Association of Time-of-Day Energy Intake Patterns with Nutrient Intakes, Diet Quality, and Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
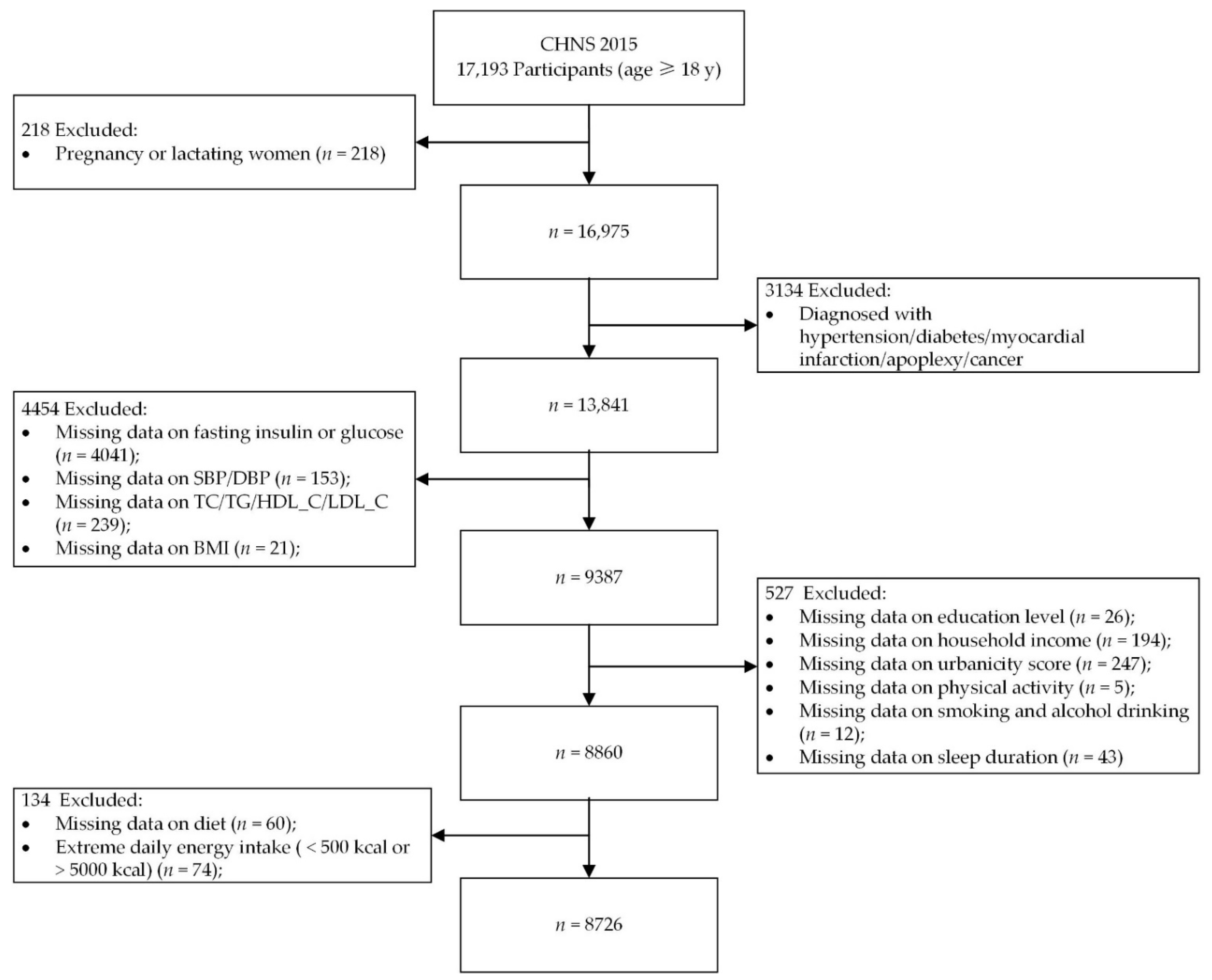
2.1. Study Population
2.2. Dietary Assessment
2.3. Definition of Meals and Snacks and Calculation of Proportion of Energy Intake from Meals, Snacks, and EOs
2.4. Time-of-Day Energy Intake Patterns
2.5. Diet Quality
2.6. Blood Biochemical Measurements and Insulin Resistance Assessment
2.7. Anthropometric Measurements
2.8. Covariates
2.9. Statistical Analysis
2.9.1. Latent Classes of Time-of-Day Energy Intake Patterns
2.9.2. Associations between Latent Classes and Sociodemographic Characteristics, Lifestyles, Eating Pattern Profiles, and Cardiometabolic Factors
2.9.3. Associations between Latent Classes and Energy-Adjusted Nutrient Intakes and Diet Quality Score
2.9.4. Associations between Latent Classes and Insulin Resistance
3. Results
3.1. Basic Characteristics of Participants
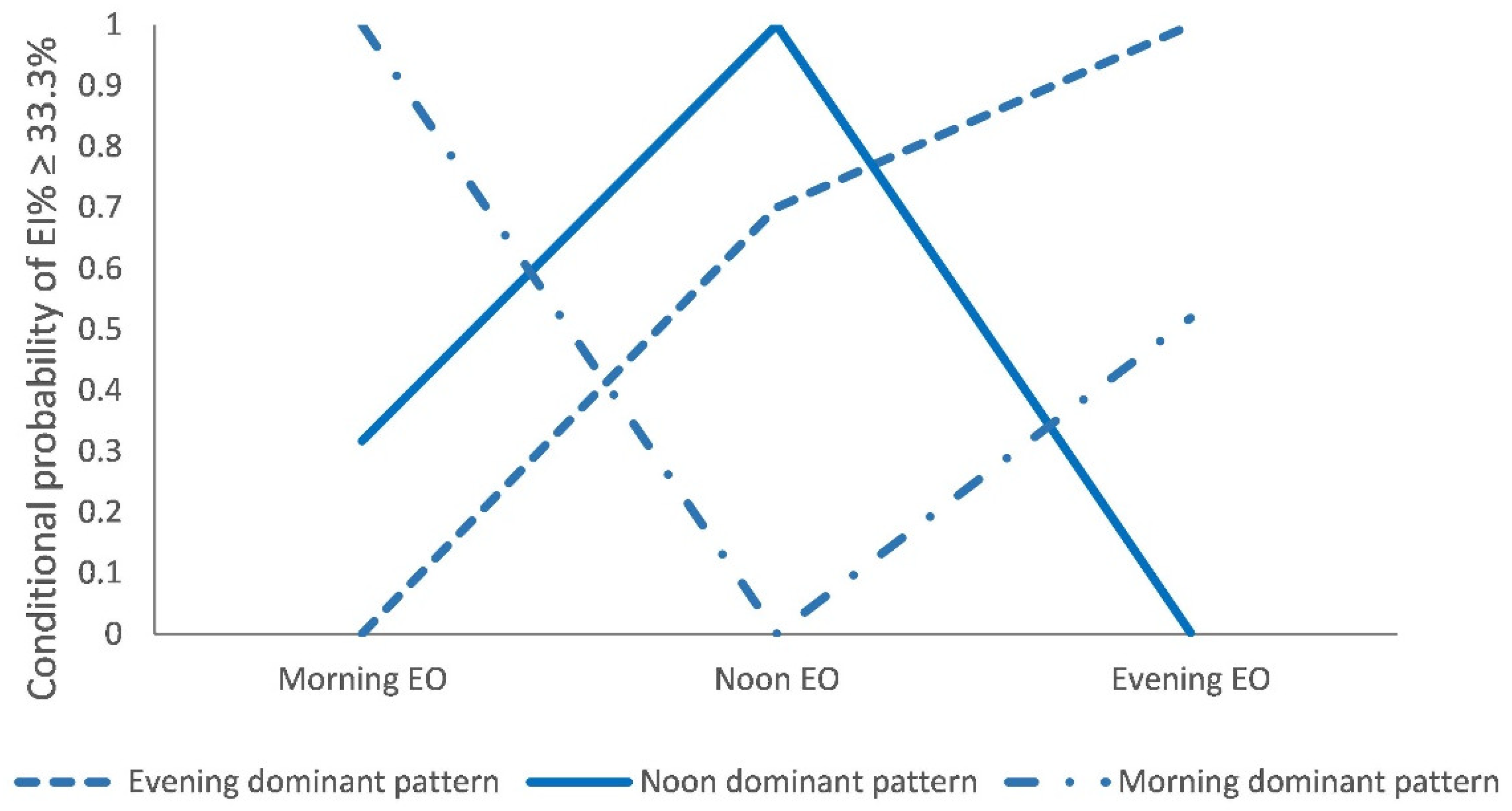
3.2. Latent Classes of Time-of-Day Energy Intake Patterns
3.3. Sociodemographic Characteristics, Lifestyles, and Cardiometabolic Risk Factors of Latent Classes
3.4. Eating Pattern Profile of Latent Classes
3.5. Daily Energy, Energy-Adjusted Nutrient Intakes, and Diet Quality Score of Latent Classes
3.6. Association between Latent Classes and Insulin Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Eicher-Miller, H.A.; Khanna, N.; Boushey, C.J.; Gelfand, S.B.; Delp, E.J. Temporal Dietary Patterns Derived among the Adult Participants of the National Health and Nutrition Examination Survey 1999–2004 Are Associated with Diet Quality. J. Acad. Nutr. Diet. 2016, 116, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Worsley, A.; Timperio, A.; McNaughton, S. Temporal eating patterns: A latent class analysis approach. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 3. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jonkers, C.F.; Fliers, E.; Bisschop, P.; Kalsbeek, A. Nutrition and the circadian timing system. Prog. Brain Res. 2012, 199, 359–376. [Google Scholar] [CrossRef]
