Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review
Abstract
1. Introduction
2. A Synopsis of Nutraceuticals and Supplements Amid the Pandemic
3. Vitamin D and SARS-CoV-2
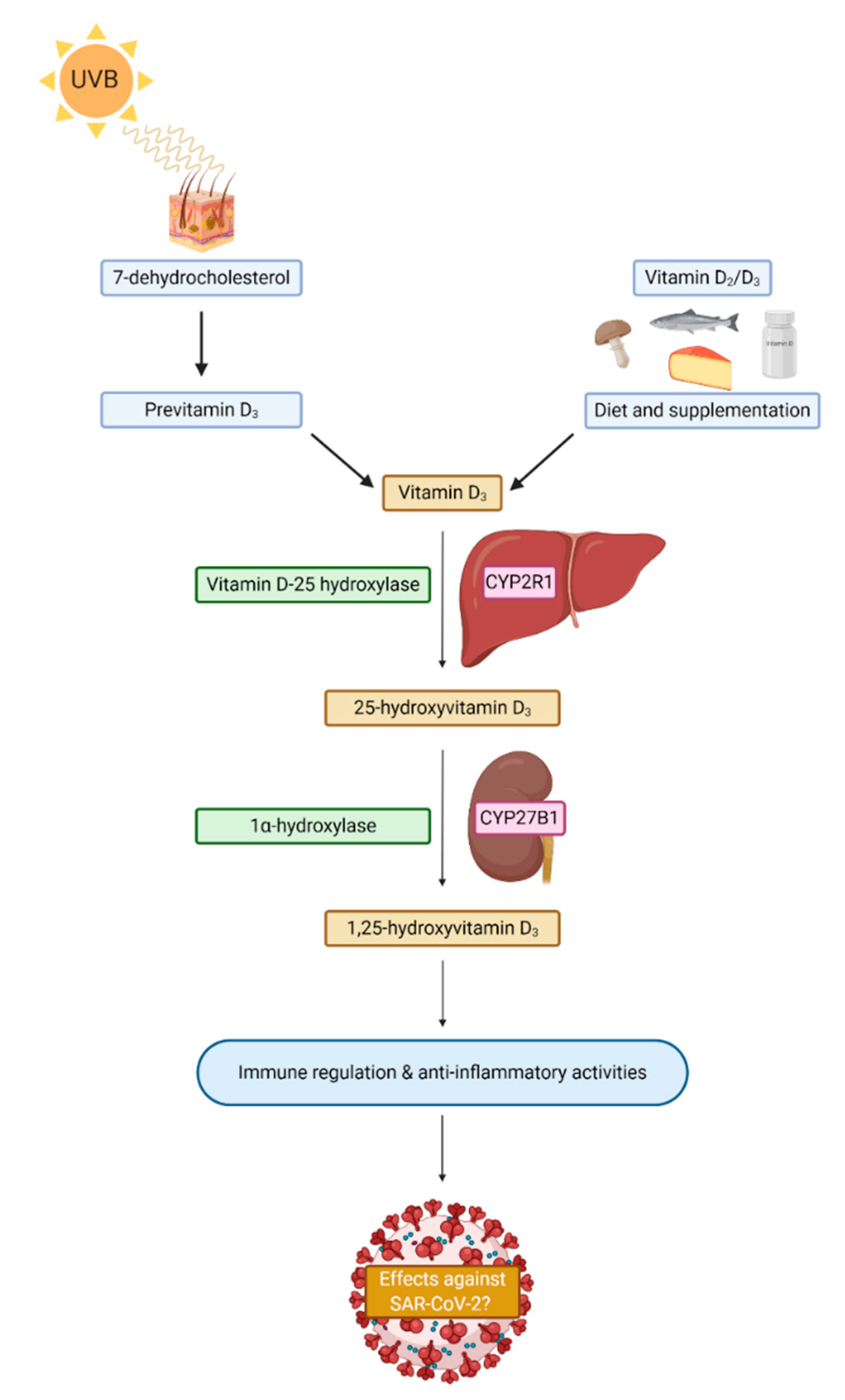
3.1. Vitamin D
3.2. Vitamin D Status
3.3. Vitamin D and the Pandemic
3.4. Plausible Mechanisms for Vitamin D against SARS-CoV-2
3.5. Prophylaxis: To Supplement, or Not to Supplement for Vitamin D
3.6. The Therapeutic Potential of Vitamin D
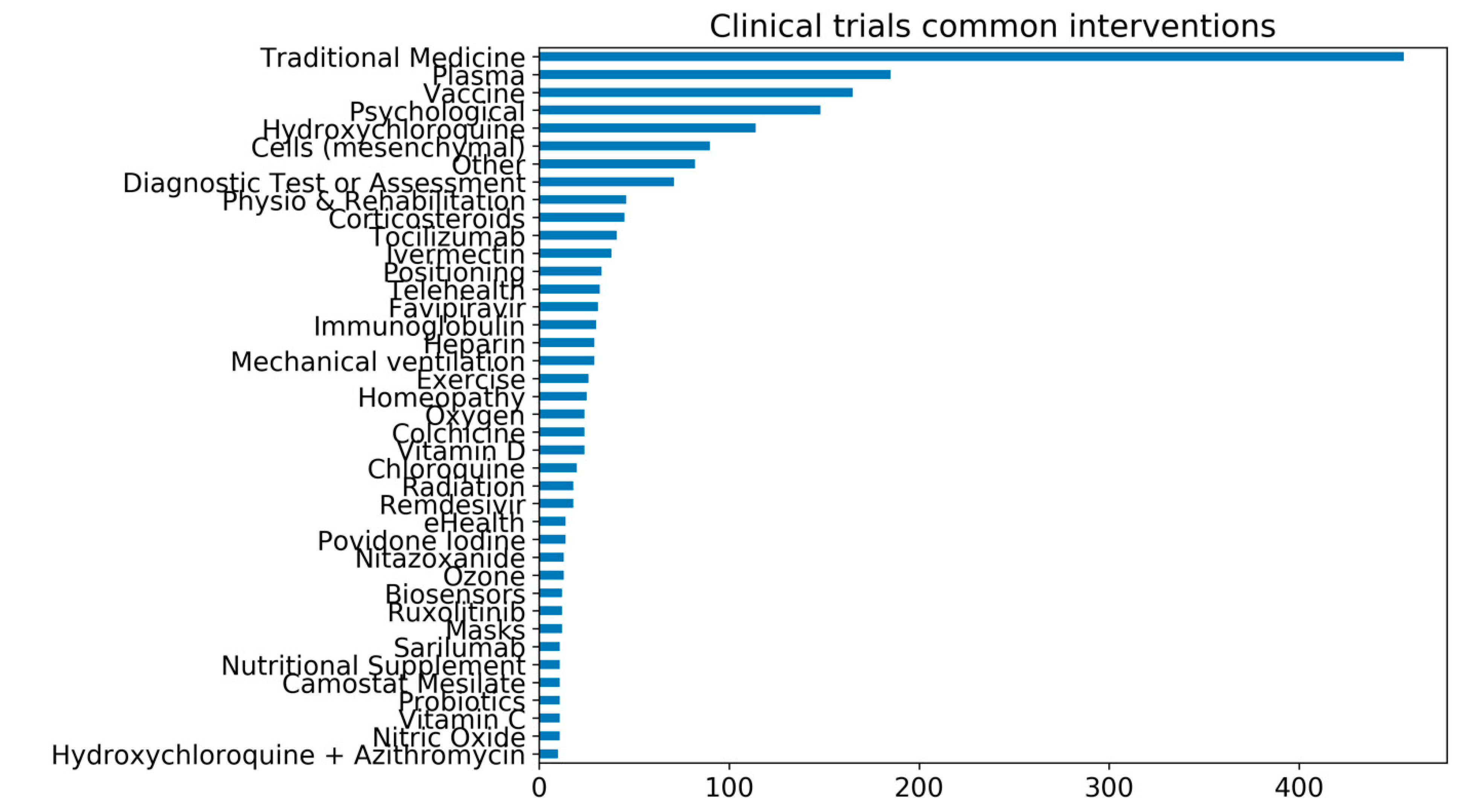
3.7. Perspectives on Vitamin D Clinical Trials and COVID-19
4. Regulatory Issues and Misinformation during the Pandemic
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Yang, X.; Yang, L.; Zou, X.; Wang, Y.; Wu, Y.; Zhou, T.; Yuan, Y.; Qi, H.; Fu, S.; et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: A multicenter retrospective study from Wuhan, China. Crit. Care 2020, 24, 394. [Google Scholar] [CrossRef]
- Rando, H.M.; MacLean, A.L.; Lee, A.J.; Ray, S.; Bansal, V.; Skelly, A.N.; Sell, E.; Dziak, J.J.; Shinholster, L.; McGowan, L.D.A. Pathogenesis, Symptomatology, and Transmission of SARS-CoV-2 through analysis of Viral Genomics and Structure. arXiv 2021, arXiv:2102.01521. [Google Scholar]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Avula, A.; Nalleballe, K.; Narula, N.; Sapozhnikov, S.; Dandu, V.; Toom, S.; Glaser, A.; Elsayegh, D. COVID-19 presenting as stroke. Brain Behav. Immun. 2020, 87, 115–119. [Google Scholar] [CrossRef]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; Pearson, C.A.B.; Quilty, B.J.; Kucharski, A.J.; Gibbs, H.; Clifford, S.; et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.M.; Anyane-Yeboa, A.; Balzora, S.; Issaka, R.B.; May, F.P. COVID-19 and the other pandemic: Populations made vulnerable by systemic inequity. