Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Article
2.2. Enterotoxin Screen (In Silico)
2.3. Enterotoxin Screen (In Vitro)
2.4. Antibiotic Sensitivity Testing
2.5. Minimum Inhibitory Concentration
2.6. Oral Toxicity Study in Rats
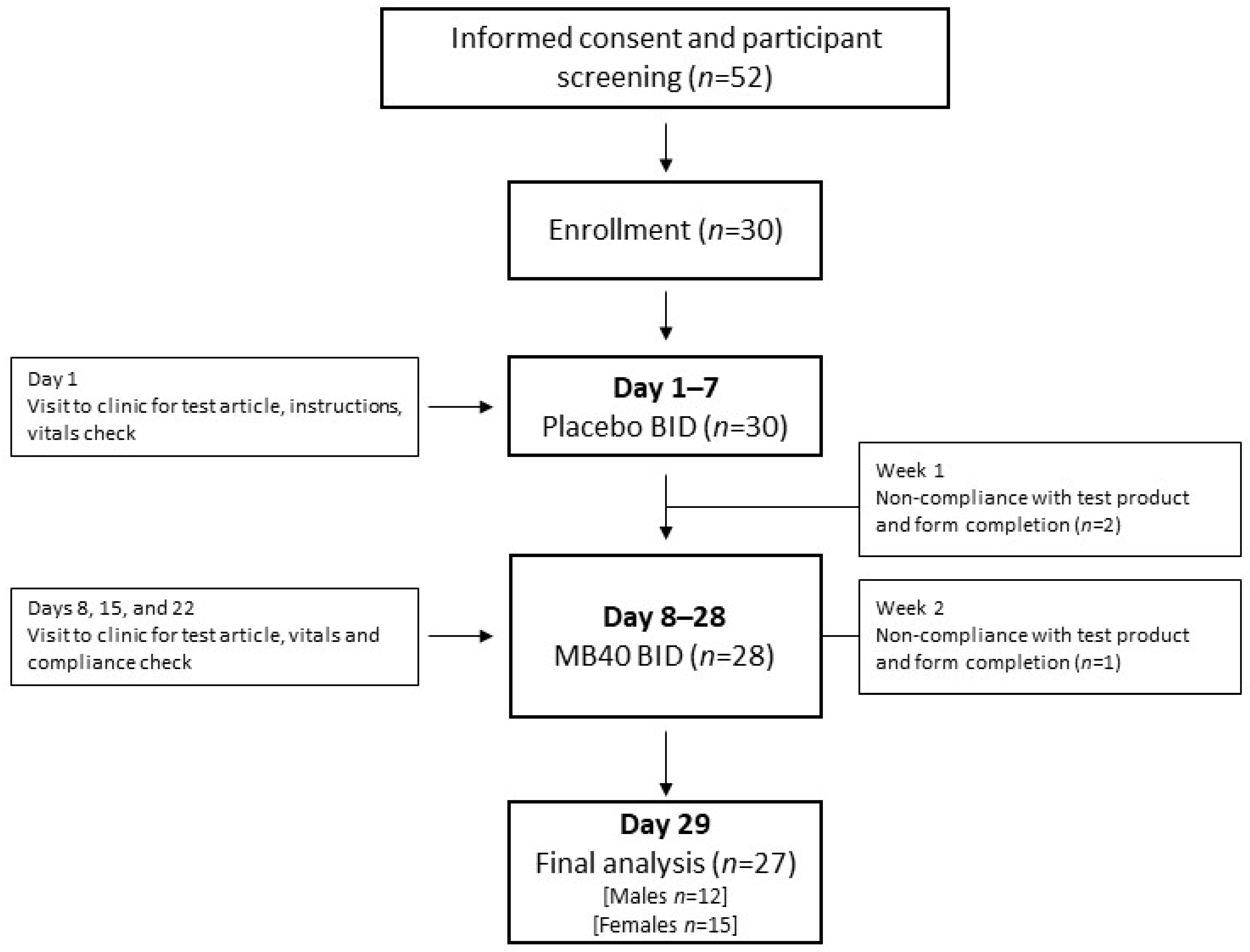
2.7. Human Clinical Safety and Tolerability Trial (Single-Blind Design)
3. Results
3.1. Subsection Enterotoxin Screens (In Silico)
3.2. Enterotoxin Screens (In Vitro)
3.3. Antibiotic Sensitivity Testing (AST)
3.4. Oral Toxicity Study in Rats
3.5. Human Clinical Safety and Tolerability Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metchnikoff, E. The Prolongation of Life; Mitchell, P.C., Ed.; G. P. Putnam’s Sons: New York, NY, USA; London, UK, 1908. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Stecker, R.A.; Moon, J.M.; Russo, T.J.; Ratliff, K.M.; Mumford, P.W.; Jäger, R.; Purpura, M.; Kerksick, C.M. Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein. Nutr. Metab. 2020, 17. [Google Scholar] [CrossRef]
- Kalman, D.S.; Schwartz, H.I.; Alvarez, P.; Feldman, S.; Pezzullo, J.C.; Krieger, D.R. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Penet, C.; Kramer, R.; Little, R.; Spears, J.L.; Parker, J.; Iyer, J.K.; Guthrie, N.; Evans, M. A randomized, double-blind, placebo-controlled, parallel study evaluating the efficacy of Bacillus subtilis MB40 to reduce abdominal discomfort, gas, and bloating. Altern. Ther. Health Med. 2019, 25. (Online ahead of print). [Google Scholar]
- Fakhry, S.; Sorrentini, I.; Ricca, E.; De Felice, M.; Baccigalupi, L. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J. Appl. Microbiol. 2008, 105, 2178–2186. [Google Scholar] [CrossRef]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef]
- Hoyles, L.; Honda, H.; Logan, N.A.; Halket, G.; La Ragione, R.M.; McCartney, A.L. Recognition of greater diversity of Bacillus species and related bacteria in human faeces. Res. Microbiol. 2012, 163, 3–13. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. History of Natto and Its Relatives; Soyinfo Center: Lafayette, CA, USA, 2012; ISBN 9781928914426. [Google Scholar]
- Jeon, H.L.; Lee, N.K.; Yang, S.J.; Kim, W.S.; Paik, H.D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Kotb, E. Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl. Biochem. Microbiol. 2015, 51, 34–43. [Google Scholar] [CrossRef]
- Chantawannakul, P.; Oncharoen, A.; Klanbut, K.; Chukeatirote, E.; Lumyong, S. Characterization of proteases of Bacillus subtilis strain 38 isolated from traditionally fermented soybean in Northern Thailand. ScienceAsia 2002, 28, 241–245. [Google Scholar] [CrossRef]
