Qualitative Nitrogen Malnutrition Damages Gut and Alters Microbiome in Adult Mice. A Preliminary Histopathological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Mice
2.2. Samples Collections
2.3. Histology
2.4. Immunohistochemistry
2.5. Microbiota
2.6. Blood and Urine Analysis
2.7. Statistics
3. Results
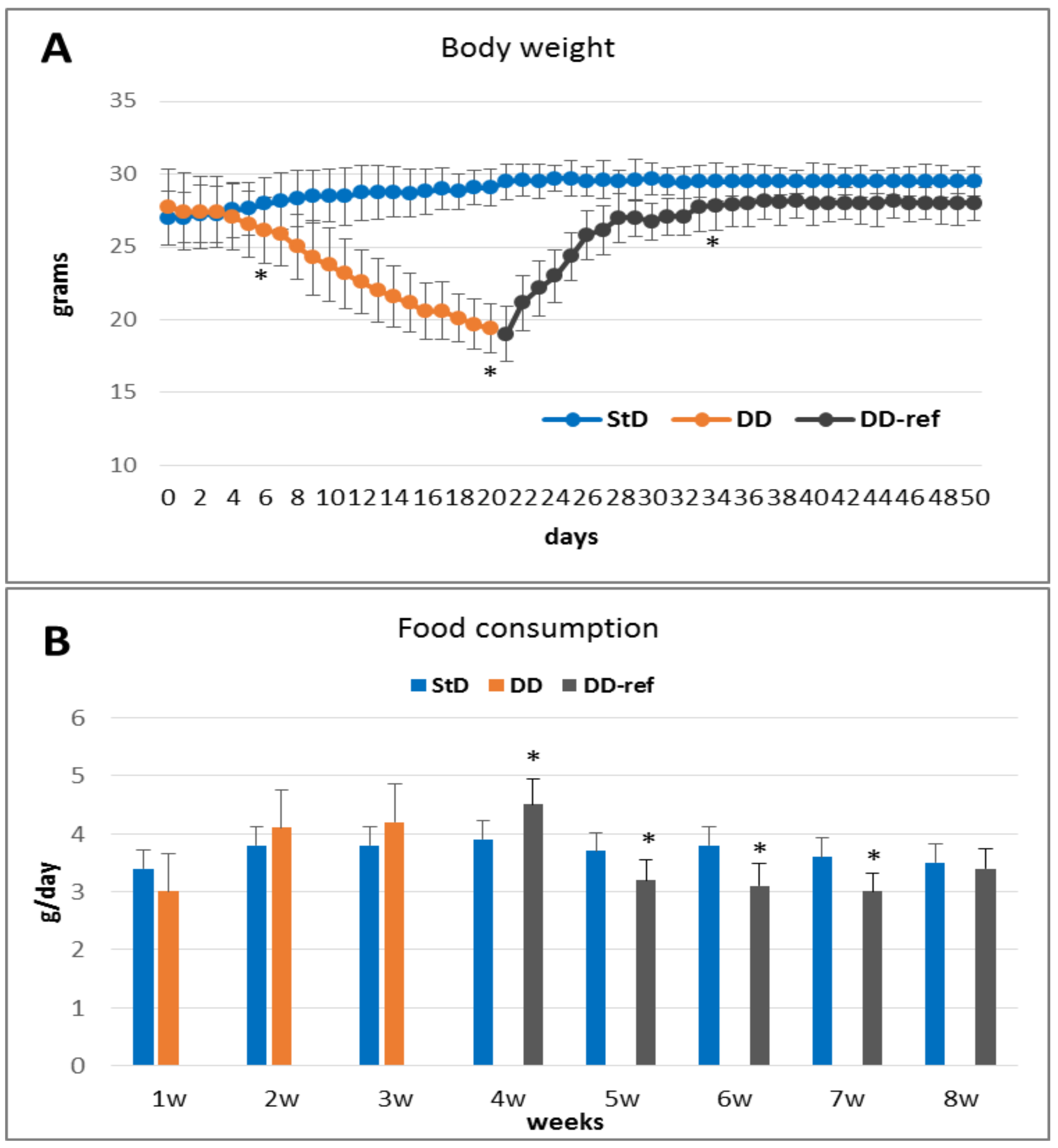
3.1. Phenotypical Modifications and Food Consumption
3.1.1. DD Fed Animals
3.1.2. DD-Ref Animals
3.2. Blood Analysis
3.3. Urine Analysis
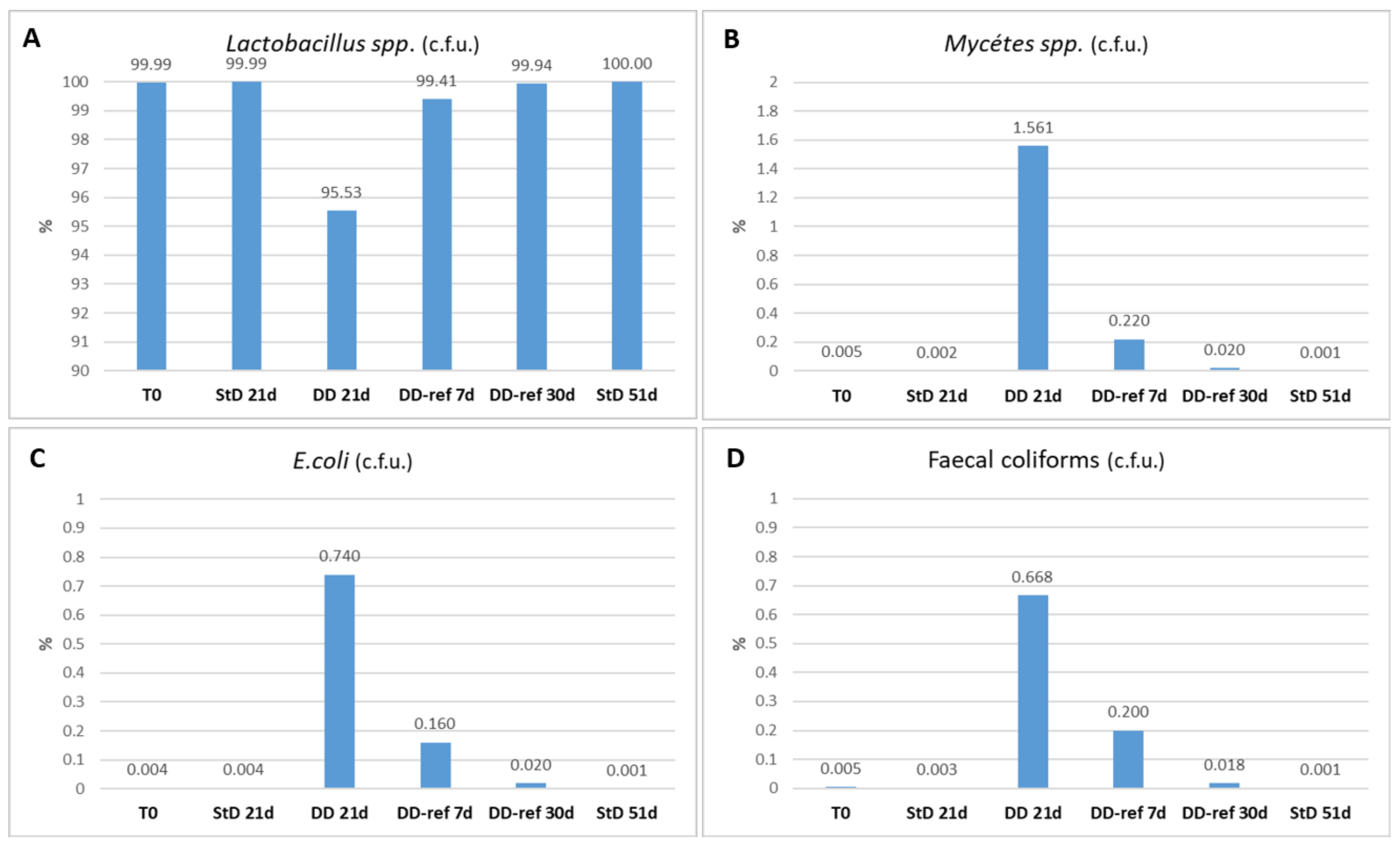
3.4. Fecal Microbiome
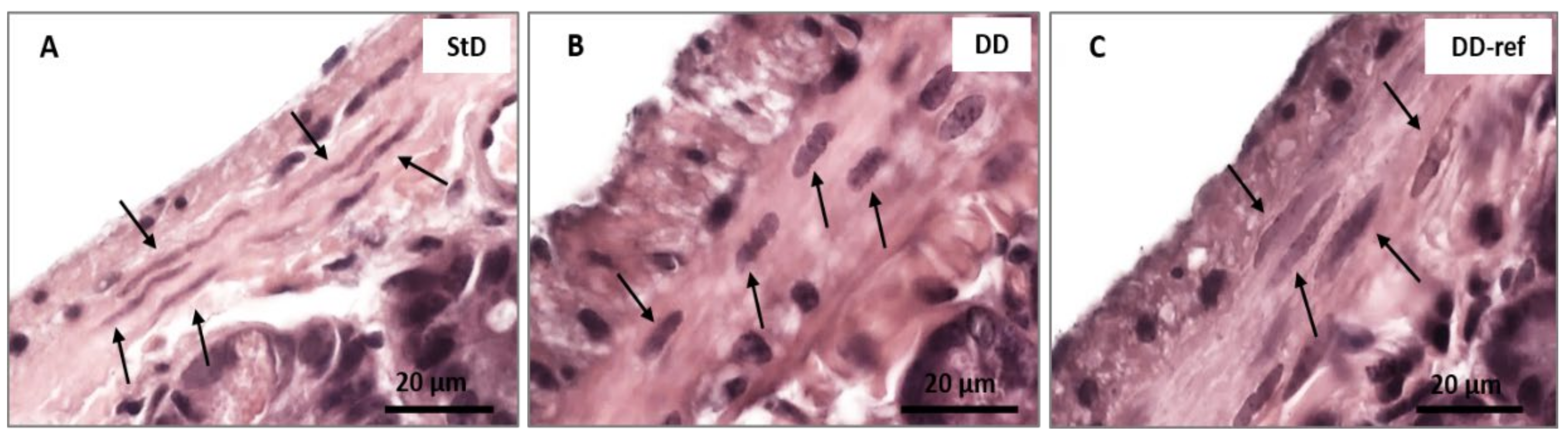
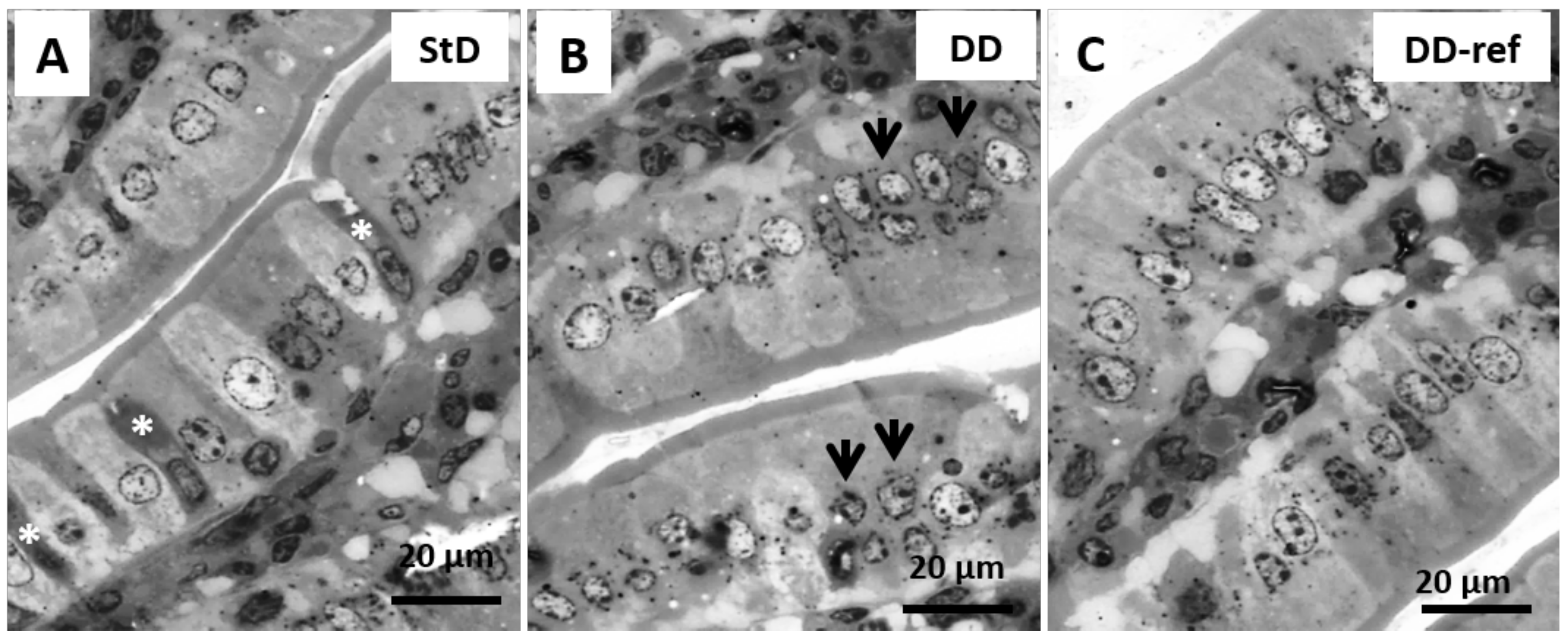
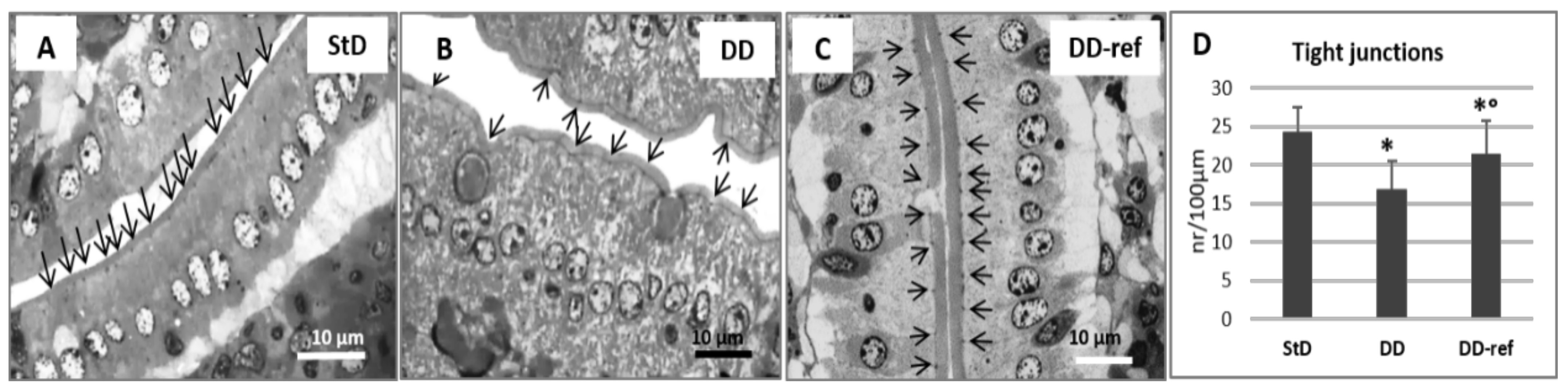
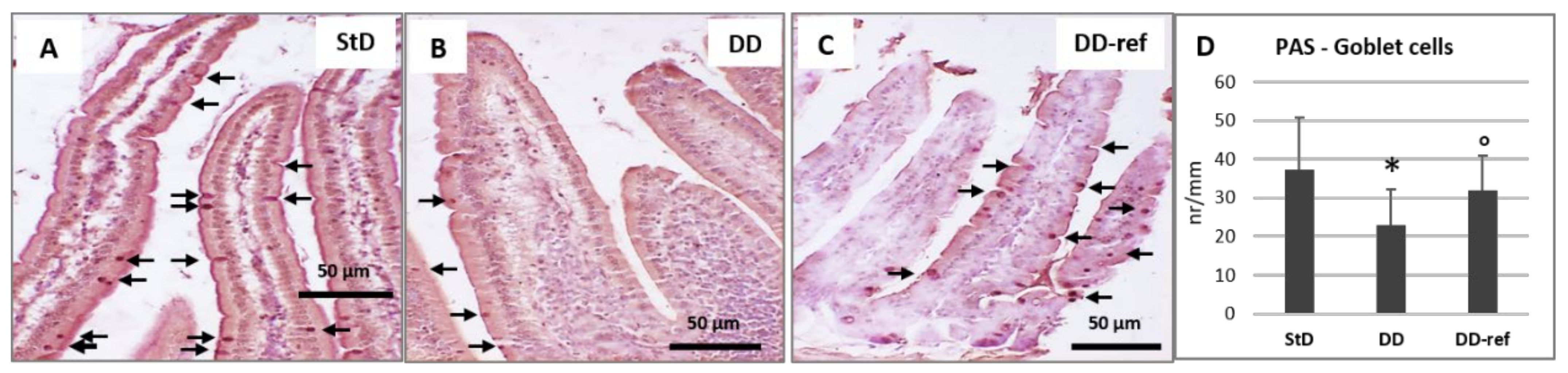
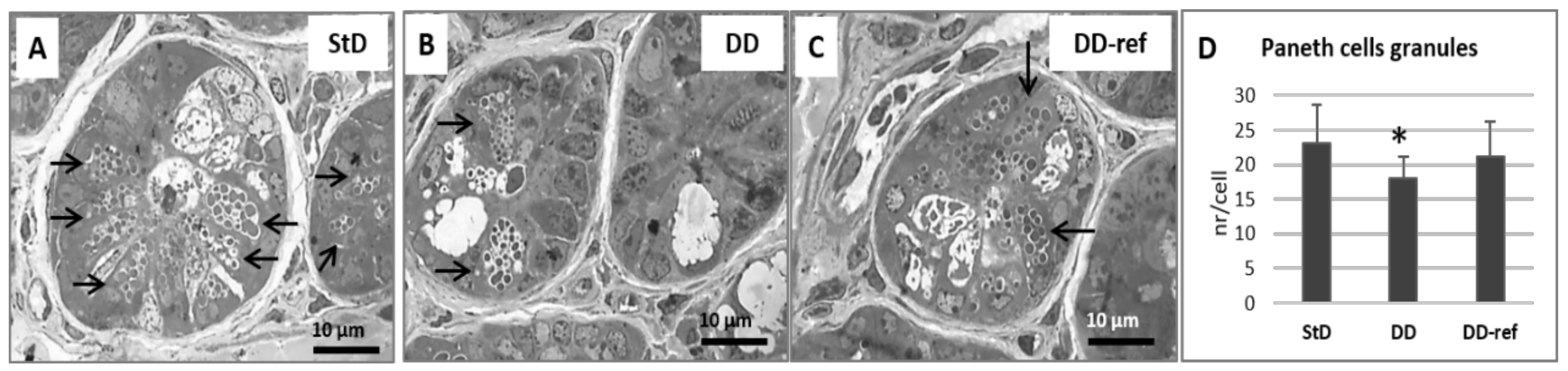
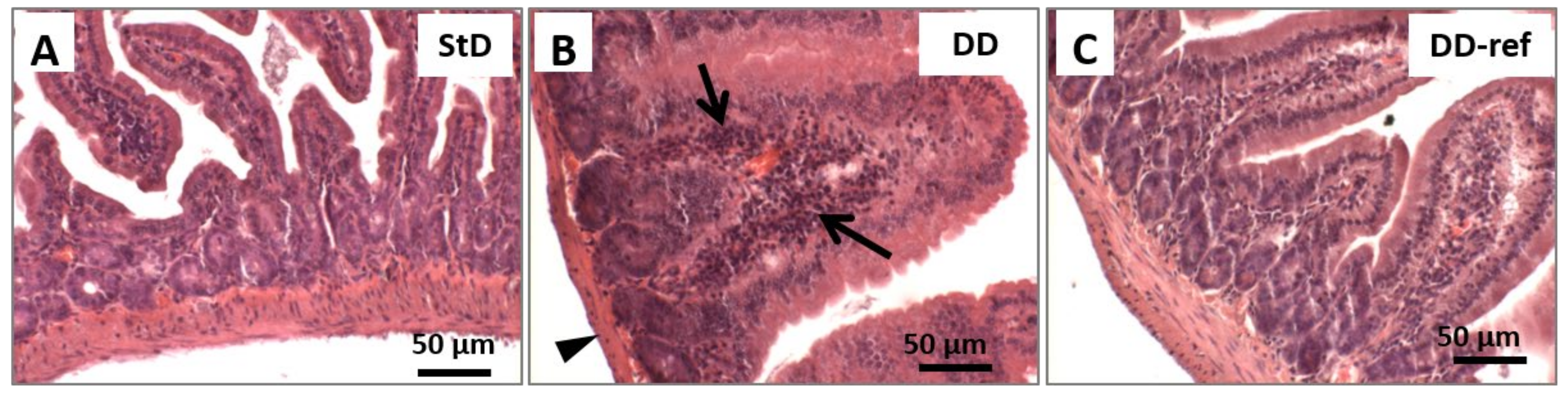
3.5. Morphology and Histopathology of the Jejunum
3.5.1. DD-Fed Animals
3.5.2. DD-Ref Animals
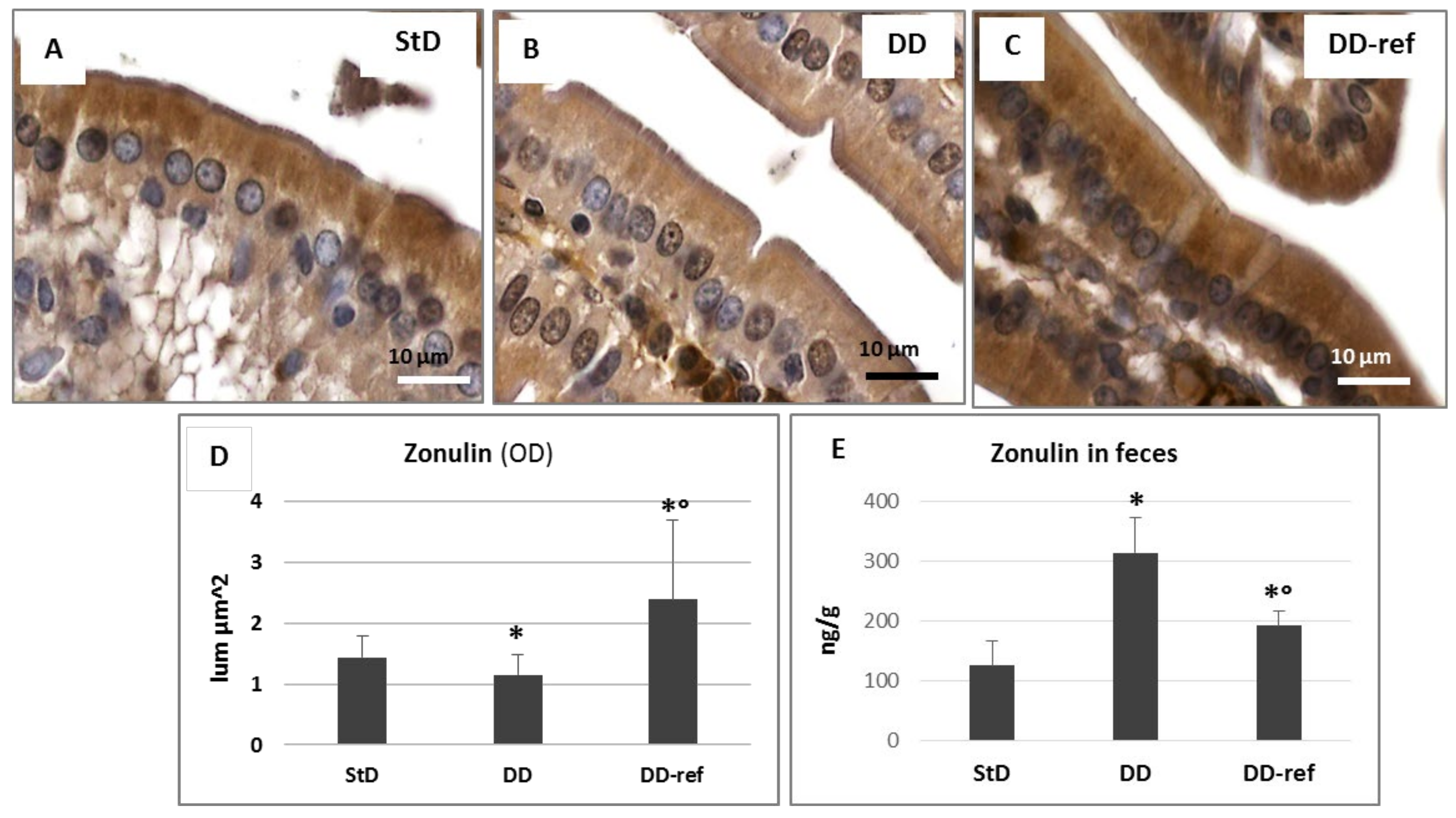
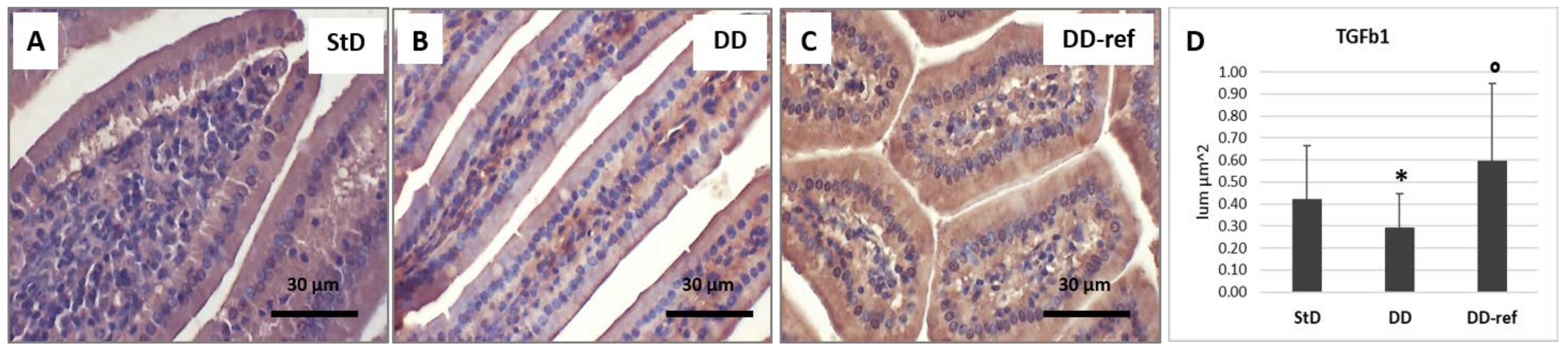
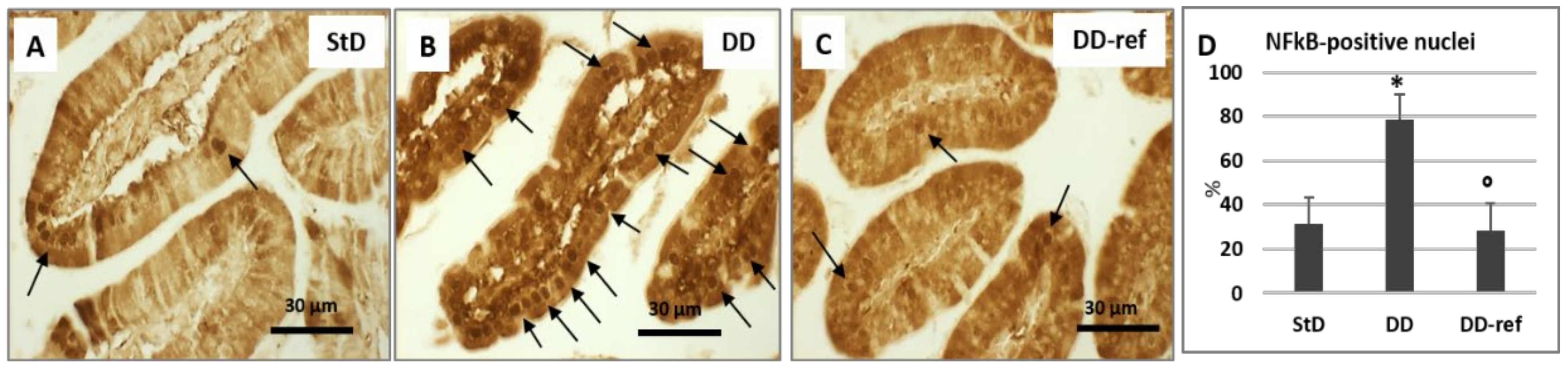
3.6. Immunohistochemistry
4. Discussion
Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corsetti, G.; Pasini, E.; Romano, C.; Calvani, R.; Picca, A.; Marzetti, E.; Flati, V.; Dioguardi, F.S. Body weight loss and tissue wasting in late middle-aged mice on slightly imbalanced Essential/Non-essential Amino Acids diet. Front. Med. 2018, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Corsetti, G.; Pasini, E.; Flati, V.; Dioguardi, F.S. Dietary modification of nitrogen intake decreases inflammation and promotes rejuvenation of spleen in aged mice. J. Food Nutr. Res. 2018, 6, 419–432. [Google Scholar] [CrossRef][Green Version]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of diets with varying essential/nonessential amino acid ratios on mouse lifespan. Nutrients 2019, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, A.; Turner, J.R.; Madara, J.L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G851–G857. [Google Scholar] [CrossRef]
- Khan, J.; Islam, M.N. Morphology of the Intestinal barrier in different physiological and pathological conditions. In Histopathology—Reviews and Recent Advances; Martinez, E.P., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 133–152. [Google Scholar] [CrossRef][Green Version]
