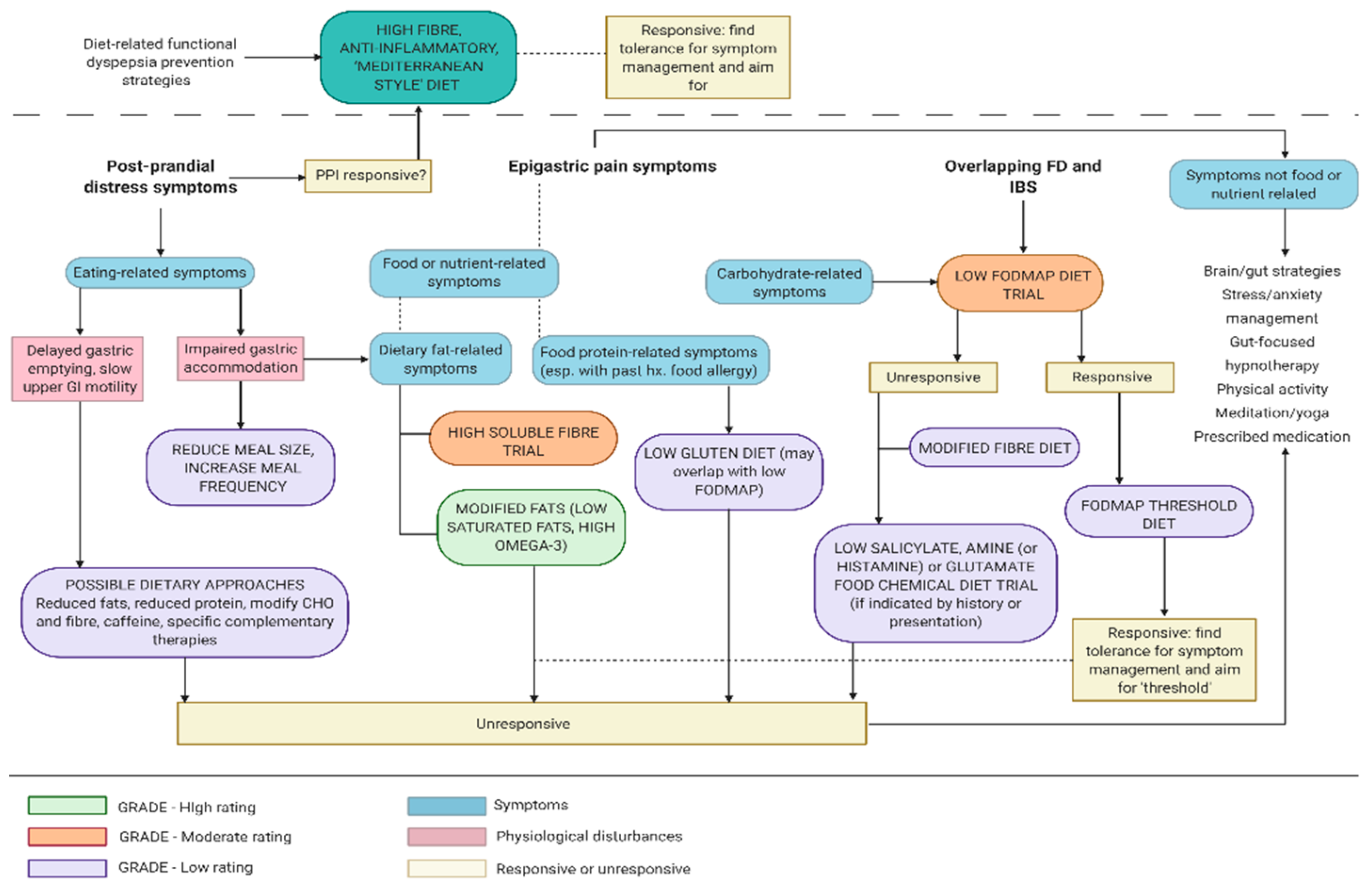
9. Guidance on FD Dietetic Management based on Existing Research
9.1. Establishing Therapeutic Dietary Management Relationship
9.2. Carbohydrate, FODMAP and Fibre-Focused Dietary Management of Functional Dyspepsia
9.3. Protein, ‘Leaky Gut’ and Immune System Approaches to FD Dietary Management
9.4. Dietary Fats and ‘Anti-Inflammatory’ Diet in FD Management
9.5. Delineation of Roles in FD Dietary Education and Advice
9.6. Training in FGID Dietary Management
10. Conclusions
Funding
Conflicts of Interest
References
- Koloski, N.A.; Talley, N.J.; Boyce, P.M. Epidemiology and health care seeking in the functional GI disorders: A population-based study. Am. J. Gastroenterol. 2002, 97, 2290–2299. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional dyspepsia. Lancet 2020. [Google Scholar] [CrossRef]
- Talley, N.J.; Walker, M.M.; Aro, P.; Ronkainen, J.; Storskrubb, T.; Hindley, L.A.; Harmsen, W.S.; Zinsmeister, A.R.; Agréus, L. Non-ulcer dyspepsia and duodenal eosinophilia: An adult endoscopic population-based case-control study. Clin. Gastroenterol. Hepatol. 2007, 5, 1175–1183. [Google Scholar] [CrossRef]
- De Bortoli, N.; Tolone, S.; Frazzoni, M.; Martinucci, I.; Sgherri, G.; Albano, E.; Ceccarelli, L.; Stasi, C.; Bellini, M.; Savarino, V. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: Common overlapping gastrointestinal disorders. Ann. Gastroenterol. 2018, 31, 639. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Aro, P.; Walker, M.M.; Agréus, L.; Johansson, S.E.; Jones, M.; Talley, N.J. Duodenal eosinophilia is associated with functional dyspepsia and new onset gastro-oesophageal reflux disease. Aliment. Pharm. Ther. 2019, 50, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Vicario, M.; Ramos, L.; Lobo, B.; Mosquera, J.L.; Alonso, C.; Sánchez, A.; Guilarte, M.; Antolín, M.; de Torres, I.; et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am. J. Gastroenterol. 2012, 107, 736–746. [Google Scholar] [CrossRef]
- Allen, A.; Flemström, G. Gastroduodenal mucus bicarbonate barrier: Protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 2005, 288, C1–C19. [Google Scholar] [CrossRef]
- Seidler, U.E. Gastrointestinal HCO3- transport and epithelial protection in the gut: New techniques, transport pathways and regulatory pathways. Curr. Opin. Pharm. 2013, 13, 900–908. [Google Scholar] [CrossRef]
- Rønnestad, I.; Akiba, Y.; Kaji, I.; Kaunitz, J.D. Duodenal luminal nutrient sensing. Curr Opin Pharm. 2014, 19, 67–75. [Google Scholar] [CrossRef]
- Grundy, D.; Al–Chaer, E.D.; Aziz, Q.; Collins, S.M.; Ke, M.; Taché, Y.; Wood, J.D. Fundamentals of Neurogastroenterology: Basic Science. Gastroenterology 2006, 130, 1391–1411. [Google Scholar] [CrossRef]
- Tortora, G.J.; Derrickson, B.H. Principles of Anatomy and Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Burns, G.; Carroll, G.; Mathe, A.; Horvat, J.; Foster, P.; Walker, M.M.; Talley, N.J.; Keely, S. Evidence for local and systemic immune activation in functional dyspepsia and the irritable bowel syndrome: A systematic review. Am. J. Gastroenterol. 2019, 114, 429–436. [Google Scholar] [CrossRef]