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Watanasiritum, L.; Kawamoto, S. Characterization of Bacillus subtilis strains in Thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 2006, 43, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sorokulova, I.B.; Pinchuk, I.; Denayrolles, M.; Osipova, I.G.; Huang, J.M.; Cutting, S.M.; Urdaci, M.C. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008, 53, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Kim, W.S.; Paik, H.D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Horosheva, T.V.; Vodyanoy, V.; Sorokulova, I. Efficacy of Bacillus probiotics in prevention of antibiotic-associated diarrhoea: A randomized, double-blind, placebo-controlled clinical trial. JMM Case Rep. 2014, 1. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef]
- Paytuví-Gallart, A.; Sanseverino, W.; Winger, A.M. Daily intake of probiotic strain Bacillus subtilis DE111 supports a healthy microbiome in children attending day-care. Benef. Microbes 2020, 11, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e06174. [Google Scholar] [CrossRef]
- Rowan, N.J.; Deans, K.; Anderson, J.G.; Gemmell, C.G.; Hunter, I.S.; Chaithong, T. Putative Virulence Factor Expression by Clinical and Food Isolates of Bacillus spp. after Growth in Reconstituted Infant Milk Formulae. Appl. Environ. Microbiol. 2001, 67, 3873–3881. [Google Scholar] [CrossRef][Green Version]
- Pariza, M.W.; Gillies, K.O.; Kraak-Ripple, S.F.; Leyer, G.; Smith, A.B. Determining the safety of microbial cultures for consumption by humans and animals. Regul. Toxicol. Pharmacol. 2015, 73, 164–171. [Google Scholar] [CrossRef] [PubMed]
- San Millán, R.M.; Martínez-Ballesteros, I.; Rementeria, A.; Garaizar, J.; Bikandi, J. Online exercise for the design and simulation of PCR and PCR-RFLP experiments. BMC Res. Notes 2013, 6, 513. [Google Scholar] [CrossRef] [PubMed]
- Agata, N.; Ohta, M.; Mori, M.; Isobe, M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 1995, 129, 17–20. [Google Scholar] [CrossRef]
- Asano, S.-I.; Nukumizu, Y.; Bando, H.; Iizuka, T.; Yamamoto, T. Cloning of novel enterotoxin genes from Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 1997, 63, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Mäntynen, V.; Lindström, K. A Rapid PCR-Based DNA Test for Enterotoxic Bacillus cereus A Rapid PCR-Based DNA Test for Enterotoxic Bacillus cereus. Appl. Environ. Microbiol. 1998, 64, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M02-A11: Performance Standards for Antimicrobial Disk Susceptibility Tests, 11th ed.; Clincial and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, ISBN 1562384856. [Google Scholar]
- Patel, J.B.; Cockerill, R.F.; Bradford, A.P.; Eliopoulos, M.G.; Hindler, A.J.; Jenkins, G.S.; Lewis, S.J.; Limbago, B.; Miller, A.L.; Nicolau, P.D. M07-A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35, ISBN 1562389874. [Google Scholar]
- US Food and Drug Administration. Redbook 2000 Guidance for Industry and Other Stakeholders Toxicological Principles for the Safety Assessment of Food Ingredients Redbook 2000 Page 1 of 4 Redbook 2000; US Food and Drug Administration: Washington, DC, USA, 2007; Volume 3.