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. NEJM 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19—Preliminary Report. NEJM 2020. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; Chu, D.K.; Akl, E.A.; El-harakeh, A.; Bognanni, A.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Lordan, R.; FitzGerald, G.A.; Grosser, T. Reopening schools during COVID-19. Science 2020, 369, 1146. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A.; Burgess, R.A.; Ashworth, S.; Beale, R.; Bhadelia, N.; Bogaert, D.; Dowd, J.; Eckerle, I.; Goldman, L.R.; Greenhalgh, T.; et al. Scientific consensus on the COVID-19 pandemic: We need to act now. Lancet 2020, 396, e71–e72. [Google Scholar] [CrossRef]
- Furlong, C. 5 Food and Beverage Trends in Europe During COVID-19. Available online: https://kerry.com/insights/kerrydigest/2020/5-food-and-beverage-trends-in-europe-during-covid-19 (accessed on 10 November 2020).
- Lordan, R.; Rando, H.M.; Consortium, C.-R.; Greene, C.S. Dietary Supplements and Nutraceuticals Under Investigation for COVID-19 Prevention and Treatment. arXiv 2021, arXiv:2102.02250v02251. [Google Scholar]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical-definition and introduction. Aaps Pharmsci. 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, Z.; Ke, Y.; Huo, S.; Ma, Y.; Zhang, Y.; Zhang, J.; Ren, Z. Dietary Diversity among Chinese Residents during the COVID-19 Outbreak and Its Associated Factors. Nutrients 2020, 12, 1699. [Google Scholar] [CrossRef]
- NUTRA Ingrediets-Asia. Lockdown Impact: Grocery Stores Bolstered NZ Supplements Sales as Pharmacies Slumped. Available online: https://www.nutraingredients-asia.com/Article/2020/07/06/Lockdown-impact-Grocery-stores-bolstered-NZ-supplements-sales-as-pharmacies-slumped (accessed on 5 August 2020).