- Cirillo, C.; Bessissow, T.; Desmet, A.-S.; Vanheel, H.; Tack, J.; Berghe, P.V. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am. J. Gastroenterol. 2015, 110, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Palsson, O.S.; Törnblom, H.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 2018, 3, 252–262. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Gururatsakul, M.; Pilkington, K.R.; Brierley, S.M.; Blackshaw, A.L.; Gerken, G.; Talley, N.J.; Holtmann, G. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am. J. Gastroenterol. 2011, 106, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P. The low-FODMAP diet in the management of functional dyspepsia in East and Southeast Asia. J. Gastroenterol. Hepatol. 2017, 32, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, K.; Talley, N.; Walker, M.; Burrows, T. Food and functional dyspepsia: A systematic review. J. Hum. Nutr. Diet. 2018, 31, 390–407. [Google Scholar] [CrossRef]
- Talley, N. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol. Motil. 2008, 20, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Ferch, C.; Shaw, M.; Chey, W.D. Use of dietary management in irritable bowel syndrome: Results of a survey of over 1500 United States gastroenterologists. J. Neurogastroenterol. Motil. 2018, 24, 437. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e65. [Google Scholar] [CrossRef] [PubMed]
- Hookway, C.; Buckner, S.; Crosland, P.; Longson, D. Irritable bowel syndrome in adults in primary care: Summary of updated NICE guidance. BMJ 2015, 350, h701. [Google Scholar] [CrossRef]
- Mutsekwa, R.N.; Larkins, V.; Canavan, R.; Ball, L.; Angus, R.L. A dietitian-first gastroenterology clinic results in improved symptoms and quality of life in patients referred to a tertiary gastroenterology service. Clin. Nutr. Espen 2019, 33, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Corsello, A.; Pugliese, D.; Gasbarrini, A.; Armuzzi, A. Diet and Nutrients in Gastrointestinal Chronic Diseases. Nutrients 2020, 12, 2693. [Google Scholar] [CrossRef]
- Enck, P.; Azpiroz, F.; Boeckxstaens, G.; Elsenbruch, S.; Feinle-Bisset, C.; Holtmann, G.; Lackner, J.M.; Ronkainen, J.; Schemann, M.; Stengel, A. Functional dyspepsia. Nat. Rev. Dis. Primers 2017, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Futagami, S.; Itoh, T.; Sakamoto, C. Systematic review with meta-analysis: Post-infectious functional dyspepsia. Aliment. Pharm. Ther. 2015, 41, 177–188. [Google Scholar] [CrossRef]
- Basnayake, C.; Kamm, M.A.; Stanley, A.; Wilson-O’Brien, A.; Burrell, K.; Lees-Trinca, I.; Khera, A.; Kantidakis, J.; Wong, O.; Fox, K. Standard gastroenterologist versus multidisciplinary treatment for functional gastrointestinal disorders (MANTRA): An open-label, single-centre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020, 5, 890–899. [Google Scholar] [CrossRef]