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Snedecor, G.W.; George, W.; Cochran, W.G.; William, G. Statistical Methods; Iowa State University Press: Iowa City, IA, USA, 1980; ISBN 0813815606. [Google Scholar]
- Dunnett, C.W. New Tables for Multiple Comparisons with a Control. Biometrics 1964, 20, 482. [Google Scholar] [CrossRef]
- Sander, C.; Schneider, R. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins 1991, 9, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Beecher, D.J.; Wong, A.C.L. Identification and analysis of the antigens detected by two commercial Bacillus cereus diarrheal enterotoxin immunoassay kits. Appl. Environ. Microbiol. 1994, 60, 4614–4616. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 1–10. [CrossRef]
- Adimpong, D.B.; Sørensen, K.I.; Thorsen, L.; Stuer-Lauridsen, B.; Abdelgadir, W.S.; Nielsen, D.S.; Derkx, P.M.F.; Jespersen, L. Antimicrobial susceptibility of bacillus strains isolated from primary starters for african traditional bread production and characterization of the bacitracin operon and bacitracin biosynthesis. Appl. Environ. Microbiol. 2012, 78, 7903–7914. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement Clinical and Laboratory Standards Institute; Clincial and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 32, ISBN 6106880700. [Google Scholar]
- Jackson, S.A.; Schoeni, J.L.; Vegge, C.; Pane, M.; Stahl, B.; Bradley, M.; Goldman, V.S.; Burguière, P.; Atwater, J.B.; Sanders, M.E. Improving end-user trust in the quality of commercial probiotic products. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef] [PubMed]
- Morovic, W.; Roper, J.M.; Smith, A.B.; Mukerji, P.; Stahl, B.; Rae, J.C.; Ouwehand, A.C. Safety evaluation of HOWARU® Restore (Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lpc-37, Bifidobacterium animalis subsp. lactis Bl-04 and B. lactis Bi-07) for antibiotic resistance, genomic risk factors, and acute toxicity. Food Chem. Toxicol. 2017, 110, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; Huang, J.M.; Khaneja, R.; Hiep, L.V.; Urdaci, M.C.; Cutting, S.M. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl. Microbiol. 2008, 105, 510–520. [Google Scholar] [CrossRef]
- Tompkins, T.A.; Hagen, K.E.; Wallace, T.D.; Fillion-Forté, V. Safety evaluation of two bacterial strains used in asian probiotic products. Can. J. Microbiol. 2008, 54, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, T.; Xu, X.; Ahmarani, J. A comprehensive review of post-market clinical studies performed in adults with an Asian probiotic formulation. Benef. Microbes 2010, 1, 93–106. [Google Scholar] [CrossRef]
- Hanifi, A.; Culpepper, T.; Mai, V.; Anand, A.; Ford, A.L.; Ukhanova, M.; Christman, M.; Tompkins, T.A.; Dahl, W.J. Evaluation of Bacillus subtilis R0179 on gastrointestinal viability and general wellness: A randomised, double-blind, placebo-controlled trial in healthy adults. Benef. Microbes 2015, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
