Temporal Development of Dyslipidemia and Nonalcoholic Fatty Liver Disease (NAFLD) in Syrian Hamsters Fed a High-Fat, High-Fructose, High-Cholesterol Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
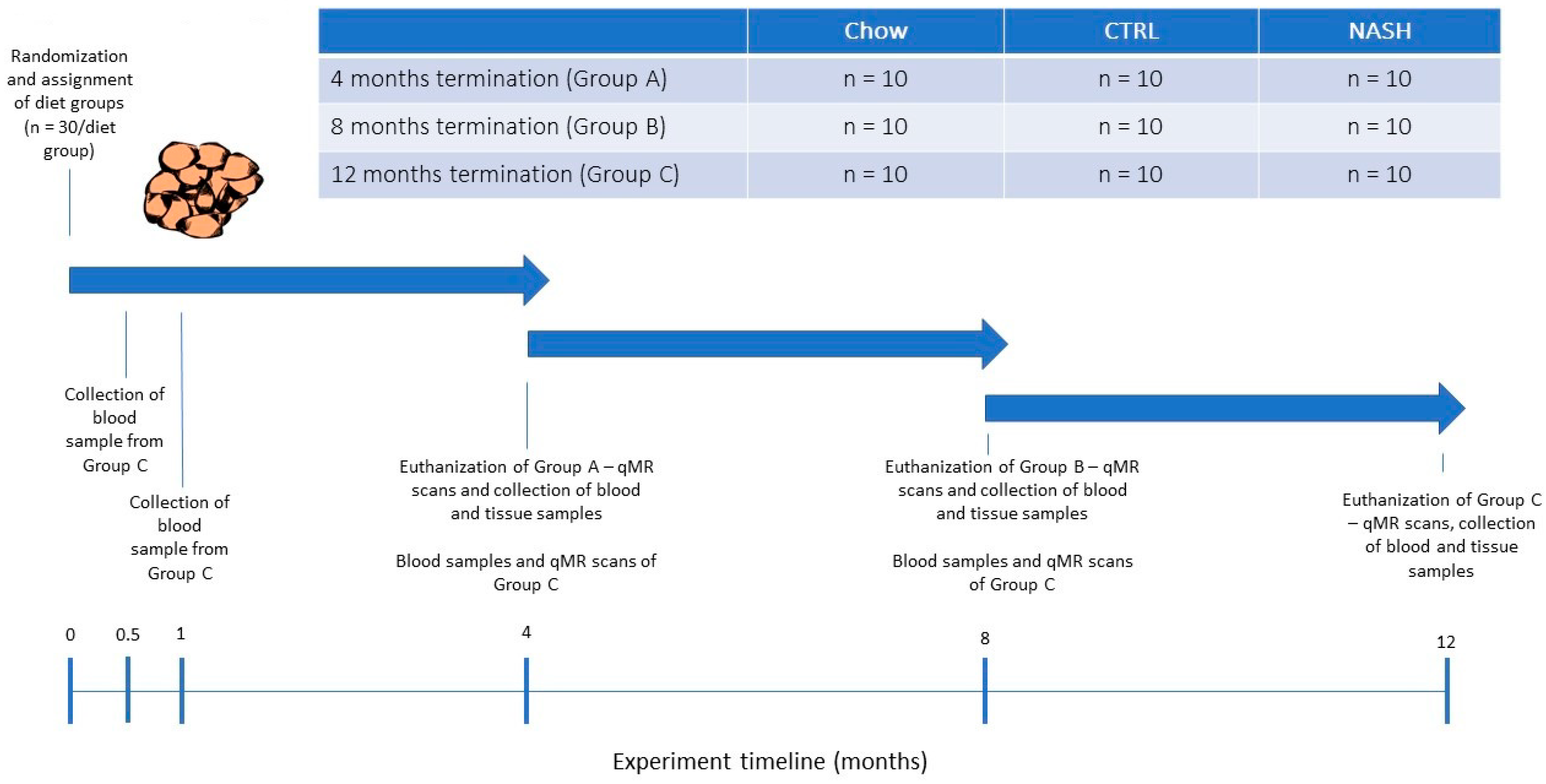
2.2. Experimental Design
2.3. Quantitative Magnetic Resonance
2.4. Plasma Parameters
2.5. Liver Biochemistry
2.6. Liver Histology
2.7. Statistics
3. Results
3.1. The NASH Diet Did Not Induce Hyperglycemia/Hyperinsulinemia But Affected Body Composition
3.2. The NASH Diet Induced Marked Dyslipidemia in Syrian Hamsters
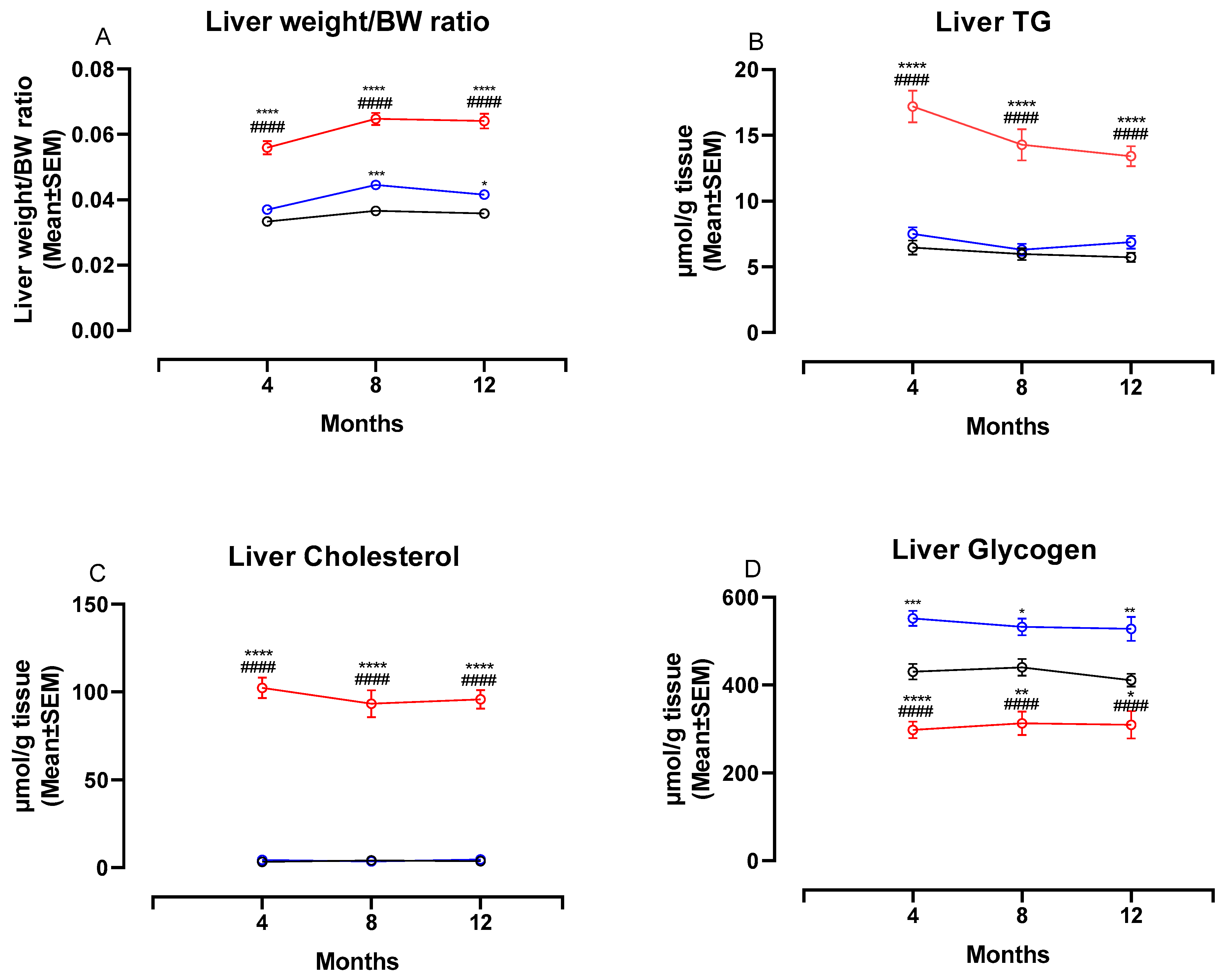
3.3. The NASH Diet Increased Liver Weight, Hepatic Triglyceride and Cholesterol Content and Reduced Glycogen Content in Syrian Hamsters
3.4. The NASH Diet Induced Hepatic Steatosis, Inflammation and Fibrosis in Syrian Hamsters
3.5. The NASH-Diet Increases Circulating Markers of Liver Dysfunction, β-Oxidation and Inflammation in Syrian Hamsters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Wong, V.W.; Wong, G.L.; Choi, P.C.; Chan, A.W.; Li, M.K.; Chan, H.Y.; Chim, A.M.-L.; Yu, J.; Sung, J.J.-Y. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut 2010, 59, 969–974. [Google Scholar] [CrossRef]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal Models of Nonalcoholic Fatty Liver Disease-A Starter’s Guide. Nutrients 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Cheah, I.K.; Halliwell, B. High fat diets and pathology in the guinea pig. Atherosclerosis or liver damage? Biochim. Biophys. Acta 2013, 1832, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Guyard-Dangremont, V.; Desrumaux, C.; Gambert, P.; Lallemant, C.; Lagrost, L. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 517–525. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Animal Models of Fibrosis in Nonalcoholic Steatohepatitis: Do They Reflect Human Disease? Adv. Nutr. 2020, 11, 1696–1711. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef]