- Nutrition Insight. COVID-19 Temporarily Bolsters European Interest in Supplements. Available online: https://www.nutritioninsight.com/news/covid-19-temporarily-bolsters-european-interest-in-supplements.html (accessed on 8 August 2020).
- Nutra Ingrediets.Com. India’s Immune Health Surge: Nation Leads APAC in Number of New Product Launches—New Data. Available online: https://www.nutraingredients.com/Article/2020/07/21/India-s-immune-health-surge-Nation-leads-APAC-in-number-of-new-product-launches-new-data (accessed on 8 August 2020).
- Ayseli, Y.I.; Aytekin, N.; Buyukkayhan, D.; Aslan, I.; Ayseli, M.T. Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: Advice for healthcare professionals. Trends Food Sci. Technol. 2020, 105, 186–199. [Google Scholar] [CrossRef]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2021, 13, 54. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The inflammation link and the role of nutrition in potential mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020. [Google Scholar] [CrossRef]
- Cena, H.; Chieppa, M. Coronavirus disease (COVID-19–SARS-CoV-2) and nutrition: Is infection in Italy suggesting a connection? Front Immunol. 2020, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S. Nutritional status and COVID-19: An opportunity for lasting change? Clin. Med. (Lond.) 2020, 20, 270–273. [Google Scholar] [CrossRef]
- Silverio, R.; Gonçalves, D.C.; Andrade, M.F.; Seelaender, M. Coronavirus Disease 2019 (COVID-19) and Nutritional Status: The Missing Link? Adv. Nutr. 2020. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Ong, M.M.; Ong, R.M.; Reyes, G.K.; Sumpaico-Tanchanco, L.B. Addressing the COVID-19 Nutrition Crisis in Vulnerable Communities: Applying a Primary Care Perspective. J Prim. Care Community Health 2020, 11, 2150132720946951. [Google Scholar] [CrossRef]
- Beck, M.A.; Handy, J.; Levander, O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004, 12, 417–423. [Google Scholar] [CrossRef]
- Beck, M.A. Increased Virulence of Coxsackievirus B3 in Mice Due to Vitamin E or Selenium Deficiency. J. Nutr. 1997, 127, 966S–970S. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A. Host Nutritional Status and Its Effect on a Viral Pathogen. J. Infect. Dis. 2000, 182, S93–S96. [Google Scholar] [CrossRef]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Ortoski, R.A.; Razzaque, M.S. Effects of sunlight exposure and vitamin D supplementation on HIV patients. J Steroid Biochem. Mol. Biol. 2020, 200, 105664. [Google Scholar] [CrossRef]
- Vergori, A.; Pinnetti, C.; Lorenzini, P.; Brita, A.; Libertone, R.; Mastrorosa, I.; Cicalini, S.; Antinori, A.; Ammassari, A. Vitamin D deficiency is associated with neurocognitive impairment in HIV-infected subjects. Infection 2019, 47, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Manion, M.; Hullsiek, K.H.; Wilson, E.M.P.; Rhame, F.; Kojic, E.; Gibson, D.; Hammer, J.; Patel, P.; Brooks, J.T.; Baker, J.V.; et al. Vitamin D deficiency is associated with IL-6 levels and monocyte activation in HIV-infected persons. PLoS ONE 2017, 12, e0175517. [Google Scholar] [CrossRef]
- Alvarez, N.; Aguilar-Jimenez, W.; Rugeles, M.T. The Potential Protective Role of Vitamin D Supplementation on HIV-1 Infection. Front. Immunol. 2019, 10, 2291. [Google Scholar] [CrossRef]
- Jiménez-Sousa, M.Á.; Martínez, I.; Medrano, L.M.; Fernández-Rodríguez, A.; Resino, S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front. Immunol. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Viard, J.-P.; Souberbielle, J.-C.; Kirk, O.; Reekie, J.; Knysz, B.; Losso, M.; Gatell, J.; Pedersen, C.; Bogner, J.R.; Lundgren, J.D.; et al. Vitamin D and clinical disease progression in HIV infection: Results from the EuroSIDA study. AIDS 2011, 25, 1305–1315. [Google Scholar] [CrossRef]
- Abraham, A.G.; Zhang, L.; Calkins, K.; Tin, A.; Hoofnagle, A.; Palella, F.J., Jr.; Estrella, M.M.; Jacobson, L.P.; Witt, M.D.; Kingsley, L.A.; et al. Vitamin D status and immune function reconstitution in HIV-infected men initiating therapy. AIDS 2018, 32, 1069–1076. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Vitamin D Supplementation Could Prevent and Treat Influenza, Coronavirus, and Pneumonia Infections. Preprints 2020. [Google Scholar] [CrossRef]
- DeVito, N.; Inglesby, P. Evidence-Based Medicine Data Lab COVID-19 TrialsTracker. Available online: https://github.com/ebmdatalab/covid_trials_tracker-covid (accessed on 10 November 2020).