| Test Group | Disc Code | Inhibition Zone (mm) | Zone Interpretation |
|---|---|---|---|
| Aminoglycosides | |||
| Kanamycin | K 30 | 35 | S |
| Gentamicin | GM 10 | 33 | S |
| Neomycin | N 30 | 29 | S |
| Streptomycin | S 10 | 15 | S |
| b-Lactams: Penicillins | |||
| Penicillin | P 10 | 29 | S |
| Ampicillin | AM 10 | 28 | Int |
| Amoxicillin/Clavulanic Acid | AmC 30 | 31 | S |
| b-Lactams: Cephems | |||
| Cephalothin | CF 30 | 52 | S |
| Cefotaxime | CTX 30 | 25 | S |
| Cefaclor | CEC 30 | 50 | S |
| Ceftriaxone | CRO 30 | 26 | S |
| Fluorquinolones | |||
| Ciprofloxacin | CIP 5 | 30 | S |
| Fosfomycins | |||
| Fosfomycin + Glucose-6-Phosphate | FOS 200 | 6 | R |
| Folate Pathway Inhibitors | |||
| Sulfamethoxazole Trimethorprim | SXT | 32 | S |
| Glycopeptides | |||
| Vancomycin | Va 5 | 17 | S |
| Macrolides, Lincosamides, Streptogramins | |||
| Clindamycin | CC 2 | 22 | S |
| Erythromycin | E 15 | 33 | S |
| Quinupristin/Dalfopristin | SYN 15 | 20 | S |
| Phenicols | |||
| Chloramphenicol | C 30 | 30 | S |
| Rifampin | |||
| Rifampin | RA 5 | 18 | Int |
| Tetracyclines | |||
| Tetracycline | Te 30 | 19 | S |
| Antibiotic | MIC (µg/mL) | |||
|---|---|---|---|---|
| EFSA Cut-off Values for Bacillus [36] | MB40 | S. aureus | E. faecalis | |
| Vancomycin | 4 | 0.5 | 1 | |
| Gentamicin | 4 | 0.125 | 0.25 | |
| Kanamycin | 8 | 1 | 2 | |
| Streptomycin | 8 | >32 | N/A | 32 * |
| Erythromycin | 4 | 0.125 | 0.25 | |
| Clindamycin | 4 | 2 | 0.125 | |
| Tetracycline | 8 | 4 | 0.5 | |
| Chloramphenicol | 8 | 4 | 8 | |
| Parameter | Units | Group and Dose (mg/kg bw/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| Control n 2 = 9 | 500 n = 10 | 1000 n 2 = 9 | 2000 n = 10 | Control n = 10 | 500 n 2 = 8 | 1000 n 2 = 8 | 2000 n = 10 | ||
| WBC | ×103/µL | 10.36 ± 1.70 | 10.22 ± 2.47 | 10.27 ± 2.43 | 9.91 ± 2.24 | 6.84 ± 2.08 | 6.86 ± 1.79 | 7.10 ± 1.77 | 7.66 ± 2.00 |
| RBC | ×106/µL | 7.45 ± 0.35 | 7.83 ± 0.29 * | 7.71 ± 0.34 | 7.53 ± 0.28 | 7.98 ± 0.33 | 7.73 ± 0.52 | 7.89 ± 0.37 | 7.86 ± 0.36 |
| HGB | g/dL | 15.2 ± 0.56 | 15.6 ± 0.62 | 15.5 ± 0.57 | 15.2 ± 0.56 | 16.1 ± 0.74 | 15.6 ± 0.49 | 15.8 ± 0.41 | 15.7 ± 0.59 |
| HCT | % | 46.2 ± 1.65 | 47.7 ± 2.04 | 47.3 ± 1.42 | 46.5 ± 1.60 | 47.2 ± 2.68 | 45.7 ± 1.69 | 46.7 ± 1.12 | 46.8 ± 1.92 |
| MCV | fL | 62.2 ± 1.23 | 60.9 ± 2.25 | 61.4 ± 1.19 | 61.8 ± 1.58 | 59.1 ± 1.29 | 59.3 ± 2.23 | 59.3 ± 1.66 | 59.6 ± 1.68 |
| MCH | pg | 20.4 ± 0.45 | 19.9 ± 0.81 | 20.2 ± 0.47 | 20.3 ± 0.55 | 20.1 ± 0.39 | 20.3 ± 0.96 | 20.1 ± 0.66 | 20.0 ± 0.51 |
| MCHC | g/dL | 32.9 ± 0.52 | 32.7 ± 0.61 | 32.8 ± 0.65 | 32.8 ± 0.51 | 34.1 ± 0.54 | 34.2 ± 0.64 | 33.9 ± 0.57 | 33.6 ± 0.44 |
| RDW | % | 12.4 ± 0.30 | 12.6 ± 0.41 | 12.6 ± 0.57 | 12.4 ± 0.42 | 11.3 ± 0.35 | 11.3 ± 0.35 | 11.2 ± 0.23 | 11.3 ± 0.32 |
| HDW | g/dL | 1.96 ± 0.07 | 1.96 ± 0.25 | 1.86 ± 0.13 | 1.90 ± 0.07 | 1.97 ± 0.10 | 1.92 ± 0.12 | 1.95 ± 0.11 | 1.93 ± 0.08 |
| MPV | fL | 7.44 ± 0.37 | 7.39 ± 0.23 | 7.39 ± 0.28 | 7.22 ± 0.38 | 6.95 ± 0.31 | 6.83 ± 0.23 | 6.98 ± 0.36 | 6.87 ± 0.26 |