- Nickles, M.A.; Hasan, A.; Shakhbazova, A.; Wright, S.; Chambers, C.J.; Sivamani, R.K. Alternative Treatment Approaches to Small Intestinal Bacterial Overgrowth: A Systematic Review. J. Altern. Complementary Med. 2020, 27, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Reed, D.E.; Muir, J.G.; Vanner, S.J. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterol. Motil. 2020, 32, e13730. [Google Scholar] [CrossRef]
- Farrall, E.; Collins, J.; Turnbull, D.A.; Holtmann, G.; Hetzel, D.J.; Andrews, J.M. Do We Know What Patients Want? the Communication/Understanding Gap Between Patients with Functional Gastrointestinal Disorders (FGIDs) and Gastroenterologists. Gastroenterology 2009, 7, 1252–1254.e2. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e1262. [Google Scholar] [CrossRef]
- Wauters, L.; Ceulemans, M.; Frings, D.; Lambaerts, M.; Accarie, A.; Toth, J.; Mols, R.; Augustijns, P.; De Hertogh, G.; Van Oudenhove, L. Proton pump inhibitors reduce duodenal eosinophilia, mast cells and permeability in patients with functional dyspepsia. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Yuan, Y.; Bercik, P.; Moayyedi, P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst. Rev. 2017, 8, CD011194. [Google Scholar]
- Potter, M.D.; Wood, N.K.; Walker, M.M.; Jones, M.P.; Talley, N.J. Proton pump inhibitors and suppression of duodenal eosinophilia in functional dyspepsia. Gut 2019, 68, 1339–1340. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Feinle-Bisset, C. Upper gastrointestinal sensitivity to meal-related signals in adult humans–relevance to appetite regulation and gut symptoms in health, obesity and functional dyspepsia. Physiol. Behav. 2016, 162, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Tome, D.; Schwarz, J.; Darcel, N.; Fromentin, G. Protein, amino acids, vagus nerve signaling, and the brain. Am. J. Clin. Nutr. 2009, 90, 838S–843S. [Google Scholar] [CrossRef]
- Masuy, I.; Van Oudenhove, L.; Tack, J.; Biesiekierski, J. Effect of intragastric FODMAP infusion on upper gastrointestinal motility, gastrointestinal, and psychological symptoms in irritable bowel syndrome vs healthy controls. Neurogastroenterol. Motil. 2018, 30, e13167. [Google Scholar] [CrossRef]
- Keshteli, A.; Haghighatdoost, F.; Azadbakht, L.; Daghaghzadeh, H.; Feinle-Bisset, C.; Afshar, H.; Feizi, A.; Esmaillzadeh, A.; Adibi, P. Dietary glycaemic index and glycaemic load and upper gastrointestinal disorders: Results from the SEPAHAN study. J. Hum. Nutr. Diet. 2017, 30, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Chedid, V.; Ford, A.C.; Haruma, K.; Horowitz, M.; Jones, K.L.; Low, P.A.; Park, S.-Y.; Parkman, H.P.; Stanghellini, V. Gastroparesis. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D. The role of food in the functional gastrointestinal disorders: Introduction to a manuscript series. Am. J. Gastroenterol. 2013, 108, 694–697. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Dietary Guidelines; NHMRC, Ed.; Australian Government: Canberra, Australia, 2013.