- Gasbarrini, G.; Montalto, M. Structure and function of tight junctions. Role in intestinal barrier. Ital. J. Gastroenterol. Hepatol. 1999, 31, 481–488. [Google Scholar]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Saudi, W.S.W.; Khan, J.; Islam, M.N. Small intestinal morphology and permeability in chronic water avoidance stress in rats. IMJ 2009, 16, 87–91. [Google Scholar]
- Suzuki, T.; Hara, H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr. Metab. 2010, 12, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenet. 2015, 7, 112. [Google Scholar] [CrossRef]
- Chen, J.; Yue, Y.; Wang, L.; Deng, Z.; Yuan, Y.; Zhao, M.; Yuan, Z.; Tan, C.; Cao, Y. Altered gut microbiota correlated with systemic inflammation in children with Kawasaki disease. Sci. Rep. 2020, 10, 14525. [Google Scholar] [CrossRef]
- Pasini, E.; Opasich, C.; Pastoris, O.; Aquilani, R. Inadequate nutritional intake for daily life activity of clinically stable patients with chronic heart failure. Am. J. Cardiol. 2004, 93, 41A–43A. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Corsetti, G.; Dioguardi, F.S. Malnutrition and gut flora dysbiosis: Specific therapies for emerging comorbidities in heart failure. BioMed Res. Int. 2015, 2015, 382585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Huang, J. Diet effects in gut microbiome and obesity. J. Food Sci. 2014, 79, R442–R451. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.S.; Terentis, A.C.; King, N.J.C.; Thomas, S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Pan, C.; Chuang, C.; Liu, H.; Sun, F.; Wang, T.; Chen, H.; Wu, C. Gastrointestinal-related Uremic Toxins in Peritoneal Dialysis: A Pilot Study with a 5-year Follow-up. Arch. Med. Res. 2013, 44, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Navarrete, J.; Vásquez, B.; del Sol, M. Morphoquantitative analysis of the Ileum of C57BL/6 mice (Mus musculus) fed with a high-fat diet. Int. J. Clin. Exp. Pathol. 2015, 8, 14649–14657. [Google Scholar] [PubMed]
- Berkeveld, M.; Langendijk, P.; Soede, N.M.; Kemp, B.; Taverne, M.A.M.; Verheijden, J.H.M.; Kuijken, N.; Koets, A.P. Improving adaptation to weaning: Effect of intermittent suckling regimens on piglet feed intake, growth, and gut characteristics. J. Anim. Sci. 2009, 87, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Middleton, C. Crypts, villi and microvilli in the small intestine of the rat. A stereological study of their variability within and between animals. J. Anat. 1985, 141, 1–17. [Google Scholar] [PubMed]