| PLT | ×10³/µL | 948 ± 108.6 | 929 ± 171.3 | 762 ± 240.1 | 997 ± 212.9 | 898 ± 155.2 | 1007 ± 139.5 | 887 ± 310.2 | 1047 ± 119.5 |
| ANEU | ×10³/µL | 1.38 ± 0.22 | 2.09 ± 1.99 | 1.79 ± 0.68 | 1.51 ± 0.22 | 1.04 ± 0.26 | 1.26 ± 0.41 | 1.18 ± 0.40 | 1.21 ± 0.43 |
| ALYM | ×10³/µL | 8.59 ± 1.48 | 7.68 ± 1.85 | 8.09 ± 1.90 | 8.02 ± 2.17 | 5.55 ± 2.17 | 5.37 ± 1.67 | 5.68 ± 1.60 | 6.13 ± 1.88 |
| AMON | ×10³/µL | 0.23 ± 0.07 | 0.23 ± 0.10 | 0.21 ± 0.07 | 0.22 ± 0.09 | 0.10 ± 0.02 | 0.11 ± 0.04 | 0.10 ± 0.03 | 0.16 ± 0.10 |
| AEOS | ×10³/µL | 0.07 ± 0.03 | 0.13 ± 0.07 * | 0.11 ± 0.07 | 0.07 ± 0.03 | 0.11 ± 0.06 | 0.07 ± 0.02 | 0.09 ± 0.05 | 0.09 ± 0.06 |
| ABAS | ×10³/µL | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| ALUC | ×10³/µL | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.03 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.03 |
| ARET | ×10³/µL | 257 ± 25.8 | 290 ± 83.2 | 252 ± 37.3 | 242 ± 35.7 | 185 ± 38.4 | 195 ± 33.9 | 177 ± 20.2 | 188.8 ± 30.9 |
| PT 1 | sec | 16.5 ± 0.54 | 17.8 ± 0.71 † | 18.2 ± 0.61 † | 18.0 ± 0.78 † | 16.0 ± 0.81 | 16.9 ± 1.09 * | 16.5 ± 0.66 | 16.9 ± 0.63 |
| APTT 1 | sec | 11.7 ± 0.80 | 10.9 ± 0.87 | 10.9 ± 0.79 | 11.0 ± 1.07 | 11.5 ± 1.12 | 11.2 ± 0.58 | 11.6 ± 1.17 | 12.1 ± 1.51 |
| Parameter | Units | Group and Dose (mg/kg bw/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| Control n = 10 | 500 n = 10 | 1000 n = 10 | 2000 n = 10 | Control n = 10 | 500 n = 10 | 1000 n = 10 | 2000 n = 10 | ||
| ALB | g/dL | 3.7 ± 0.13 | 3.8 ± 0.16 | 3.6 ± 0.12 | 3.6 ± 0.13 | 4.1 ± 0.18 | 4.1 ± 0.13 | 4.2 ± 0.13 | 4.2 ± 0.16 |
| TP | g/dL | 6.0 ± 0.23 | 6.0 ± 0.24 | 5.9 ± 0.19 | 5.8 ± 0.26 | 6.5 ± 0.26 | 6.5 ± 0.21 | 6.6 ± 0.21 | 6.5 ± 0.23 |
| GLOB | g/dL | 2.3 ± 0.14 | 2.3 ± 0.13 | 2.3 ± 0.16 | 2.2 ± 0.13 | 2.4 ± 0.11 | 2.4 ± 0.09 | 2.4 ± 0.13 | 2.4 ± 0.16 |
| ALB/GLOB | 1.66 ± 0.10 | 1.67 ± 0.10 | 1.63 ± 0.13 | 1.62 ± 0.06 | 1.72 ± 0.08 | 1.73 ± 0.07 | 1.77 ± 0.11 | 1.76 ± 0.14 | |
| ALT | U/L | 43 ± 10.0 | 39 ± 3.9 | 38 ± 3.4 | 39 ± 4.2 | 24 ± 3.0 | 29 ± 4.4 * | 26 ± 4.1 | 29 ± 3.6 * |
| AST | U/L | 115 ± 16.7 | 109 ± 34.7 | 106 ± 21.7 | 102 ± 20.7 | 104 ± 18.4 | 125 ± 30.7 | 103 ± 25.0 | 116 ± 21.6 |
| ALP | U/L | 256 ± 48.5 | 291 ± 53.1 | 291 ± 60.3 | 260 ± 45.3 | 151 ± 35.7 | 167 ± 53.7 | 153 ± 30.6 | 151 ± 52.5 |
| GGT | U/L | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| SDH | U/L | 10 ± 5.6 | 9 ± 2.7 | 8 ± 2.5 | 7 ± 2.2 | 12 ± 7.8 | 9 ± 4.6 | 10 ± 3.2 | 12 ± 7.4 |
| TBIL | mg/dL | 0.00 ± 0.00 | 0.01 ± 0.03 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| CREAT | mg/dL | 0.22 ± 0.03 | 0.22 ± 0.03 | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.33 ± 0.07 | 0.32 ± 0.04 | 0.30 ± 0.02 | 0.34 ± 0.06 |
| BUN | mg/dL | 12.5 ± 0.83 | 12.6 ± 1.55 | 12.4 ± 1.66 | 12.6 ± 1.49 | 15.8 ± 1.69 | 17.4 ± 1.70 | 16.9 ± 3.12 | 16.8 ± 1.87 |
| Ca | mg/dL | 10.7 ± 0.28 | 10.6 ± 0.33 | 10.5 ± 0.26 | 10.5 ± 0.27 | 10.3 ± 0.16 | 10.2 ± 0.16 | 10.3 ± 0.21 | 10.3 ± 0.25 |