- Briand, F.; Maupoint, J.; Brousseau, E.; Breyner, N.; Bouchet, M.; Costard, C.; Leste-Lasserre, T.; Petitjean, M.; Chen, L.; Chabrat, A.; et al. Elafibranor improves diet-induced nonalcoholic steatohepatitis associated with heart failure with preserved ejection fraction in Golden Syrian hamsters. Metabolism 2021, 117, 154707. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Volek, J.S. Guinea pigs: A suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr. Metab. 2006, 3, 17. [Google Scholar] [CrossRef]
- Wang, Z.; Niimi, M.; Ding, Q.; Liu, Z.; Wang, L.; Zhang, J.; Xu, J.; Fan, J. Comparative studies of three cholesteryl ester transfer proteins and their interactions with known inhibitors. PLoS ONE 2017, 12, e0180772. [Google Scholar] [CrossRef]
- Cohen, D.E.; Fisher, E.A. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin. Liver Dis. 2013, 33, 380–388. [Google Scholar]
- Chassaing, B.; Miles-Brown, J.; Pellizzon, M.; Ulman, E.; Ricci, M.; Zhang, L.; Patterson, A.D.; Vijay-Kumar, M.; Gewirtz, A.T. Lack of soluble fiber drives diet-induced adiposity in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G528–G541. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Age of laboratory hamster and human: Drawing the connexion. Biomed. Pharmacol. J. 2019, 12, 49–56. [Google Scholar] [CrossRef]
- Metzinger, M.N.; Miramontes, B.; Zhou, P.; Liu, Y.; Chapman, S.; Sun, L.; Sasser, T.A.; Duffield, G.E.; Stack, M.S.; Leevy, W.M. Correlation of X-ray computed tomography with quantitative nuclear magnetic resonance methods for pre-clinical measurement of adipose and lean tissues in living mice. Sensors 2014, 14, 18526–18542. [Google Scholar] [CrossRef]
- Hvid, H.; Blouin, M.J.; Birman, E.; Damgaard, J.; Poulsen, F.; Fels, J.J.; Fledelius, C.; Hansen, B.F.; Pollak, M. Treatment with insulin analog X10 and IGF-1 increases growth of colon cancer allografts. PLoS ONE 2013, 8, e79710. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Glass, L.M.; Hunt, C.M.; Fuchs, M.; Su, G.L. Comorbidities and Nonalcoholic Fatty Liver Disease: The Chicken, the Egg, or Both? Fed. Pract. 2019, 36, 64–71. [Google Scholar] [PubMed]