- Bi, H.; Gan, Y.; Yang, C.; Chen, Y.; Tong, X.; Lu, Z. Breakfast skipping and the risk of type 2 diabetes: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Owen, A.J.; Liew, D. Skipping Breakfast and the Risk of Cardiovascular Disease and Death: A Systematic Review of Prospective Cohort Studies in Primary Prevention Settings. J. Cardiovasc. Dev. Dis. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Chuang, S.Y.; Chang, H.Y.; Pan, W.H. Energy intake at different times of the day: Its association with elevated total and LDL cholesterol levels. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Suwa, K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J. Diabetes Metab. Disord. 2015, 14, 16. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Chan, Q.; Griep, L.M.O.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Horn, L.V.; Paul Elliott, M.; Frost, G.S. The impact of eating frequency and time of intake on nutrient quality and body mass index: The INTERMAP Study, a population based study. J. Acad. Nutr. Diet. 2015, 115, 528–536.e1. [Google Scholar] [CrossRef]
- Bo, S.; Musso, G.; Beccuti, G.; Fadda, M.; Fedele, D.; Gambino, R.; Gentile, L.; Durazzo, M.; Ghigo, E.; Cassader, M. Consuming More of Daily Caloric Intake at Dinner Predisposes to Obesity. A 6-Year Population-Based Prospective Cohort Study. PloS ONE 2014, 9, e108467. [Google Scholar] [CrossRef]
- Leech, R.M.; Livingstone, K.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Meal Frequency but Not Snack Frequency Is Associated with Micronutrient Intakes and Overall Diet Quality in Australian Men and Women. J. Nutr. 2016, 146, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.M.; Timperio, A.; Worsley, A.; McNaughton, S.A. Eating patterns of Australian adults: Associations with blood pressure and hypertension prevalence. Eur. J. Nutr. 2019, 58, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Eicher-Miller, H.A.; Boushey, C.J.; Gelfand, S.B.; Delp, E.J. Temporal Dietary Patterns Using Kernel k-Means Clustering. In Proceedings of the 2011 IEEE International Symposium on Multimedia, Dana Point, CA, USA, 5–7 December 2011. [Google Scholar] [CrossRef]
- Aqeel, M.M.; Guo, J.; Lin, L.; Gelfand, S.B.; Delp, E.J.; Bhadra, A.; Richards, E.A.; Hennessy, E.; Eicher-Miller, H.A. Temporal Dietary Patterns Are Associated with Obesity in US Adults. J. Nutr. 2020, 150, 3259–3268. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wefers, J.; van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef]