- Shepherd, S.J.; Lomer, M.C.; Gibson, P.R. Short-chain carbohydrates and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.; Vanner, S. Dietary therapies for functional bowel symptoms: Recent advances, challenges, and future directions. Neurogastroenterol. Motil. 2018, 30, e13238. [Google Scholar] [CrossRef]
- Staudacher, H.M. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 16–19. [Google Scholar] [CrossRef]
- Potter, M.D.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat intolerance and chronic gastrointestinal symptoms in an Australian population-based study: Association between wheat sensitivity, celiac disease and functional gastrointestinal disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Duodenal chemosensing of short-chain fatty acids: Implications for GI diseases. Curr. Gastroenterol. Rep. 2019, 21, 35. [Google Scholar] [CrossRef]
- Pittayanon, R.; Yuan, Y.; Bollegala, N.P.; Khanna, R.; Lacy, B.E.; Andrews, C.N.; Leontiadis, G.I.; Moayyedi, P. Prokinetics for functional dyspepsia: A systematic review and meta-analysis of randomized control trials. Am. J. Gastroenterol. 2019, 114, 233–243. [Google Scholar] [CrossRef]
- Puntis, J.; Zamvar, V. Congenital sucrase–isomaltase deficiency: Diagnostic challenges and response to enzyme replacement therapy. Arch. Dis. Child. 2015, 100, 869–871. [Google Scholar] [CrossRef]
- Kim, S.B.; Calmet, F.H.; Garrido, J.; Garcia-Buitrago, M.T.; Moshiree, B. Sucrase-isomaltase deficiency as a potential masquerader in irritable bowel syndrome. Dig. Dis. Sci. 2020, 65, 534–540. [Google Scholar] [CrossRef]
- Tziatzios, G.; Giamarellos-Bourboulis, E.J.; Papanikolaou, I.S.; Pimentel, M.; Dimitriadis, G.D.; Triantafyllou, K. Is small intestinal bacterial overgrowth involved in the pathogenesis of functional dyspepsia? Med. Hypotheses 2017, 106, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Bhagatwala, J. Small intestinal bacterial overgrowth: Clinical features and therapeutic management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Pimentel, M.; Rao, S.S. How to test and treat small intestinal bacterial overgrowth: An evidence-based approach. Curr. Gastroenterol. Rep. 2016, 18, 8. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Pryor, J.; Burns, G.L.; Duncanson, K.; Horvat, J.C.; Walker, M.M.; Talley, N.J.; Keely, S. Functional Dyspepsia and Food: Immune Overlap with Food Sensitivity Disorders. Curr. Gastroenterol. Rep. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Boettcher, E.; Crowe, S.E. Dietary proteins and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 728–736. [Google Scholar] [CrossRef]
- Nejad, A.S.; MacGlashan, D.W., Jr. Dependence of Optimal Histamine Release on Cell Surface IgE Density on Human Basophils: Nature of the Stimulus. Int. Arch. Allergy Immunol. 2018, 177, 181–191. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, T.; Mösinger, M.; Ruchay, Z.; Röcken, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, D. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019, 157, 109–118.e105. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Oliveira, M.; Alegre, I.; Vinas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. Int. J. Food Microbiol. 2008, 123, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Massier, L.; Chakaroun, R.; Kovacs, P.; Heiker, J.T. Blurring the picture in leaky gut research: How shortcomings of zonulin as a biomarker mislead the field of intestinal permeability. Gut 2020. [Google Scholar] [CrossRef]