- Piel, C.; Montagne, L.; Seve, B.; Lallès, J.P. Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation. J. Nutr. 2005, 135, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, H.; Mercier, Y.; Zhang, X.; Wu, C.; Wu, X.; Tang, L.; Che, L.; Lin, Y.; Xu, S.; et al. Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue. Br. J. Nutr. 2014, 112, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeith, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Murray, P.R.; Baron, E.J.; Jorgensen, J.H.; Pfaller, M.A.; Yolken, R.H. Manual of Clinical Microbiology, 8th ed.; American Society for Microbiology: Washington, DC, USA, 2003. [Google Scholar]
- Gietzen, W.D.; Hao, S.; Anthony, T.G. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu. Rev. Nutr. 2007, 27, 63–78. [Google Scholar] [CrossRef]
- Sherman, P.; Forstner, J.; Roomi, N.; Khatri, I.; Fostner, G. Mucin depletion in the intestine of malnourished rats. Ann. J. Physiol. 1985, 248, G418–G423. [Google Scholar] [CrossRef]
- Sullivan, D.; Vaerman, J.P.; Loo, C. Influence of severe protein malnutrition on rat lacrimal, salivary and gastrointestinal immune expression during development, adulthood and ageing. Immunology 1993, 78, 308–317. [Google Scholar]
- Welsh, F.; Farmery, S.M.; MacLennan, K.; Sheridan, M.B.; Barclay, G.R.; Guillou, P.J.; Reynolds, J.V. Gut barrier function in malnourished patients. Gut 1998, 42, 396–401. [Google Scholar] [CrossRef]
- Allori, C.; Agüero, G.; De Holgado, A.P.R.; De Nader, O.M.; Perdigon, G. Gut mucosa morphology and microflora changes in malnourished mice after renutrition with milk and administration of Lactobacillus casei. J. Food Prot. 2000, 63, 83–90. [Google Scholar] [CrossRef]
- Bodiga, V.L.; Boindala, S.; Putcha, U.; Subramaniam, K.; Manchala, R. Chronic low intake of protein or vitamins increases the intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition 2005, 21, 949–960. [Google Scholar] [CrossRef]
- Srugo, S.A.; Bloise, E.; Nguyen, T.T.; Connor, K.L. Impact of maternal malnutrition on gut barrier defense: Implications for pregnancy health and fetal development. Nutrients 2019, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Samuels, S.E.; Knowles, A.L.; Tilignac, T.; Debiton, E.; Madelmont, J.C.; Attaix, D. Protein metabolism in the small intestine during cancer cachexia and chemotherapy in mice. Cancer Res. 2000, 60, 4968–4974. [Google Scholar] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Mitic, L.L.; Anderson, J.M. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998, 60, 121–142. [Google Scholar] [CrossRef]