- Rangaraj, V.R.; Siddula, A.; Burgess, H.J.; Pannain, S.; Knutson, K.L. Association between Timing of Energy Intake and Insulin Sensitivity: A Cross-Sectional Study. Nutrients 2020, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, Z.; Wang, L.; Wang, H.; Zhang, J.; Du, W.; Su, C.; Jia, X.; Ouyang, Y.; Wang, Y.; et al. Evaluating adherence to recommended diets in adults 1991–2015: Revised China dietary guidelines index. Nutr. J. 2019, 18, 70. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Su, C.; Song, X.; Hu, H.; Du, W.; Wang, H.; Zhang, B. Longitudinal Association between Urbanicity and Total Dietary Fat Intake in Adults in Urbanizing China from 1991 to 2015: Findings from the CHNS. Nutrients 2020, 12, 1597. [Google Scholar] [CrossRef]
- Collins, L.M.; Lanza, S.T. Latent Class and Latent Transition Analysis with Applications in the Social, Behavioral and Health Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 6–7. [Google Scholar]
- De Castro, J.M. The time of day of food intake influences overall intake in humans. J. Nutr. 2004, 134, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Schatzkin, A.; Ballard-Barbash, R. Evening eating and subsequent long-term weight change in a national cohort. Int. J. Obes. 1997, 21, 407–412. [Google Scholar] [CrossRef][Green Version]
- Wang, J.B.; Patterson, R.E.; Ang, A.; Emond, J.A.; Shetty, N.; Arab, L. Timing of energy intake during the day is associated with the risk of obesity in adults. J. Hum. Nutr. Diet. 2014, 27, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.M.; Timperio, A.; Livingstone, K.M.; Worsley, A.; McNaughton, S.A. Temporal eating patterns: Associations with nutrient intakes, diet quality, and measures of adiposity. Am. J. Clin. Nutr. 2017, 106, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Rodriguez Lopez, S.; Carmenate Moreno, M. Association between meal intake behavior and blood pressure in Spanish adults. Nutr. Hosp. 2017, 34, 654–660. [Google Scholar] [CrossRef][Green Version]
- Almoosawi, S.; Prynne, C.J.; Hardy, R.; Stephen, A.M. Time-of-day of energy intake. J. Hypertens. 2013, 31, 882–892. [Google Scholar] [CrossRef]
- Beccuti, G.; Monagheddu, C.; Evangelista, A.; Ciccone, G.; Broglio, F.; Soldati, L.; Bo, S. Timing of food intake: Sounding the alarm about metabolic impairments? A systematic review. Pharmacol. Res. 2017, 125, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.M.; Shi, J.W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin. Sci. 2013, 125, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.; Jarrett, R.J. Diurnal variation in insulin sensitivity. Lancet 1972, 2, 947–948. [Google Scholar] [CrossRef]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.F.; Mistlberger, R.E. Circadian adaptations to meal timing: Neuroendocrine mechanisms. Front. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef]