- Rao, R.; Samak, G. Role of glutamine in protection of intestinal epithelial tight junctions. J. Epithel. Biol. Pharmacol. 2012, 5, 47. [Google Scholar]
- Klimberg, V.; Souba, W. The importance of intestinal glutamine metabolism in maintaining a healthy gastrointestinal tract and supporting the body’s response to injury and illness. Surg. Annu. 1990, 22, 61–76. [Google Scholar] [PubMed]
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019, 68, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; Kater, C.E. Adrenal fatigue does not exist: A systematic review. Bmc Endocr. Disord. 2016, 16, 48. [Google Scholar]
- Potter, M.D.; Duncanson, K.; Jones, M.P.; Walker, M.M.; Keely, S.; Talley, N.J. Wheat Sensitivity and Functional Dyspepsia: A Pilot, Double-Blind, Randomized, Placebo-Controlled Dietary Crossover Trial with Novel Challenge Protocol. Nutrients 2020, 12, 1947. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nakae, H.; Matsuoka, T.; Takahashi, S.; Hisada, T.; Tomita, J.; Koga, Y. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol. 2017, 4. [Google Scholar] [CrossRef]
- Beeckmans, D.; Farré, R.; Riethorst, D.; Keita, Å.V.; Augustijns, P.; Söderholm, J.D.; Vanuytsel, T.; Vanheel, H.; Tack, J. Relationship between bile salts, bacterial translocation, and duodenal mucosal integrity in functional dyspepsia. Neurogastroenterol. Motil. 2020, 32, e13788. [Google Scholar] [CrossRef]
- Feinle-Bisset, C.; Azpiroz, F. Dietary lipids and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 737–747. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 1–9. [Google Scholar] [CrossRef]
- Zhong, L.; Shanahan, E.R.; Raj, A.; Koloski, N.A.; Fletcher, L.; Morrison, M.; Walker, M.M.; Talley, N.J.; Holtmann, G. Dyspepsia and the microbiome: Time to focus on the small intestine. Gut 2017, 66, 1168–1169. [Google Scholar] [CrossRef] [PubMed]
- Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. Adherence to the pro-inflammatory diet in relation to prevalence of irritable bowel syndrome. Nutr. J. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keshteli, A.H.; Madsen, K.; Nickurak, C.; Kroeker, K.; Mandal, R.; Wishart, D.S.; van Zanten, S.V.; Halloran, B.P.; Fedorak, R.N.; Valcheva, R. Adherence to an Anti-Inflammatory Diet Prevents Increases in Colonic Inflammation in Ulcerative Colitis Patients in Remission. Gastroenterology 2017, 152, S599. [Google Scholar] [CrossRef]
- Hodge, L.; Swain, A.; Faulkner-Hogg, K. Food allergy and intolerance. Aust. Fam. Physician 2009, 38, 705. [Google Scholar]
- Malakar, S. Bioactive food chemicals and gastrointestinal symptoms: A focus of salicylates. J. Gastroenterol. Hepatol. 2017, 32, 73–77. [Google Scholar] [CrossRef]
- Skypala, I.J.; Williams, M.; Reeves, L.; Meyer, R.; Venter, C. Sensitivity to food additives, vaso-active amines and salicylates: A review of the evidence. Clin. Transl. Allergy 2015, 5, 34. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Sampson, H.A.; Simon, R.A. Food Allergy: Adverse Reactions to Foods and Food Additives; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gibson, P.R.; Varney, J.; Malakar, S.; Muir, J.G. Food components and irritable bowel syndrome. Gastroenterology 2015, 148, 1158–1174.e1154. [Google Scholar] [CrossRef]
- Vojdani, A.; Gushgari, L.R.; Vojdani, E. Interaction between food antigens and the immune system: Association with autoimmune disorders. Autoimmun. Rev. 2020, 19, 102459. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Additives and Processing Aids. Available online: https://www.foodstandards.gov.au/consumer/additives/Pages/default.aspx (accessed on 31 December 2020).