- Pang, B.; Zhang, J.; Lee, M.S.; Zheng, W. Enlightenment from clinical trials on Chinese medicine for coronavirus disease 2019 (COVID-19). Integr. Med. Res. 2020, 9, 100481. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C Can Shorten the Length of Stay in the ICU: A Meta-Analysis. Nutrients 2019, 11, 708. [Google Scholar] [CrossRef]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 2636. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat. Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef] [PubMed]
- Hagel, A.F.; Layritz, C.M.; Hagel, W.H.; Hagel, H.-J.; Hagel, E.; Dauth, W.; Kressel, J.; Regnet, T.; Rosenberg, A.; Neurath, M.F.; et al. Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn Schmiedeberg’s Arch. Pharm. 2013, 386, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020, 69, 1228–1234. [Google Scholar] [CrossRef]
- Rentsch, C.T.; DeVito, N.J.; MacKenna, B.; Morton, C.E.; Bhaskaran, K.; Brown, J.P.; Schultze, A.; Hulme, W.J.; Croker, R.; Walker, A.J.; et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: A population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol. 2020. [Google Scholar] [CrossRef]
- Mitjà, O.; Corbacho-Monné, M.; Ubals, M.; Tebe, C.; Peñafiel, J.; Tobias, A.; Ballana, E.; Alemany, A.; Riera-Martí, N.; Pérez, C.A.; et al. Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Hulisz, D. Efficacy of Zinc Against Common Cold Viruses: An Overview. J. E Am. Pharm. Assoc. 2004, 44, 594–603. [Google Scholar] [CrossRef]
- Hemilä, H. Zinc lozenges may shorten the duration of colds: A systematic review. Open Resp. Med. J. 2011, 5, 51–58. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. Npj Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Rogero, M.M.; Leão, M.d.C.; Santana, T.M.; Pimentel, M.V.D.M.B.; Carlini, G.C.G.; da Silveira, T.F.F.; Gonçalves, R.C.; Castro, I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Shen, J.; Mehra, R.; Kallianpur, A.; Culver, D.A.; Gack, M.U.; Farha, S.; Zein, J.; Comhair, S.; et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020, 18, e3000970. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Thrombosis and COVID-19: The Potential Role of Nutrition. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- SharesMagazine. Market & Company News: Fruitflow. Available online: https://www.sharesmagazine.co.uk/news/market/7044546/Fruitflow (accessed on 9 November 2020).
- O’Kennedy, N.; Duttaroy, A.K. Platelet hyperactivity in COVID-19: Can the tomato extract Fruitflow® be used as an antiplatelet regime? Med Hypotheses 2021, 147, 110480. [Google Scholar] [CrossRef]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced immuno-protection against Covid-19. Ir Med J 2020, 113, 58. [Google Scholar] [PubMed]
- Ebadi, M.; Montano-Loza, A.J. Perspective: Improving vitamin D status in the management of COVID-19. Euro. J. Clin. Nutr. 2020, 74, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Lanham-New, S.A.; Webb, A.R.; Cashman, K.D.; Buttriss, J.L.; Fallowfield, J.L.; Masud, T.; Hewison, M.; Mathers, J.C.; Kiely, M.; Welch, A.A.; et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. Bmj Nutr. Prev. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Griffin, G.; Kenny, R.A. Perspective: Vitamin D deficiency and COVID-19 severityplausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. New York Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Hyppönen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [CrossRef]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D Stored in Fat Tissue During a 5-Year Intervention Affects Serum 25-Hydroxyvitamin D Levels the Following Year. J. Clin. Endocrinol. Metab. 2017, 102, 3731–3738. [Google Scholar] [CrossRef]
- Yousefzadeh, P.; Shapses, S.A.; Wang, X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int. J. Endocrinol. 2014, 2014, 981581. [Google Scholar] [CrossRef]
- L Bishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. Jbmr Plus 2020, e10405. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D.; Drezner, M.K. Evaluation of Ergocalciferol or Cholecalciferol Dosing, 1,600 IU Daily or 50,000 IU Monthly in Older Adults. J. Clin. Endocrinol. Metab. 2011, 96, 981–988. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- Hewison, M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011, 7, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Seuter, S.; de Mello, V.D.F.; Schwab, U.; Voutilainen, S.; Pulkki, K.; Nurmi, T.; Virtanen, J.; Tuomainen, T.-P.; Uusitupa, M. Primary Vitamin D Target Genes Allow a Categorization of Possible Benefits of Vitamin D3 Supplementation. PLoS ONE 2013, 8, e71042. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Martucci, G.; Tuzzolino, F.; Arcadipane, A.; Pieber, T.R.; Schnedl, C.; Urbanic Purkart, T.; Treiber, G.; Amrein, K. The effect of high-dose cholecalciferol on bioavailable vitamin D levels in critically ill patients: A post hoc analysis of the VITdAL-ICU trial. Intensive Care Med. 2017, 43, 1732–1734. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Quraishi, S.A. Vitamin D status in critically ill patients: The evidence is now bioavailable! Crit. Care 2014, 18, 449. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Camargo, C.A., Jr. Vitamin D in acute stress and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 625. [Google Scholar] [CrossRef]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144, 132–137. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; McCarthy, C.M.; Bhan, I.; Camargo, C.A., Jr. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit. Care. Med. 2014, 42, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Delanghe, J.R. Importance of the Lipid-Bound Character of Vitamin D Binding Protein in the Evaluation of Vitamin D Status in COVID-19 Patients. Am. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Soule, S.; Gaddam, R.R.; Elder, P.; Chambers, S.; Doogue, M. Unbound Vitamin D Concentrations Are Not Decreased in Critically Ill Patients. Intern. Med. J. 2021. [Google Scholar] [CrossRef]