- Utzschneider, K.M.; Kahn, S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F. Hepatic Steatosis as a Marker of Metabolic Dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef]
- Min, H.K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Lucero, D.; Zago, V.; López, G.I.; Graffigna, M.; López, G.H.; Fainboim, H.; Miksztowicz, V.; Rosso, L.G.; Belli, S.; Levalle, O.; et al. Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome? Clin. Chim. Acta 2011, 412, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Miller, A.E.; Naples, M.; Baker, C.; Kohen, R.; Xu, E.; Su, Q.; Allister, E.M.; Wheeler, M.B.; Adeli, K. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E462–E473. [Google Scholar] [CrossRef] [PubMed]
- Briand, F.; Thieblemont, Q.; Muzotte, E.; Sulpice, T. High-fat and fructose intake induces insulin resistance, dyslipidemia, and liver steatosis and alters in vivo macrophage-to-feces reverse cholesterol transport in hamsters. J. Nutr. 2012, 142, 704–709. [Google Scholar] [CrossRef]
- Briand, F.; Brousseau, E.; Quinsat, M.; Burcelin, R.; Sulpice, T. Obeticholic acid raises LDL-cholesterol and reduces HDL-cholesterol in the Diet-Induced NASH (DIN) hamster model. Eur. J. Pharmacol. 2018, 818, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, C.; Zhang, X.; Li, N.; Dong, Z.; Sun, G.; Sun, X. Atorvastatin promotes AMPK signaling to protect against high fat diet-induced non-alcoholic fatty liver in golden hamsters. Exp. Ther. Med. 2020, 19, 2133–2142. [Google Scholar] [CrossRef]
- Lai, Y.S.; Yang, T.C.; Chang, P.Y.; Chang, S.F.; Ho, S.L.; Chen, H.L.; Lu, S.-C. Electronegative LDL is linked to high-fat, high-cholesterol diet-induced nonalcoholic steatohepatitis in hamsters. J. Nutr. Biochem. 2016, 30, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Lu, L.G. Nonalcoholic Fatty Liver Disease: Dyslipidemia, Risk for Cardiovascular Complications, and Treatment Strategy. J. Clin. Transl. Hepatol. 2015, 3, 78–84. [Google Scholar]