| Model Fit Statistics | 1 Class | 2 Classes | 3 Classes | 4 Classes |
|---|---|---|---|---|
| AIC | 36,053.953 | 33,478.537 | 31,640.972 | 31,402.251 |
| BIC | 36,096.397 | 33,570.743 | 31,782.453 | 31,593.250 |
| adjusted BIC | 36,077.330 | 33,529.431 | 31,718.896 | 31,507.449 |
| LMR-LRT | NA | <0.001 | <0.001 | <0.001 |
| BS-LRT | NA | <0.001 | <0.001 | <0.001 |
| Entropy | NA | 1.000 | 0.997 | 0.992 |
| Variables | “Evening Dominant Pattern” (n = 4887) | “Noon Dominant Pattern” (n = 2479) | “Morning Dominant Pattern” (n = 1360) | p-Value |
|---|---|---|---|---|
| Age (year, mean (SD)) | 49.27 (14.03) | 51.22 (14.31) | 52.72 (14.33) | <0.001 |
| Gender (n, %) | ||||
| Man | 2279 (46.63) | 1042 (42.03) | 592 (43.53) | <0.001 |
| Woman | 2608 (53.37) | 1437 (57.97) | 768 (56.47) | |
| Education level (n, %) | ||||
| Primary school | 1301 (26.62) | 749 (30.21) | 460 (33.82) | <0.001 |
| Middle school | 1780 (36.42) | 779 (31.42) | 446 (32.79) | |
| High school | 1109 (22.69) | 567 (22.87) | 294 (21.62) | |
| College and above | 697 (14.26) | 384 (15.49) | 160 (11.76) | |
| Geographic region (n, %) | ||||
| City | 918 (18.78) | 545 (21.98) | 247 (18.16) | <0.001 |
| Suburban | 946 (19.36) | 382 (15.41) | 165 (12.13) | |
| County | 832 (17.02) | 437 (17.63) | 207 (15.22) | |
| Rural village | 2191 (44.83) | 1115 (44.98) | 741 (54.49) | |
| Physical activity (n, %) | ||||
| Low | 1253 (25.64) | 677 (27.31) | 387 (28.46) | 0.077 |
| Middle | 1744 (35.69) | 894 (36.06) | 449 (33.01) | |
| High | 1890 (38.67) | 908 (36.63) | 524 (38.53) | |
| Sleep duration (n, %) | ||||
| 6–8 h | 3940 (80.62) | 1968 (79.39) | 1063 (78.16) | 0.063 |
| <6 h | 110 (2.25) | 77 (3.11) | 33 (2.43) | |
| >8 h | 837 (17.13) | 434 (17.51) | 264 (19.41) | |
| Smoking (n, %) | ||||
| Nonsmoker | 3536 (72.36) | 1918 (77.37) | 1018 (74.85) | <0.001 |
| Ex-smoker | 111 (2.27) | 46 (1.86) | 32 (2.35) | |
| Current smoker | 1240 (25.37) | 515 (20.77) | 310 (22.79) | |
| Alcohol drinking (n, %) | ||||
| Nondrinker | 3414 (69.86) | 1845 (74.43) | 1015 (74.63) | <0.001 |
| Drink ≤1 time/month | 273 (5.59) | 128 (5.16) | 59 (4.34) | |
| Drink 1–2 times/month | 355 (7.26) | 139 (5.61) | 66 (4.85) | |