- Jassil, N.K.; Sharma, A.; Bikle, D.; Wang, X. Vitamin D Binding Protein and 25-Hydroxyvitamin D Levels: Emerging Clinical Applications. Endocr. Pract. 2017, 23, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Taes, Y.E.; De Buyzere, M.L.; Christophe, A.B.; Kaufman, J.M.; Delanghe, J.R. Investigation of the potential association of vitamin D binding protein with lipoproteins. Ann. Clin. Biochem. 2010, 47, 143–150. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Speeckaert, R.; Delanghe, J.R. Vitamin D binding protein in COVID-19. Clin. Med. 2020, 20, e136–e137. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu Batur, L.; Hekim, N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2021, 93, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; De Buyzere, M.L.; Delanghe, J.R. Vitamin D binding protein polymorphism and COVID-19. J. Med. Virol. 2021, 93, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [PubMed]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low Vitamin D Status: Definition, Prevalence, Consequences, and Correction. Endocrinol. Metab. Clin. North Am. 2010, 39, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Malatesta, K.; Norris, K. Vitamin D and chronic kidney disease. Ethn. Dis. 2009, 19, S5–S11. [Google Scholar]
- National Institutes of Health: Office of Dietary Supplements. Vitamin D: Fact Sheet For Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 18 February 2021).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27. [Google Scholar] [CrossRef]
- Weishaar, T.; Rajan, S.; Keller, B. Probability of Vitamin D Deficiency by Body Weight and Race/Ethnicity. J. Am. Board Fam. Med. 2016, 29, 226–232. [Google Scholar] [CrossRef]
- Parva, N.R.; Tadepalli, S.; Singh, P.; Qian, A.; Joshi, R.; Kandala, H.; Nookala, V.K.; Cheriyath, P. Prevalence of Vitamin D Deficiency and Associated Risk Factors in the US Population (2011-2012). Cureus 2018, 10, e2741. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Zhou, A.; Leach, M.J.; Hyppönen, E. Differences and determinants of vitamin D deficiency among UK biobank participants: A cross-ethnic and socioeconomic study. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Chapter 59—Worldwide Vitamin D Status. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 15–40. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Frost, P. Vitamin D deficiency among northern Native Peoples: A real or apparent problem? Int. J. Circumpolar Health 2012, 71, 18001. [Google Scholar] [CrossRef]
- Arrazola, J.; Masiello, M.M.; Joshi, S.; Dominguez, A.E.; Poel, A.; Wilkie, C.M.; Bressler, J.M.; McLaughlin, J.; Kraszewski, J.; Komatsu, K.K. COVID-19 Mortality Among American Indian and Alaska Native Persons—14 States, January–June 2020. Morb. Mort. Wkly. Rep. 2020, 69, 1853. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis. Int. J. Env. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef]
- Sabetta, J.R.; DePetrillo, P.; Cipriani, R.J.; Smardin, J.; Burns, L.A.; Landry, M.L. Serum 25-Hydroxyvitamin D and the Incidence of Acute Viral Respiratory Tract Infections in Healthy Adults. PLoS ONE 2010, 5, e11088. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Houston, D.K.; Bandinelli, S.; Sun, K.; Cherubini, A.; Cappola, A.R.; Guralnik, J.M.; Ferrucci, L. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur. J. Clin. Nutr. 2010, 64, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Targher, G.; Smits, G.; Chonchol, M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 2009, 205, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Tjønneland, A.; Olsen, A.; Halkjær, J.; Lind, B.; Heegaard, A.-M.; Schwarz, P. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J. Clin. Endocrinol. Metab. 2015, 100, 2339–2346. [Google Scholar] [CrossRef]
- Lim, S.; Kim, M.J.; Lim, S.; Kim, M.J.; Choi, S.H.; Shin, C.S.; Park, K.S.; Jang, H.C.; Billings, L.K.; Meigs, J.B.; et al. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am. J. Clin. Nutr. 2013, 97, 524–530. [Google Scholar] [CrossRef]
- Ke, L.; Graubard, B.I.; Albanes, D.; Fraser, D.R.; Weinstein, S.J.; Virtamo, J.; Brock, K.E. Hypertension, Pulse, and Other Cardiovascular Risk Factors and Vitamin D Status in Finnish Men. Am. J. Hypertens. 2013, 26, 951–956. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Kronenberger, B.; Köberle, V.; Bojunga, J.; Zeuzem, S.; Trojan, J.; Piiper, A.; Waidmann, O. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma—a prospective cohort study. Aliment. Pharm. Ther. 2014, 39, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Buring, J.E.; Manson, J.E.; Giovannucci, E.L.; Moorthy, M.V.; Zhang, S.; Lee, I.-M.; Lin, J.H. Circulating Vitamin D Levels and Risk of Colorectal Cancer in Women. Cancer Prev. Res. 2015, 8, 675–682. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-Hydroxyvitamin D Concentrations ≥40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Bleizgys, A. Vitamin D and COVID-19: It is time to act. Int. J. Clin. Pr. 2020, e13748. [Google Scholar] [CrossRef]