| Cl | mEq/L | 104 ± 1.1 | 104 ± 1.4 | 104 ± 1.2 | 104 ± 0.7 | 108 ± 1.0 | 108 ± 0.8 | 107 ± 1.5 | 106 ± 2.0 |
| PHOS | mg/dL | 9.9 ± 0.42 | 9.7 ± 0.53 | 9.7 ± 0.57 | 9.4 ± 0.31 | 7.5 ± 0.64 | 7.7 ± 0.57 | 7.4 ± 0.66 | 8.0 ± 0.84 |
| K | mEq/L | 5.63 ± 0.46 | 5.40 ± 0.43 | 5.37 ± 0.42 | 5.38 ± 0.28 | 5.31 ± 0.56 | 5.08 ± 0.56 | 5.04 ± 0.34 | 5.11 ± 0.40 |
| Na | mEq/L | 144 ± 0.7 | 144 ± 1.2 | 144 ± 1.2 | 144 ± 1.2 | 145 ± 1.4 | 145 ± 0.7 | 144 ± 1.1 | 144 ± 1.2 |
| GLU | mg/dL | 97 ± 4.8 | 106 ± 13.3 | 101 ± 8.3 | 104 ± 11.0 | 105 ± 6.9 | 106 ± 8.3 | 106 ± 7.9 | 103 ± 12.7 |
| CHOL | mg/dL | 67 ± 12.7 | 72 ± 13.6 | 64 ± 8.1 | 64 ± 12.1 | 62 ± 15.7 | 55 ± 7.4 | 60 ± 16.3 | 64 ± 9.4 |
| TRIG | mg/dL | 60 ± 17.0 | 62 ± 19.0 | 62 ± 21.5 | 56 ± 10.9 | 25 ± 3.5 | 22 ± 4.6 | 26 ± 7.1 | 26 ± 8.1 |
| Parameter | Units | Group and Dose (mg/kg bw/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| Control n = 10 | 500 n = 10 | 1000 n = 10 | 2000 n = 10 | Control n = 10 | 500 n = 10 | 1000 n = 10 | 2000 n = 10 | ||
| SPGRAV | 1.023 ± 0.011 | 1.028 ± 0.013 | 1.028 ± 0.012 | 1.032 ± 0.016 | 1.032 ± 0.014 | 1.034 ± 0.016 | 1.029 ± 0.007 | 1.027 ± 0.009 | |
| pH | 7.2 ± 0.48 | 7.3 ± 0.54 | 7.3 ± 0.42 | 7.1 ± 0.37 | 6.0 ± 0.28 | 5.9 ± 0.30 | 6.0 ± 0.24 | 5.9 ± 0.22 | |
| UROBIL | mg/dL | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 | 0.2 ± 0.00 |
| TVOL | mL | 9.1 ± 3.70 | 9.1 ± 5.93 | 8.2 ± 4.85 | 6.8 ± 3.91 | 6.1 ± 4.86 | 7.1 ± 7.39 | 6.0 ± 2.49 | 7.0 ± 4.18 |
| Parameter | Units | Group and Dose (mg/kg bw/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| Control n = 10 | 500 n = 10 | 1000 n = 10 | 2000 n = 10 | Control n = 10 | 500 n = 10 | 1000 n = 10 | 2000 n = 10 | ||
| TBW | g | 311 ± 28.8 | 322 ± 24.2 | 318 ± 24.0 | 312 ± 20.2 | 204 ± 15.2 | 206 ± 15.4 | 208 ± 11.4 | 208 ± 17.5 |
| Adrenals | g | 0.048 ± 0.006 | 0.056 ± 0.005 † | 0.054 ± 0.004 | 0.055 ± 0.007 * | 0.061 ± 0.008 | 0.066 ± 0.007 | 0.060 ± 0.007 | 0.066 ± 0.007 |
| Adrenals/TBW | g/100 g | 0.016 ± 0.002 | 0.018 ± 0.002 | 0.017 ± 0.001 | 0.018 ± 0.002 | 0.030 ± 0.003 | 0.032 ± 0.004 | 0.029 ± 0.004 | 0.032 ± 0.002 |
| Adrenals/TBRW | g/100 g | 2.550 ± 0.282 | 2.965 ± 0.310 † | 2.769 ± 0.201 | 2.836 ± 0.344 | 3.306 ± 0.434 | 3.545 ± 0.384 | 3.232 ± 0.336 | 3.620 ± 0.314 |
| Brain | g | 1.88 ± 0.08 | 1.91 ± 0.07 | 1.94 ± 0.08 | 1.94 ± 0.06 | 1.85 ± 0.07 | 1.87 ± 0.08 | 1.86 ± 0.07 | 1.83 ± 0.07 |
| Brain/TBW | g/100 g | 0.609 ± 0.052 | 0.595 ± 0.050 | 0.614 ± 0.048 | 0.622 ± 0.038 | 0.910 ± 0.053 | 0.910 ± 0.081 | 0.897 ± 0.044 | 0.885 ± 0.066 |
| Heart | g | 1.32 ± 0.15 | 1.39 ± 0.17 | 1.32 ± 0.16 | 1.31 ± 0.10 | 0.90 ± 0.10 | 0.91 ± 0.06 | 0.99 ± 0.14 | 0.94 ± 0.09 |
| Heart/TBW | g/100 g | 0.424 ± 0.034 | 0.431 ± 0.037 | 0.414 ± 0.038 | 0.420 ± 0.026 | 0.442 ± 0.034 | 0.441 ± 0.030 | 0.478 ± 0.083 | 0.451 ± 0.040 |
| Heart/TBRW | g/100 g | 69.964 ± 8.010 | 72.815 ± 8.187 | 67.761 ± 7.615 | 67.699 ± 5.146 | 48.757 ± 4.738 | 48.616 ± 3.470 | 53.345 ± 8.818 | 51.131 ± 4.620 |