- Schnabel, L.; Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Monteiro, C.A.; Hercberg, S.; Benamouzig, R. Association between ultra-processed food consumption and functional gastrointestinal disorders: Results from the French NutriNet-Santé cohort. Am. J. Gastroenterol. 2018, 113, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D. Re-evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA J. 2017, 15, e04910. [Google Scholar]
- Afonso, A.; Garcia Matas, R.; Germini, A.; Merten, C.; Robinson, T. EFSA’s Activities on Emerging Risks in 2015. 2016; Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2016.EN-1100 (accessed on 1 January 2021). [CrossRef]
- Lerner, A.; Matthias, T. Microbial transglutaminase is beneficial to food industries but a caveat to public health. Med. ONE 2019, 4, e190001. [Google Scholar]
- Elli, L.; Bergamini, C.; Bardella, M.; Schuppan, D. Transglutaminases in inflammation and fibrosis of the gastrointestinal tract and the liver. Dig. Liver Dis. 2009, 41, 541–550. [Google Scholar] [CrossRef]
- Spencer, M.; Gupta, A.; Van Dam, L.; Shannon, C.; Menees, S.; Chey, W.D. Artificial sweeteners: A systematic review and primer for gastroenterologists. J. Neurogastroenterol. Motil. 2016, 22, 168. [Google Scholar] [CrossRef]
- Bryant, C.; Mclaughlin, J. Low calorie sweeteners: Evidence remains lacking for effects on human gut function. Physiol. Behav. 2016, 164, 482–485. [Google Scholar] [CrossRef]
- Shanahan, E.; Zhong, L.; Talley, N.; Morrison, M.; Holtmann, G. Characterisation of the gastrointestinal mucosa-associated microbiota: A novel technique to prevent cross-contamination during endoscopic procedures. Aliment. Pharm. Ther. 2016, 43, 1186–1196. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2015, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Tsuda, A.; Matsuoka, T.; Mine, T.; Koga, Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol. 2016, 1, e000109. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Mizushima, K.; Inada, Y.; Inoue, K.; Harusato, A.; Dohi, O. Higher levels of Streptococcus in upper gastrointestinal mucosa associated with symptoms in patients with functional dyspepsia. Digestion 2020, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Paula, H.; Grover, M.; Halder, S.L.; Locke, G.R., 3rd; Schleck, C.D.; Zinsmeister, A.R.; Talley, N.J. Non-enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neurogastroenterol. Motil. 2015, 27, 1580–1586. [Google Scholar] [CrossRef]
- Villarreal, A.A.; Aberger, F.J.; Benrud, R.; Gundrum, J.D. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ 2012, 111, 17–20. [Google Scholar] [PubMed]
- Tan, V.P.; Liu, K.S.; Lam, F.Y.; Hung, I.F.; Yuen, M.F.; Leung, W.K. Randomised clinical trial: Rifaximin versus placebo for the treatment of functional dyspepsia. Aliment. Pharmacol. Ther. 2017, 1, 767–776. [Google Scholar] [CrossRef]
- Fransen, F.; Sahasrabudhe, N.M.; Elderman, M.; Bosveld, M.; El Aidy, S.; Hugenholtz, F.; Borghuis, T.; Kousemaker, B.; Winkel, S.; van der Gaast-de Jongh, C. β2→ 1-fructans modulate the immune system in vivo in a microbiota-dependent and-independent fashion. Front. Immunol. 2017, 8, 154. [Google Scholar] [CrossRef]
- Takagi, A.; Yanagi, H.; Ozawa, H.; Uemura, N.; Nakajima, S.; Inoue, K.; Kawai, T.; Ohtsu, T.; Koga, Y. Effects of Lactobacillus gasseri OLL2716 on Helicobacter pylori-Associated Dyspepsia: A Multicenter Randomized Double-Blind Controlled Trial. Gastroenterol. Res. Pract. 2016, 2016, 7490452. [Google Scholar] [CrossRef]
- Ohtsu, T.; Takagi, A.; Uemura, N.; Inoue, K.; Sekino, H.; Kawashima, A.; Uchida, M.; Koga, Y. The Ameliorating Effect of Lactobacillus gasseri OLL2716 on Functional Dyspepsia in Helicobacter pylori-Uninfected Individuals: A Randomized Controlled Study. Digestion 2017, 96, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Microba. Understanding the Role of Gut Microbiome Analysis in Clinical Practice. 2019. Available online: https://insight.microba.com/healthcare/ (accessed on 10 March 2021).