| Drink 1–4 times/week | 459 (9.39) | 221 (8.91) | 112 (8.24) | |
| Drink everyday | 386 (7.90) | 146 (5.89) | 108 (7.94) | |
| Per capita household income (n, %) | ||||
| Low | 2925 (59.85) | 1455 (58.69) | 872 (64.12) | 0.007 |
| Medium | 1828 (37.41) | 959 (38.68) | 465 (34.19) | |
| High | 134 (2.74) | 65 (2.62) | 23 (1.69) | |
| Urbanicity score (mean (SD)) | 72.70 (17.06) | 71.29 (18.23) | 69.58 (17.80) | <0.001 |
| BMI (mg/kg2, mean (SD)) | 23.70 (3.57) | 23.94 (3.74) | 23.79 (3.53) | 0.029 |
| SBP (mmHg, mean (SD)) | 123.84 (16.50) | 124.30 (17.45) | 125.03 (17.23) | 0.063 |
| DBP (mmHg, mean (SD)) | 79.57 (10.38) | 79.56 (10.26) | 80.13 (10.30) | 0.179 |
| TC (mmol/L, mean (SD)) | 4.94 (1.06) | 4.84 (1.06) | 4.82 (0.97) | <0.001 |
| TG (log mmol/L, mean (SD)) 1 | 0.18 (0.59) | 0.13 (0.57) | 0.17 (0.55) | 0.010 |
| LDL_C (mmol/L, mean (SD)) | 3.12 (0.89) | 3.06 (0.92) | 3.04 (0.85) | 0.002 |
| HDL_C (mmol/L, mean (SD)) | 1.29 (0.33) | 1.28 (0.34) | 1.29 (0.32) | 0.866 |
| Glucose (log mmol/L, mean (SD)) 1 | 1.65 (0.18) | 1.65 (0.18) | 1.65 (0.19) | 0.637 |
| Insulin (log μU/mL, mean (SD)) 1 | 1.79 (0.64) | 1.74 (0.62) | 1.74 (0.66) | 0.003 |
| HOMA-IR (log mean (SD)) 1 | 0.32 (0.71) | 0.28 (0.69) | 0.27 (0.72) | 0.018 |
| Eating Pattern Profile | “Evening Dominant Pattern” (n = 4887) | “Noon Dominant Pattern” (n = 2479) | “Morning Dominant Pattern” (n = 1360) | p-Value 2 |
|---|---|---|---|---|
| EI from Morning EO (%) 3 | 22.64 (17.75, 27.04) | 30.25 (25.25, 34.39) | 39.39 (36.27, 44.08) | <0.001 |
| EI from Noon EO (%) | 36.60 (32.33, 40.95) | 42.02 (37.92, 46.79) | 27.52 (22.69, 30.43) | <0.001 |
| EI from Evening EO (%) | 40.49 (36.96, 45.05) | 28.55 (24.59, 31.09) | 33.63 (29.75, 38.16) | <0.001 |
| Variables | “Evening Dominant Pattern” (n = 4887) | “Noon Dominant Pattern” (n = 2479) | “Morning Dominant Pattern” (n = 1360) | p-Value |
|---|---|---|---|---|
| Energy and Nutrient Intakes 1 | ||||
| Energy, kcal | 2094.63 (10.06) a | 1997.50 (14.10) b | 1895.06 (19.07) c | <0.001 |
| Carbohydrate, g | 241.16 (0.99) c | 257.52 (1.39) b | 271.70 (1.88) a | <0.001 |
| Carbohydrate, EI% | 48.04 (0.17) c | 51.32 (0.24) b | 53.48 (0.33) a | <0.001 |
| Total fat, g | 84.82 (0.44) a | 79.70 (0.61) b | 74.34 (0.83) c | <0.001 |
| Total fat, EI% | 37.29 (0.17) a | 34.96 (0.24) b | 33.29 (0.32) c | <0.001 |
| Protein, g | 70.16 (0.27) a | 66.90 (0.38) b | 65.00 (0.51) c | <0.001 |