- Ghasemian, R.; Shamshirian, A.; Heydari, K.; Malekan, M.; Alizadeh-Navaei, R.; Ebrahimzadeh, M.A.; Jafarpour, H.; Shahmirzadi, A.R.; Khodabandeh, M.; Seyfari, B.; et al. The Role of Vitamin D in The Age of COVID-19: A Systematic Review and Meta-Analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Björkhem-Bergman, L.; Ekström, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. Bmj Open 2012, 2. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Med. (Baltim.) 2019, 98, e17252. [Google Scholar] [CrossRef]
- Kohlmeier, M. Avoidance of vitamin D deficiency to slow the COVID-19 pandemic. Bmj Nutr Prev Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, P.B. COVID-19 fatalities, latitude, sunlight, and vitamin D. Am. J. Infect. Control 2020, 48, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharm. Ther. 2020. [Google Scholar] [CrossRef]
- Walrand, S. Autumn COVID-19 surge dates in Europe correlated to latitudes, not to temperature-humidity, pointing to vitamin D as contributing factor. Sci. Rep. 2021, 11, 1981. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Ann. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020, 93, 508–511. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Di Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2020. [Google Scholar] [CrossRef]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.-H.; Kwon, H.Y.; Lee, J.-S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Cacho, P.M.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J.; et al. Vitamin D Status in Hospitalized Patients With SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Pizzini, A.; Aichner, M.; Sahanic, S.; Böhm, A.; Egger, A.; Hoermann, G.; Kurz, K.; Widmann, G.; Bellmann-Weiler, R.; Weiss, G.; et al. Impact of Vitamin D Deficiency on COVID-19—A Prospective Analysis from the CovILD Registry. Nutrients 2020, 12, 2775. [Google Scholar] [CrossRef]
- Ye, K.; Tang, F.; Liao, X.; Shaw, B.A.; Deng, M.; Huang, G.; Qin, Z.; Peng, X.; Xiao, H.; Chen, C.; et al. Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity?-A Case-Control Study. J. Am. Coll. Nutr. 2020. [Google Scholar] [CrossRef]
- Israel, A.; Cicurel, A.A.; Feldhamer, I.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. The link between vitamin D deficiency and Covid-19 in a large population. medRxiv 2020. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Jahaj, E.; Pratikaki, M.; Orfanos, S.E.; Dimopoulou, I.; Kotanidou, A. Low 25-Hydroxyvitamin D Levels on Admission to the Intensive Care Unit May Predispose COVID-19 Pneumonia Patients to a Higher 28-Day Mortality Risk: A Pilot Study on a Greek ICU Cohort. Nutrients 2020, 12, 3773. [Google Scholar] [CrossRef] [PubMed]
- Karahan, S.; Katkat, F. Impact of Serum 25(OH) Vitamin D Level on Mortality in Patients with COVID-19 in Turkey. J. Nutr. Health Aging 2021, 25, 189–196. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Belanger, M.J.; Lorinsky, M.K.; Karamanis, D.; Chamorro-Pareja, N.; Ognibene, J.; Palaiodimos, L.; Mantzoros, C.S. Vitamin D Status is Associated With In-hospital Mortality and Mechanical Ventilation: A Cohort of COVID-19 Hospitalized Patients. Mayo Clin. Proceed. 2021. [Google Scholar] [CrossRef]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. Clin. Res. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Van Den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2020. [Google Scholar] [CrossRef]
- Liu, N.; Sun, J.; Wang, X.; Zhang, T.; Zhao, M.; Li, H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 58–64. [Google Scholar] [CrossRef]
- Tabrizi, R.; Moosazadeh, M.; Akbari, M.; Dabbaghmanesh, M.H.; Mohamadkhani, M.; Asemi, Z.; Heydari, S.T.; Akbari, M.; Lankarani, K.B. High Prevalence of Vitamin D Deficiency among Iranian Population: A Systematic Review and Meta-Analysis. Iran J. Med. Sci. 2018, 43, 125–139. [Google Scholar]
- Larijani, B.; Hossein-Nezhad, A.; Feizabad, E.; Maghbooli, Z.; Adibi, H.; Ramezani, M.; Taheri, E. Vitamin D deficiency, bone turnover markers and causative factors among adolescents: A cross-sectional study. J. Diabetes Metab. Disord. 2016, 15, 46. [Google Scholar] [CrossRef]
- John Hopkins University. John Hopkins University & Medicine: Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 9 November 2020).
- Pereira-Santos, M.; Santos, J.; Carvalho, G.Q.; Santos, D.B.D.; Oliveira, A.M. Epidemiology of vitamin D insufficiency and deficiency in a population in a sunny country: Geospatial meta-analysis in Brazil. Crit. Rev. Food Sci. Nutr. 2019, 59, 2102–2109. [Google Scholar] [CrossRef]
- Pango lineages. P.1 Report 31 January 2021. Available online: https://cov-lineages.org/global_report_P.1.html (accessed on 31 January 2021).
- Faria, N.R.; Claro, I.M.; Candido, D.; Moyses Franco, L.A.; Andrade, P.S.; Coletti, T.M.; Silva, C.A.M.; Sales, F.C.; Manuli, E.R.; Aguiar, R.S.; et al. Genomic Characterisation of an Emergent SARS-CoV-2 Lineage in Manaus: Preliminary Findings. Available online: https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586 (accessed on 31 January 2021).