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y. American Gut: An open platform for citizen science microbiome research. Msystems 2018, 3, e00031-18. [Google Scholar] [CrossRef]
- Rasool, S.; Abid, S.; Iqbal, M.P.; Mehboobali, N.; Haider, G.; Jafri, W. Relationship between vitamin B 12, folate and homocysteine levels and H. Pylori infection in patients with functional dyspepsia: A cross-section study. BMC Res. Notes 2012, 5, 206. [Google Scholar] [CrossRef]
- May, B.; Köhler, S.; Schneider, B. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Aliment. Pharm. Ther. 2000, 14, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Cargiolli, M.; Cassarano, S.; Polese, B.; De Conno, B.; Aurino, L.; Mancino, N.; Sarnelli, G. Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World J. Gastroenterol. 2020, 26, 456–465. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Dorairaj, R.; Sali, A. Herbal formula improves upper and lower gastrointestinal symptoms and gut health in Australian adults with digestive disorders. Nutr. Res. 2020, 76, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Monash FODMAP. Monash FODMAP app; Monash FODMAP: Melbourne, Australia, 2019. [Google Scholar]
- Holtmann, G.; Schrenk, D.; Madisch, A.; Allescher, H.D.; Ulrich-Merzenich, G.; Mearin, F.; Larrey, D.; Malfertheiner, P. Use of evidence-based herbal medicines for patients with functional gastrointestinal disorders: A conceptional framework for risk-benefit assessment and regulatory approaches. Dig. Dis. 2020, 38, 269–279. [Google Scholar] [CrossRef]
- Rodiño-Janeiro, B.K.; Alonso-Cotoner, C.; Pigrau, M.; Lobo, B.; Vicario, M.; Santos, J. Role of corticotropin-releasing factor in gastrointestinal permeability. J. Neurogastroenterol. Motil. 2015, 21, 33. [Google Scholar] [CrossRef]
- Calvert, E.L.; Houghton, L.A.; Cooper, P.; Morris, J.; Whorwell, P.J. Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology 2002, 123, 1778–1785. [Google Scholar] [CrossRef]
- Haag, S.; Senf, W.; Tagay, S.; Langkafel, M.; Braun-Lang, U.; Pietsch, A.; Heuft, G.; Talley, N.; Holtmann, G. Is there a benefit from intensified medical and psychological interventions in patients with functional dyspepsia not responding to conventional therapy? Aliment. Pharm. Ther. 2007, 25, 973–986. [Google Scholar] [CrossRef]
- Ljótsson, B.; Andréewitch, S.; Hedman, E.; Rück, C.; Andersson, G.; Lindefors, N. Exposure and mindfulness based therapy for irritable bowel syndrome–an open pilot study. J. Behav. Ther. Exp. Psychiatry 2010, 41, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sebastián Sánchez, B.; Gil Roales-Nieto, J.; Ferreira, N.B.; Gil Luciano, B.; Sebastián Domingo, J.J. New psychological therapies for irritable bowel syndrome: Mindfulness, acceptance and commitment therapy (ACT). Rev. Española De Enferm. Dig. 2017, 109, 648–657. [Google Scholar] [CrossRef]
- Edebol-Carlman, H.; Ljótsson, B.; Linton, S.J.; Boersma, K.; Schrooten, M.; Repsilber, D.; Brummer, R.J. Face-to-face cognitive-behavioral therapy for irritable bowel syndrome: The effects on gastrointestinal and psychiatric symptoms. Gastroenterol. Res. Pract. 2017, 2017, 8915872. [Google Scholar] [CrossRef] [PubMed]
- Van Oudenhove, L.; Aziz, Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 158–167. [Google Scholar] [CrossRef]
- Duncanson, K.; Burrows, T.; Keely, S.; Potter, M.; Das, G.; Walker, M.; Talley, N.J. The Alignment of Dietary Intake and Symptom-Reporting Capture Periods in Studies Assessing Associations between Food and Functional Gastrointestinal Disorder Symptoms: A Systematic Review. Nutrients 2019, 11, 2590. [Google Scholar] [CrossRef] [PubMed]