| Protein, EI% | 14.02 (0.05) a | 13.35 (0.07) b | 12.88 (0.10) c | <0.001 |
| Fiber, g | 12.46 (0.11) b | 12.55 (0.16) a,b | 13.15 (0.21) a | 0.016 |
| Vitamin C, mg | 93.00 (2.20) a | 82.33 (3.09) b | 81.70 (4.19) b | 0.005 |
| Calcium, mg | 375.88 (2.76) | 369.57 (3.86) | 369.50 (5.24) | 0.323 |
| Iron, mg | 22.14 (0.14) | 22.40 (0.19) | 21.87 (0.26) | 0.255 |
| Zinc, mg | 11.02 (0.04) a | 10.41 (0.06) b | 10.46 (0.07) b | <0.001 |
| Sodium, mg | 4659.45 (100.10) | 4835.83 (140.18) | 5083.83 (190.06) | 0.129 |
| Potassium, mg | 1711.98 (8.87) a | 1664.58 (12.42) b | 1658.83 (16.85) b | 0.001 |
| Phosphorus, mg | 966.00 (3.19) | 959.28 (4.47) | 961.88 (6.06) | 0.461 |
| Diet Quality Score 2 | ||||
| CDGI (2019)-A score | 50.93 (0.14) b | 51.72 (0.20) a | 50.67 (0.27) b | <0.001 |
| Models | “Noon Dominant Pattern” (n = 2479) | “Evening Dominant Pattern” (n = 4887) | “Morning Dominant Pattern” (n = 1360) |
|---|---|---|---|
| Model 1 | 1 | 1.16(1.02–1.32) * | 1.01(0.85–1.20) |
| Model 2 | 1 | 1.15(1.01–1.31) * | 1.05(0.88–1.25) |
| Model 3 | 1 | 1.14(1.00–1.30) * | 1.05(0.88–1.26) |
| Model 4 | 1 | 1.21(1.05–1.40) * | 1.08(0.89–1.31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Wang, H.; Su, C.; Wang, Z.; Huang, F.; Zhang, J.; Du, W.; Jia, X.; Jiang, H.; Ouyang, Y.; et al. Association of Time-of-Day Energy Intake Patterns with Nutrient Intakes, Diet Quality, and Insulin Resistance. Nutrients 2021, 13, 725. https://doi.org/10.3390/nu13030725
Song X, Wang H, Su C, Wang Z, Huang F, Zhang J, Du W, Jia X, Jiang H, Ouyang Y, et al. Association of Time-of-Day Energy Intake Patterns with Nutrient Intakes, Diet Quality, and Insulin Resistance. Nutrients. 2021; 13(3):725. https://doi.org/10.3390/nu13030725
Chicago/Turabian StyleSong, Xiaoyun, Huijun Wang, Chang Su, Zhihong Wang, Feifei Huang, Jiguo Zhang, Wenwen Du, Xiaofang Jia, Hongru Jiang, Yifei Ouyang, and et al. 2021. "Association of Time-of-Day Energy Intake Patterns with Nutrient Intakes, Diet Quality, and Insulin Resistance" Nutrients 13, no. 3: 725. https://doi.org/10.3390/nu13030725
APA StyleSong, X., Wang, H., Su, C., Wang, Z., Huang, F., Zhang, J., Du, W., Jia, X., Jiang, H., Ouyang, Y., Wang, Y., Li, L., Ding, G., & Zhang, B. (2021). Association of Time-of-Day Energy Intake Patterns with Nutrient Intakes, Diet Quality, and Insulin Resistance. Nutrients, 13(3), 725. https://doi.org/10.3390/nu13030725