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef]
- Itkonen, S.T.; Andersen, R.; Björk, A.K.; Brugård Konde, Å.; Eneroth, H.; Erkkola, M.; Holvik, K.; Madar, A.A.; Meyer, H.E.; Tetens, I.; et al. Vitamin D status and current policies to achieve adequate vitamin D intake in the Nordic countries. Scand. J. Public Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and inflammation: Potential implications for severity of COVID-19. Ir. Med. J. 2020, 113, 81. [Google Scholar]
- Brenner, H. Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths—Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action. Nutrients 2021, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Hussein, M.H.; Toraih, E.A.; Elshazli, R.M.; Jardak, C.; Sultana, N.; Youssef, M.R.; Omar, M.; Attia, A.S.; Fawzy, M.S.; et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J. Med. Virol. 2021, 93, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D Deficiency Is Inversely Associated with COVID-19 Incidence and Disease Severity in Chinese People. T. J. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.A.; Drenos, F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: A Mendelian randomisation study of open data. Bmj Nutr. Prev. Health 2021. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, T.; Heianza, Y.; Qi, L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: A prospective study in UK Biobank. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. Jama Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Med. (KaunasLith. ) 2019, 55, 541. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Inserra, F.; Ferder, L.; García, J.; Manucha, W. Vitamin D deficiency in African Americans is associated with a high risk of severe disease and mortality by SARS-CoV-2. J. Hum. Hypertens. 2020. [Google Scholar] [CrossRef] [PubMed]
- Danik, J.S.; Manson, J.E. Vitamin d and cardiovascular disease. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 414–424. [Google Scholar] [CrossRef]
- de Paula, T.P.; Moreira, J.S.R.; Sperb, L.F.; Muller, M.E.P.; Steemburgo, T.; Viana, L.V. Efficacy of single-dose cholecalciferol in the blood pressure of patients with type 2 diabetes, hypertension and hypovitaminoses D. Sci. Rep. 2020, 10, 19611. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020, 32, 2141–2158. [Google Scholar] [CrossRef]
- Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M. Vitamin D and Influenza—Prevention or Therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.; García-Sastre, A.; Ruchala, P.; Lehrer, R.I.; Chang, T.; Klotman, M.E. β-Defensin Inhibits Influenza Virus Replication by Cell-Mediated Mechanism(s). J Infect. Dis. 2007, 196, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Vargas, J., Jr.; DelPortillo, A.; Klotman, M.E. Dual role of α-defensin-1 in anti–HIV-1 innate immunity. J. Clin. Invest. 2005, 115, 765–773. [Google Scholar] [CrossRef]
- Dixon, B.M.; Barker, T.; McKinnon, T.; Cuomo, J.; Frei, B.; Borregaard, N.; Gombart, A.F. Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults. Bmc Res. Notes 2012, 5, 575. [Google Scholar] [CrossRef]
- Jeng, L.; Yamshchikov, A.V.; Judd, S.E.; Blumberg, H.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Assessing vitamin D status. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 440–444. [Google Scholar] [CrossRef]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo Jr, C.A.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Wang, J.; Kaplan, N.; Wysocki, J.; Yang, W.; Lu, K.; Peng, H.; Batlle, D.; Lavker, R.M. The ACE2-deficient mouse: A model for a cytokine storm-driven inflammation. Faseb J. 2020, 34, 10505–10515. [Google Scholar] [CrossRef] [PubMed]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K. Vitamin D deficiency and co-morbidities in COVID-19 patients—A fatal relationship? Nfs J. 2020, 20, 10–21. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gupta, P.; Banerjee, D. Letter: Does vitamin D have a potential role against COVID-19? Aliment. Pharm. 2020, 52, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.J. Renin-Angiotensin System Blockade in COVID-19: Good, Bad, or Indifferent? J. Am. Coll. Cardiol. 2020, 76, 277–279. [Google Scholar] [CrossRef]
- Hadizadeh, F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Young, D.; Coupland, C.; Channon, K.M.; Tan, P.S.; Harrison, D.A.; Rowan, K.; Aveyard, P.; Pavord, I.D.; Watkinson, P.J. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart 2020, 106, 1503–1511. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. NEJM 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. (Lond) 2020, 20, e107–e108. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2020. [Google Scholar] [CrossRef]
- Song, W.-C.; FitzGerald, G.A. COVID-19, microangiopathy, hemostatic activation, and complement. J. Clin. Invest. 2020, 130, 3950–3953. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yuan, J.; Li, X.; Bao, Y.; Hou, Y.; Li, Z.; Tan, S.C.; Low, T.Y.; Chu, Y. Association between serum vitamin D levels and venous thromboembolism (VTE): A systematic review and meta-analysis of observational studies. Complementary Ther. Med. 2020, 54, 102579. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, A.C. Forty years since the structural elucidation of platelet-activating factor (PAF): Historical, current, and future research perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Benskin, L. A Basic Review of the Preliminary Evidence That COVID-19 Risk and Severity Is Increased in Vitamin D Deficiency. Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Brenner, H.; Schöttker, B. Vitamin D Insufficiency May Account for Almost Nine of Ten COVID-19 Deaths: Time to Act. Comment on: “Vitamin D Deficiency and Outcome of COVID-19 Patients”. Nutrients 2020, 12, 3642. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 323, 1891–1892. [Google Scholar] [CrossRef]
- Belanger, M.J.; Hill, M.A.; Angelidi, A.M.; Dalamaga, M.; Sowers, J.R.; Mantzoros, C.S. Covid-19 and Disparities in Nutrition and Obesity. NEJM 2020, 383, e69. [Google Scholar] [CrossRef]
- Médicine, A.N.D. Press release from the National Academy of Medicine: Vitamin D and Covid-19. Available online: http://www.academie-medecine.fr/communique-de-lacademie-nationale-de-medecine-vitamine-d-et-covid-19/ (accessed on 20 November 2020).
- Torjesen, I. Covid-19: Public health agencies review whether vitamin D supplements could reduce risk. BMJ 2020, 369, m2475. [Google Scholar] [CrossRef]
- NHS. Vitamin D. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/ (accessed on 20 November 2020).
- Scottish Government. Vitamin D: Advice for all Age Groups. Available online: https://www.gov.scot/publications/vitamin-d-advice-for-all-age-groups/ (accessed on 20 November 2020).
- The Times. Coronavirus in Scotland: Vulnerable will Receive Vitamin D Supplements. Available online: https://www.thetimes.co.uk/article/coronavirus-in-scotland-vulnerable-will-receive-vitamin-d-supplements-zc8stdpkh (accessed on 20 November 2020).
- Pinter, P. Rapid Response: Covid-19: Vitamin D a risk-assessment tool and to reduce morbidity and mortality in covid-19 pandemic. BMJ 2020, 369, m1820. [Google Scholar]
- Laird, E.; Kenny, R.A. Vitamin D deficiency in Ireland—Implications for COVID-19. Results from The Irish Longitudinal Study on Ageing (TILDA); Trinity College Dublin: Dublin, Ireland, 2020. [Google Scholar]
- McCartney, D.M.; O’Shea, P.M.; Faul, J.L.; Healy, M.J.; Byrne, G.; Griffin, T.P.; Walsh, J.B.; Byrne, D.G.; Kenny, R.A. Vitamin D and SARS-CoV-2 infection—evolution of evidence supporting clinical practice and policy development. Irish Journal of Medical Science (1971) 2020. [Google Scholar] [CrossRef] [PubMed]
- Health Service Executive Ireland. Can your Diet Prevent Covid-19? Available online: https://www.hse.ie/eng/services/news/media/pressrel/can-your-diet-prevent-covid-19-.html (accessed on 20 November 2020).