| Kidneys | g | 2.71 ± 0.27 | 2.74 ± 0.22 | 2.69 ± 0.18 | 2.76 ± 0.27 | 1.69 ± 0.13 | 1.78 ± 0.19 | 1.73 ± 0.08 | 1.77 ± 0.14 |
| Kidneys/TBW | g/100 g | 0.871 ± 0.051 | 0.852 ± 0.039 | 0.848 ± 0.054 | 0.882 ± 0.049 | 0.829 ± 0.042 | 0.863 ± 0.075 | 0.836 ± 0.031 | 0.851 ± 0.065 |
| Kidneys/TBRW | g/100 g | 143.784 ± 13.822 | 144.088 ± 14.090 | 138.610 ± 10.194 | 142.450 ± 14.074 | 91.257 ± 4.822 | 95.578 ± 11.958 | 93.285 ± 4.083 | 96.257 ± 6.595 |
| Liver | g | 12.03 ± 1.44 | 12.17 ± 1.06 | 11.69 ± 1.12 | 11.83 ± 1.24 | 7.30 ± 0.75 | 7.70 ± 0.63 | 7.59 ± 0.61 | 7.91 ± 0.66 |
| Liver/TBW | g/100 g | 3.862 ± 0.224 | 3.788 ± 0.304 | 3.677 ± 0.167 | 3.784 ± 0.253 | 3.569 ± 0.216 | 3.738 ± 0.216 | 3.655 ± 0.208 | 3.810 ± 0.255 |
| Liver/TBRW | g/100 g | 638.137 ± 65.444 | 639.713 ± 64.238 | 602.730 ± 59.382 | 611.639 ± 69.043 | 393.042 ± 28.612 | 413.189 ± 36.288 | 407.813 ± 22.574 | 431.661 ± 31.625 |
| Pituitary | g | 0.012 ± 0.002 | 0.014 ± 0.002 | 0.014 ± 0.002 | 0.0142 ± 0.003 | 0.015 ± 0.002 | 0.015 ± 0.002 | 0.015 ± 0.002 | 0.016 ± 0.002 |
| Pituitary/TBW | g/100 g | 0.004 ± 0.001 | 0.004 ± 0.000 | 0.004 ± 0.001 | 0.005 ± 0.001 * | 0.007 ± 0.001 | 0.007 ± 0.002 | 0.007 ± 0.001 | 0.008 ± 0.001 |
| Pituitary/TBRW | g/100 g | 0.621 ± 0.094 | 0.720 ± 0.104 | 0.696 ± 0.106 | 0.730 ± 0.126 | 0.806 ± 0.107 | 0.812 ± 0.143 | 0.813 ± 0.122 | 0.875 ± 0.087 |
| Spleen | g | 0.73 ± 0.13 | 0.78 ± 0.18 | 0.76 ± 0.12 | 0.70 ± 0.12 | 0.48 ± 0.12 | 0.47 ± 0.08 | 0.50 ± 0.09 | 0.46 ± 0.07 |
| Spleen/TBW | g/100 g | 0.233 ± 0.035 | 0.241 ± 0.048 | 0.237 ± 0.026 | 0.223 ± 0.032 | 0.234 ± 0.046 | 0.229 ± 0.035 | 0.242 ± 0.043 | 0.221 ± 0.030 |
| Spleen/TBRW | g/100 g | 38.648 ± 7.350 | 40.779 ± 9.099 | 38.966 ± 6.884 | 36.031 ± 5.488 | 25.898 ± 5.939 | 25.353 ± 4.689 | 26.979 ± 4.793 | 25.044 ± 3.166 |
| Thymus | g | 0.672 ± 0.159 | 0.725 ± 0.156 | 0.711 ± 0.164 | 0.668 ± 0.119 | 0.542 ± 0.112 | 0.573 ± 0.115 | 0.526 ± 0.097 | 0.489 ± 0.084 |
| Thymus/TBW | g/100 g | 0.215 ± 0.043 | 0.225 ± 0.042 | 0.223 ± 0.044 | 0.213 ± 0.028 | 0.264 ± 0.046 | 0.278 ± 0.052 | 0.253 ± 0.041 | 0.236 ± 0.047 |
| Thymus/TBRW | g/100 g | 35.728 ± 8.575 | 37.924 ± 7.416 | 36.619 ± 8.324 | 34.506 ± 6.080 | 29.092 ± 5.263 | 30.818 ± 6.590 | 28.233 ± 4.811 | 26.699 ± 4.758 |
| Thryoid + PTH | g | 0.015 ± 0.002 | 0.017 ± 0.003 | 0.017 ± 0.003 | 0.016 ± 0.003 | 0.015 ± 0.002 | 0.015 ± 0.002 | 0.013 ± 0.002 | 0.014 ± 0.002 |
| Thryoid + PTH/TBW | g/100 g | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.008 ± 0.001 | 0.007 ± 0.001 | 0.006 ± 0.001 * | 0.007 ± 0.001 |
| Thryoid + PTH/TBRW | g/100 g | 0.805 ± 0.110 | 0.908 ± 0.157 | 0.887 ± 0.155 | 0.812 ± 0.136 | 0.822 ± 0.084 | 0.788 ± 0.112 | 0.722 ± 0.126 | 0.758 ± 0.090 |
| Epididymides | g | 0.59 ± 0.06 | 0.62 ± 0.06 | 0.65 ± 0.04 | 0.62 ± 0.07 | ||||
| Epididymides/TBW | g/100 g | 0.190 ± 0.019 | 0.194 ± 0.021 | 0.205 ± 0.023 | 0.201 ± 0.032 | ||||
| Epididymides/TBRW | g/100 g | 31.294 ± 2.245 | 32.786 ± 3.976 | 33.387 ± 2.866 | 32.301 ± 4.294 | ||||