- Ivanov, A.I.; McCall, I.C.; Parkos, C.A.; Nusrat, A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2004, 15, 2639–2651. [Google Scholar] [CrossRef]
- Turksen, K.; Troy, T.C. Barriers built on claudins. J. Cell. Sci. 2004, 117, 2435–2447. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal zonulin: Open sesame! Gut 2001, 49, 159–162. [Google Scholar] [CrossRef][Green Version]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Goswami, P.; Das, T.K.; Nag, T.; Sreenivas, V.; Ahuja, V.; Panda, S.K.; Gupta, S.D.; Makharia, G.K. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: A new perspective. Virchows Arch. 2012, 460, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Nguyen, D.; Bui, V.; Nguyen, H.; Hoa, N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. 1999, 276, G965–G974. [Google Scholar] [CrossRef]
- Sturgeon, C.; Lan, J.; Fasano, A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann. N. Y. Acad. Sci. 2017, 1397, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chi, M.M.; Scull, B.P.; Rigby, R.; Schwerbrock, N.M.; Magness, S.; Jobin, C.; Lund, P.K. High-fat diet: Bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010, 5, e12191. [Google Scholar] [CrossRef] [PubMed]
- Suenaert, P.; Bulteel, V.; Lemmens, L.; Noman, M.; Geypens, B.; Van Assche, G.; Geboes, K.; Ceuppens, J.L.; Rutgeerts, P. Antitumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 2000–2004. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α induced increase in intestinal epithelial tight junction permeability requires NFkB activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T. Toll-like receptor signaling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef]
- Ye, D.; Ma, I.; Ma, T.Y. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G496–G504. [Google Scholar] [CrossRef]
- Ren, W.K.; Yin, J.; Zhu, X.P.; Liu, G.; Li, N.Z.; Peng, Y.Y.; Yin, Y.Y. Glutamine on intestinal inflammation: A mechanistic perspective. Eur. J. Inflamm. 2013, 11, 315–326. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.K.; Martins, C.A.; Cosme, R.; Fayer, R.; Guerrant, R.L. Transforming growth factor beta1 ameliorates intestinal epithelial barrier disruption by Cryptosporidium parvum in vitro in the absence of mucosal T lymphocytes. Infect. Immun. 2000, 68, 5635–5644. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Pasini, E.; Marzetti, E.; Calvani, R.; Picca, A.; Flati, V.; Dioguardi, F.S. Diet enrichment with a specific essential free amino acid mixture improves healing of undressed wounds in aged rats. Exp. Gerontol. 2017, 96, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; Chen, W. Balancing acts: The role of TGF-β in the mucosal immune system. Trends Mol. Med. 2011, 17, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Troncone, E.; Marafini, I.; Stolfi, C.; Monteleone, G. Transforming Growth Factor-β1/Smad7 in intestinal immunity, inflammation, and cancer. Front. Immunol. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Oshima, H.; Nakayama, M.; Han, T.S.; Naoi, K.; Ju, X.; Maeda, Y.; Robine, S.; Tsuchiya, K.; Sato, T.; Sato, H.; et al. Suppressing TGFbeta signaling in regenerating epithelia in an inflammatory microenvironment is sufficient to cause invasive intestinal cancer. Cancer Res. 2015, 75, 766–776. [Google Scholar] [CrossRef]
- Bauché, D.; Marie, J.C. Transforming growth factor β: A master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.L.; Santos, R.G.; Generoso, S.V.; Arantes, R.M.; Correia, M.I.; Cardoso, V.N. Pretreatment with arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition 2010, 26, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E. Glutamine: Role in gut protection in critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in intestinal mucosal defense and inflammation: Learning from clinical and experimental studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Gersemann, M.; Becker, S.; Kübler, I.; Koslowski, M.; Wang, G.; Herrlinger, K.R.; Griger, J.; Fritz, P.; Fellermann, K.; Schwab, M.; et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009, 77, 84–94. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, Y.; Wang, Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.F.; Ren, L.N.; Li, J.L.; Zhu, X.D.; Xing, T.; Zhang, L.; Gao, F.; Zhou, G.H. Protective effects of γ-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019, 98, 6400–6410. [Google Scholar] [CrossRef]
- Wehkamp, J.; Stange, E.F. An update review on the Paneth cell as key to ileal Crohn’s disease. Front. Immunol. 2020, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Reid, M.A.; Sanderson, S.M.; Locasale, J.W. Cancer Metabolism. In Abeloff’s Clinical Oncology, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 127–138.e4. [Google Scholar] [CrossRef]