- Healthy Ireland. Eat Well. Available online: https://www.gov.ie/en/publication/da7f19-eat-well/?referrer=http://www.gov.ie/healthyireland/eatwell (accessed on 30 November 2020).
- MacDonell, S.O.; Miller, J.C.; Harper, M.J.; Waters, D.L.; Houghton, L.A. Vitamin D status and its predictors in New Zealand aged-care residents eligible for a government-funded universal vitamin D supplementation programme. Public Health Nutr. 2016, 19, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. “Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Healthcare and Excellence (NICE). Medicines Evidence Commentary: Commentary on Important New Evidence from Medicines Awareness Weekly. Available online: https://www.nice.org.uk/advice/es28/chapter/Key-messages (accessed on 20 November 2020).
- Jungreis, I.; Kellis, M. Mathematical analysis of Córdoba calcifediol trial suggests strong role for Vitamin D in reducing ICU admissions of hospitalized COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79, 111017. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Hanotte, B.; Grandin de l’Eprevier, C.; Sabatier, J.-M.; Lafaie, L.; Célarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of Vitamin D Supplementation vs Placebo on Hospital Length of Stay in Patients with Severe COVID-19: A Multicenter, Double-blind, Randomized Controlled Trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Wang, R.; DeGruttola, V.; Lei, Q.; Mayer, K.H.; Redline, S.; Hazra, A.; Mora, S.; Willett, W.C.; Ganmaa, D.; Manson, J.E. The vitamin D for COVID-19 (VIVID) trial: A pragmatic cluster-randomized design. Contemp. Clin. Trials 2020, 100, 106176. [Google Scholar] [CrossRef]
- Martineau, A.R.; Forouhi, N.G. Vitamin D for COVID-19: A case to answer? Lancet Diabetes Endocrinol. 2020, 8, 735–736. [Google Scholar] [CrossRef]
- Guha, C.; Osawa, M.; Werner, P.A.; Galbraith, R.M.; Paddock, G.V. Regulation of human Gc (vitamin d—binding) protein levels: Hormonal and cytokine control of gene expression in vitro. Hepatology 1995, 21, 1675–1681. [Google Scholar] [CrossRef]
- Lee, P.; Eisman, J.A.; Center, J.R. Vitamin D Deficiency in Critically Ill Patients. NEJM 2009, 360, 1912–1914. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharm. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals—shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharm. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharm. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- The United States Food and Drug Administration. NDI 1157—Oleandrin from Phoenix Biotechnology, Inc. Available online: https://beta.regulations.gov/document/FDA-2020-S-0023-0068 (accessed on 20 November 2019).
- Halford, B. What is Oleandrin, the Compound Touted as a Possible COVID-19 Treatment? Available online: https://cen.acs.org/biological-chemistry/natural-products/oleandrin-compound-touted-possible-COVID/98/web/2020/08 (accessed on 12 November 2020).
- The Federal Trade Commission. FTC Sues California Marketer of $23,000 COVID-19 “Treatment” Plan. Available online: https://www.ftc.gov/news-events/press-releases/2020/07/ftc-sues-california-marketer-23000-covid-19-treatment-plan (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Noetic Nutraceuticals MARCS-CMS 607572—15 May 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/noetic-nutraceuticals-607572-05152020 (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: LVWellness & Aesthetics MARCS-CMS 609821—15 October 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/lvwellness-aesthetics-609821-10152020 (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Spartan Enterprises Inc. dba Watershed Wellness Center. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/spartan-enterprises-inc-dba-watershed-wellness-center-610876-10302020 (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Peterson Research Laboratories LLC MARCS-CMS 607439—23 October 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/peterson-research-laboratories-llc-607439-10232020 (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Predator Nutrition MARCS-CMS 607136—23 October 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/predator-nutrition-607136-10232020 (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Beepothecary LLC MARCS-CMS 608383—23 October 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/beepothecary-llc-608383-10232020 (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Pro Breath MD, LLC dba Dentist Select and OraCare MARCS-CMS 610686—18 november 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/pro-breath-md-llc-dba-dentist-select-and-oracare-610686-11182020 (accessed on 20 November 2020).
- Canada Health. Health Product Advertising Incidents Related to COVID-19. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/health-product-advertising-incidents.html (accessed on 20 November 2020).
- The United States Food and Drug Administration. Warning Letter: Fusion Health and Vitality LLC MARCS-CMS 607545—11 May 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/fusion-health-and-vitality-llc-607545-05112020 (accessed on 16 February 2021).
- The United States Food and Drug Administration. FDA News Release: Coronavirus (COVID-19) Update: 8 January 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-january-8-2021 (accessed on 16 February 2021).
- Henrina, J.; Lim, M.A.; Pranata, R. COVID-19 and misinformation: How an infodemic fuelled the prominence of vitamin D. Br. J. Nutr. 2021, 125, 359–360. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordan, R. Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review. Nutrients 2021, 13, 740. https://doi.org/10.3390/nu13030740
Lordan R. Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review. Nutrients. 2021; 13(3):740. https://doi.org/10.3390/nu13030740
Chicago/Turabian StyleLordan, Ronan. 2021. "Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review" Nutrients 13, no. 3: 740. https://doi.org/10.3390/nu13030740
APA StyleLordan, R. (2021). Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review. Nutrients, 13(3), 740. https://doi.org/10.3390/nu13030740