| Sem Ves+Prostate | g | 1.76 ± 0.20 | 1.75 ± 0.21 | 1.88 ± 0.15 | 1.84 ± 0.22 | ||||
| Sem Ves+ Prostate/TBW | g/100 g | 0.569 ± 0.083 | 0.543 ± 0.050 | 0.595 ± 0.071 | 0.593 ± 0.086 | ||||
| Sem Ves+ Prostate/TBRW | g/100 g | 93.280 ± 10.280 | 91.923 ± 11.384 | 96.746 ± 6.438 | 95.193 ± 10.821 | ||||
| Testes (g) | g | 2.84 ± 0.26 | 3.13 ± 0.27 * | 3.05 ± 0.18 | 2.92 ± 0.21 | ||||
| Testes/TBW | g/100 g | 0.915 ± 0.076 | 0.977 ± 0.112 | 0.963 ± 0.070 | 0.937 ± 0.065 | ||||
| Testes/TBRW | g/100 g | 150.543 ± 10.304 | 164.380 ± 13.689 * | 157.245 ± 11.300 | 151.047 ± 11.821 | ||||
| Ovaries | g | 0.116 ± 0.019 | 0.127 ± 0.016 | 0.119 ± 0.014 | 0.123 ± 0.024 | ||||
| Ovaries/TBW | g/100 g | 0.057 ± 0.007 | 0.062 ± 0.008 | 0.057 ± 0.007 | 0.059 ± 0.009 | ||||
| Ovaries/TBRW | g/100 g | 6.274 ± 1.014 | 6.852 ± 1.064 | 6.398 ± 0.696 | 6.706 ± 1.153 | ||||
| Uterus | g | 0.61 ± 0.16 | 0.49 ± 0.06 | 0.53 ± 0.22 | 0.54 ± 0.16 | ||||
| Uterus/TBW | g/100 g | 0.299 ± 0.082 | 0.239 ± 0.038 | 0.254 ± 0.100 | 0.263 ± 0.089 | ||||
| Uterus/TBRW | g/100 g | 32.838 ± 8.568 | 26.256 ± 3.783 | 28.250 ± 10.787 | 29.496 ± 8.388 | ||||
| Study Week | Severity Score | Nausea | Vomiting | Heartburn | Abdominal Bloating | Indigestion | Upper Abdominal Pain | Lower Abdominal Pain | Diarrhea | Constipation | Flatulence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Placebo) | Mean (S.D.) | 6.0 (NA) n = 1 | 8.0 (NA) n = 1 | 2.0 (1.4) n = 2 | 3.9 (1.8) n = 11 | 1.0 (NA) n = 1 | 3.0 (NA) n = 1 | 3.5 (1.3) n = 4 | NA (NA) n = 0 | 3.8 (1.8) n = 14 | 2.7 (1.3) n = 36 |
| 2 | Mean (S.D.) | NA (NA) n = 0 | NA (NA) n = 0 | 1.0 (0.0) n = 2 | 3.4 (2.2) n = 9 | NA (NA) n = 0 | NA (NA) n = 0 | 2.4 (0.9) n = 5 | 6.0 (NA) n = 1 | 3.2 (1.2) n = 19 | 3.1 (1.7) n = 20 |
| 3 | Mean (S.D.) | 1.0 (0.0) n = 3 | NA (NA) n = 0 | NA (NA) n = 0 | 2.0 (1.0) n = 7 | 3.0 (NA) n = 1 | NA (NA) n = 0 | NA (NA) n = 0 | NA (NA) n = 0 | 2.2 (1.1) n = 19 | 2.4 (1.1) n = 17 |
| 4 | Mean (S.D.) | NA (NA) n = 0 | NA (NA) n = 0 | NA (NA) n = 0 | 2.3 (0.4) n = 7 | NA (NA) n = 0 | NA (NA) n = 0 | 3.3 (0.6) n = 3 | 1.0 (NA) n = 1 | 2.2 (0.8) n = 13 | 2.8 (1.5) n = 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spears, J.L.; Kramer, R.; Nikiforov, A.I.; Rihner, M.O.; Lambert, E.A. Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements. Nutrients 2021, 13, 733. https://doi.org/10.3390/nu13030733
Spears JL, Kramer R, Nikiforov AI, Rihner MO, Lambert EA. Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements. Nutrients. 2021; 13(3):733. https://doi.org/10.3390/nu13030733
Chicago/Turabian StyleSpears, Jessica L., Richard Kramer, Andrey I. Nikiforov, Marisa O. Rihner, and Elizabeth A. Lambert. 2021. "Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements" Nutrients 13, no. 3: 733. https://doi.org/10.3390/nu13030733
APA StyleSpears, J. L., Kramer, R., Nikiforov, A. I., Rihner, M. O., & Lambert, E. A. (2021). Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements. Nutrients, 13(3), 733. https://doi.org/10.3390/nu13030733