- Madras, B.K.; Cohen, E.L.; Messing, R.; Munro, H.M.; Wurtman, R.J. Relevance of free tryptophan in serum to tissue tryptophan concentrations. Metabolism 1974, 23, P1107–P1116. [Google Scholar] [CrossRef]
- Metz, R.; Rust, S.; DuHadaway, J.B.; Mautino, M.R.; Munn, D.H.; Vahanian, N.N.; Link, C.J.; Prendergast, G.C. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012, 1, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Bird, A.R. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef]
- Rist, V.T.; Weiss, E.; Eklund, M.; Mosenthin, R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal 2013, 7, 1067–1078. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review article: Fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Nordin, S.A.; Basir, R.; Abdullah, M. Mycobiome in the gut: A multiperspective review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F.S. Pathogenic gut flora in patients with chronic heart failure. J. Am. Coll. Cardiol. Heart Fail. 2016, 4, 221–227. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Assanelli, D.; Testa, C.; Romano, C.; Dioguardi, F.S.; Aquilani, R. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Med. 2019, 110, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Romano, C.; Stacchiotti, A.; Pasini, E.; Dioguardi, F.S. Endoplasmic reticulum stress and apoptosis triggered by sub-chronic lead exposure in mice spleen: A histopathological study. Biol. Trace Elem. Res. 2017, 178, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, H.T.; Kone, B.C.; Mercer, D.W.; Moody, F.G.; Weisbrodt, N.W.; Moore, F.A. Post-injury multiple organ failure: The role of the gut. Shock 2001, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, O.D. Pathogenesis of multiple organ dysfunction syndrome: Gut origin, protection, and decontamination. Surg. Infect. 2000, 1, 217–225. [Google Scholar] [CrossRef]


| StD | DD | |
|---|---|---|
| KCal/Kg | 3952 | 3995 |
| Carbohydrates % | 54.61 | 61.76 |
| Lipids % | 7.5 | 6.12 |
| Nitrogen % | 21.8 ° | 20 * |
| Proteins: % of total nitrogen content | 95.93 | 0 |
| Free AA: % of total nitrogen content | 4.07 | 100 |
| EAA/NEAA (% in grams) | - | 15/85 |
| Free AA composition (%) | ||
| L-Leucine (bcaa) | - | 4.7 |
| L-Isoleucine (bcaa) | - | 2.35 |
| L-Valine (bcaa) | - | 2.35 |
| L-Lysine | 0.97 | 2.44 |
| L-Threonine | - | 13.13 |
| L-Hystidine | - | 5.65 |
| L-Phenylalanine | - | 4 |
| L-Cystine | 0.39 | - |
| L-Cysteine | - | 5.65 |
| L-Methionine | 0.45 | 1.9 |
| L-Tyrosine | - | 9.25 |
| L-Triptophan | 0.28 | 0.04 |