- Boushey, C.; Spoden, M.; Zhu, F.; Delp, E.; Kerr, D. New mobile methods for dietary assessment: Review of image-assisted and image-based dietary assessment methods. Proc. Nutr. Soc. 2017, 76, 283–294. [Google Scholar] [CrossRef]
- De Lourdes Samaniego-Vaesken, M.; Partearroyo, T.; Cano, A.; Urrialde, R.; Varela-Moreiras, G. Novel database of declared low-and no-calorie sweeteners from foods and beverages available in Spain. J. Food Compos. Anal. 2019, 82, 103234. [Google Scholar] [CrossRef]
- Linedale, E.C.; Shahzad, M.A.; Kellie, A.R.; Mikocka-Walus, A.; Gibson, P.R.; Andrews, J.M. Referrals to a tertiary hospital: A window into clinical management issues in functional gastrointestinal disorders. JGH Open 2017, 1, 84–91. [Google Scholar] [CrossRef]
| Dietary Strategy --------------------------- Considerations and Predisposing Or Risk Factors | Regular Small Meals | Modified Texture | Reduced Dietary fat | Anti-inflammatory Diet | Reduced Protein | Gluten Free Diet | Modified Carbohydrate | Low FODMAP Trial | High Soluble Fibre | Reduced Fibre | Low Chemical Diet | Low Food Additives | Probiotic Supplements | Prebiotic Supplements | Complementary Therapies | Reduced Caffeine |
| Predisposing or risk factors for FD | ||||||||||||||||
| Suspected duodenal microbiota alterations |  |  |  |  |  |  |  | |||||||||
| Small intestinal bacterial overgrowth |  |  |  | |||||||||||||
| Suspected immune or allergy-like response |  |  |  | |||||||||||||
| Intestinal permeability | .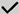 | 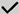 | ? L-glutamine | 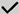 | 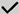 | 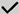 | 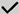 | |||||||||
| Bile acid involvement |  |  | ||||||||||||||
| FD symptom-related | ||||||||||||||||
| Delayed gastric emptying and/or impaired accommodation |  |  |  | . |  | Soluble | Ginger Iberogast |  | ||||||||
| Early satiety | 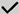 | 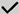 | 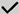 | 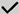 | 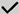 | |||||||||||
| Wheat-induced symptoms |  |  |  |  | ||||||||||||
| Pain | 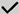 | 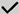 | 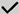 | Polyols | 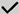 | 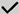 | ||||||||||
| Co-presenting extraintestinal symptoms (Atopy, migraine) |  |  |  | |||||||||||||
| Post prandial distress |  |  |  |  |  |  | ||||||||||
 dietary management approach suited to symptom or risk factor. ? dietary management option to consider (very limited evidence or only suitable for a subset of patients).
dietary management approach suited to symptom or risk factor. ? dietary management option to consider (very limited evidence or only suitable for a subset of patients).Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncanson, K.; Burns, G.; Pryor, J.; Keely, S.; Talley, N.J. Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia. Nutrients 2021, 13, 1109. https://doi.org/10.3390/nu13041109
Duncanson K, Burns G, Pryor J, Keely S, Talley NJ. Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia. Nutrients. 2021; 13(4):1109. https://doi.org/10.3390/nu13041109
Chicago/Turabian StyleDuncanson, Kerith, Grace Burns, Jennifer Pryor, Simon Keely, and Nicholas J. Talley. 2021. "Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia" Nutrients 13, no. 4: 1109. https://doi.org/10.3390/nu13041109
APA StyleDuncanson, K., Burns, G., Pryor, J., Keely, S., & Talley, N. J. (2021). Mechanisms of Food-Induced Symptom Induction and Dietary Management in Functional Dyspepsia. Nutrients, 13(4), 1109. https://doi.org/10.3390/nu13041109