| L-Alanine | - | 30 |
| L-Glycine | 0.88 | 12.7 |
| L-Arginine | 1.1 | 11.8 |
| L-Proline | - | 10.2 |
| L-Glutamine | - | 10.2 |
| L-Serine | - | 5.1 |
| L-Glutamic Acid | - | 2 |
| L-Asparagine | - | 1.4 |
| L-Aspartic Acid | - | 0.8 |
| StD | DD | DD-Ref | F | p | |
|---|---|---|---|---|---|
| Feces (g/day) | 0.66 ± 0.05 | 0.32 ± 0.02 * | 0.51 ± 0.15 *° | 29.27 | 0.000 |
| StD | DD | DD-Ref | F | p | |
|---|---|---|---|---|---|
| Body weight (g) | 29.67 ± 1.97 | 17.83 ± 1.17 * | 28.13 ± 1.2 ° | 202.84 | 0.000 |
| Body length (cm) | 10.08 ± 0.28 | 9.43 ± 0.08 * | 9.59 ± 0.09 * | 36.52 | 0.000 |
| Heart (g) | 0.18 ± 0.02 | 0.13 ± 0.01 * | 0.14 ± 0.01 * | 32.80 | 0.000 |
| Kidneys (g) | 0.58 ± 0.06 | 0.30 ± 0.04 * | 0.49 ± 0.04 ° | 87.05 | 0.000 |
| Liver (g) | 1.68 ± 0.2 | 0.65 ± 0.07 * | 1.38 ± 0.12 *° | 143.03 | 0.000 |
| Spleen (g) | 0.15 ± 0.04 | 0.05 ± 0.01 * | 0.08 ± 0.01 *° | 44.02 | 0.000 |
| rpWAT (g) | 0.21 ± 0.02 | 0.04 ± 0.01 * | 0.19 ± 0.03 ° | 173.8 | 0.000 |
| BAT (g) | 0.17 ± 0.01 | 0.08 ± 0.01 * | 0.34 ± 0.04 *° | 271.2 | 0.000 |
| Triceps surae (g) | 0.35 ± 0.03 | 0.18 ± 0.02 * | 0.28 ± 0.04 *° | 68.05 | 0.000 |
| StD | DD | DD-Ref | F | p | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 136 ± 7.16 | 108.3 ± 6.76 * | 128.2 ± 15.95 ° | 13.94 | 0.000 |
| Erythrocytes (RBC) (M/µL) | 9.7 ± 0.42 | 10.37 ± 0.3 * | 9.31 ± 0.27 ° | 20.69 | 0.000 |
| Hemoglobin (g/dL) | 15.08 ± 0.48 | 15.12 ± 0.47 | 14.91 ± 0.9 | 0.24 | 0.791 |
| Platelet (PLT) (K/µL) | 1278.0 ± 516.4 | 540.1 ± 291.32 * | 1115.3 ± 65.2 ° | 10.14 | 0.000 |
| Leucocytes (WBC) (K/µL) | 7.82 ± 1.76 | 3.76 ± 1.19 * | 6.0 ± 0.93 *° | 18.45 | 0.000 |
| Neutrophils (K/µL) | 1.78 ± 0.42 | 2.24 ± 0.6 | 1.40 ± 0.41 ° | 6.03 | 0.009 |
| Lymphocytes (K/µL) | 5.76 ± 1.37 | 1.50 ± 0.8 * | 4.42 ± 0.49 *° | 41.54 | 0.000 |
| N/L ratio (%) | 0.32 ± 0.1 | 1.83 ± 0.6 * | 0.31 ± 0.07 ° | 48.98 | 0.000 |
| Serum | |||||
| Albumin (g/L) | 28.66 ± 2.57 | 25.43 ± 1.64 | 28.42 ± 3.2 | 3.98 | 0.034 |
| Urea (mmol/L) | 8.54 ± 2.01 | 8.78 ± 1.17 | 7.58 ± 0.72 | 1.63 | 0.219 |
| Creatinine (µmol/L) | 23.83 ± 1.89 | 35.63 ± 4.4 * | 28.0 ± 4.34 ° | 20.58 | 0.000 |
| Aptoglobin (mg/mL) | 2.70 ± 1.05 | 0.14 ± 0.01 * | 2.80 ± 1.02 ° | 25.46 | 0.000 |
| StD | DD | DD-ref | F | p | |
|---|---|---|---|---|---|
| Albumin (g/L) | 2.66 ± 0.23 | 1.36 ± 0.4 * | 2.13 ± 0.7 ° | 18.3 | 0.000 |
| Urea (mmol/L) | 835.9 ± 35.85 | 1445.2 ± 181.7 * | 1396.4 ± 231.3 * | 19.59 | 0.000 |
| Creatinine (µm/L) | 6147.66 ± 957.8 | 4637.8 ± 396.5 * | 4414 ± 240.23 * | 11.78 | 0.000 |
| Total protein (g/L) | 2.29 ± 0.2 | 1.38 ± 0.11 * | 2.48 ± 0.52 ° | 16.08 | 0.000 |
| StD | DD | DD-Ref | F | p | |
|---|---|---|---|---|---|
| Wall thickness (µm) | 346.2 ± 41.6 | 319.9 ± 32.7 | 341.1 ± 32.7 | 3.02 | 0.057 |
| Muscular layer thickness (µm) | 68.6 ± 8.4 | 60.0 ± 6.9 * | 66.8 ± 6.93 ° | 7.43 | 0.001 |
| Mucosa layer thickness (µm) | 277.56 ± 44 | 259.94 ± 37.2 | 274.31 ± 33.2 | 1.19 | 0.311 |
| Villi length (µm) | 208.86 ± 38.3 | 187.21 ± 38.6 | 194.31 ± 33.1 | 1.83 | 0.169 |
| Villi diameter (µm) | 73.1 ± 11.8 | 73.6 ± 12.6 | 74.7 ± 10.2 | 0.11 | 0.898 |
| Epithelium thickness (µm) | 28.5 ± 6.5 | 20.7 ± 4.7 * | 26.3 ± 3.5 ° | 12.94 | 0.000 |
| Enterocytes (n° Nuclei/100 µm) | 18.5 ± 5.1 | 11.4 ± 2.6 * | 14.6 ± 2.1 *° | 18.2 | 0.000 |
| Enterocytes: Nuclear diameter (µm) | 4.6 ± 0.8 | 5.2 ± 0.9 | 4.5 ± 0.88 ° | 4.46 | 0.016 |
| Enterocytes: junctions (n°/100 µm) | 24.36 ± 3.4 | 16.88 ± 3.5 * | 21.41 ± 4.3 ° | 23.42 | 0.000 |
| Goblet cells (nr/mm) | 37.4 ± 13.4 | 23.0 ± 9.2 * | 31.9 ± 9.1 ° | 9.13 | 0.000 |
| Crypt depth (µm) | 76.93 ± 7.3 | 70.38 ± 8.0 | 79.99 ± 8.1 | 0.88 | 0.422 |
| Paneth cells granules (nr/cell) | 23.12 ± 5.6 | 17.94 ± 3.2 * | 21.18 ± 5.1 | 5.57 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsetti, G.; Romano, C.; Pasini, E.; Testa, C.; Dioguardi, F.S. Qualitative Nitrogen Malnutrition Damages Gut and Alters Microbiome in Adult Mice. A Preliminary Histopathological Study. Nutrients 2021, 13, 1089. https://doi.org/10.3390/nu13041089
Corsetti G, Romano C, Pasini E, Testa C, Dioguardi FS. Qualitative Nitrogen Malnutrition Damages Gut and Alters Microbiome in Adult Mice. A Preliminary Histopathological Study. Nutrients. 2021; 13(4):1089. https://doi.org/10.3390/nu13041089
Chicago/Turabian StyleCorsetti, Giovanni, Claudia Romano, Evasio Pasini, Cristian Testa, and Francesco S. Dioguardi. 2021. "Qualitative Nitrogen Malnutrition Damages Gut and Alters Microbiome in Adult Mice. A Preliminary Histopathological Study" Nutrients 13, no. 4: 1089. https://doi.org/10.3390/nu13041089
APA StyleCorsetti, G., Romano, C., Pasini, E., Testa, C., & Dioguardi, F. S. (2021). Qualitative Nitrogen Malnutrition Damages Gut and Alters Microbiome in Adult Mice. A Preliminary Histopathological Study. Nutrients, 13(4), 1089. https://doi.org/10.3390/nu13041089