- Grundy, S.M. Does Dietary Cholesterol Matter? Curr. Atheroscler. Rep. 2016, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Makadia, S.S.; Blaha, M.; Keenan, T.; Ndumele, C.; Jones, S.; DeFilippis, A.; Martin, S.; Kohli, P.; Conceicao, R.; Carvalho, J.; et al. Relation of hepatic steatosis to atherogenic dyslipidemia. Am. J. Cardiol. 2013, 112, 1599–1604. [Google Scholar] [CrossRef]
- Amor, A.J.; Perea, V. Dyslipidemia in nonalcoholic fatty liver disease. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 103–108. [Google Scholar] [CrossRef]
- Nseir, W.; Shalata, A.; Marmor, A.; Assy, N. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig. Dis. Sci. 2011, 56, 3439–3449. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Corey, K.E.; Lai, M.; Gelrud, L.G.; Misdraji, J.; Barlow, L.L.; Zheng, H.; Andersson, K.L.; Thiim, M.; Pratt, D.S.; Chung, R.T. Non-high-density lipoprotein cholesterol as a biomarker for nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 651–656. [Google Scholar] [CrossRef]
- Alkhouri, N.; Eng, K.; Lopez, R.; Nobili, V. Non-high-density lipoprotein cholesterol (non-HDL-C) levels in children with nonalcoholic fatty liver disease (NAFLD). Springerplus 2014, 3, 407. [Google Scholar] [CrossRef]
- Cui, Y.; Blumenthal, R.S.; Flaws, J.A.; Whiteman, M.K.; Langenberg, P.; Bachorik, P.S.; Bush, T.L. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch. Intern. Med. 2001, 161, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Orakzai, S.H.; Nasir, K.; Blaha, M.; Blumenthal, R.S.; Raggi, P. Non-HDL cholesterol is strongly associated with coronary artery calcification in asymptomatic individuals. Atherosclerosis 2009, 202, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, C.; Lomonaco, R.; Orsak, B.; Finch, J.; Chang, Z.; Kochunov, V.G.; Hardies, J.; Cusi, K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 2012, 35, 873–878. [Google Scholar] [CrossRef]
- Holm, C.; Osterlund, T.; Laurell, H.; Contreras, J.A. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Ann. Rev. Nutr. 2000, 20, 365–393. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Mells, J.E.; Fu, P.P.; Kumar, P.; Smith, T.; Karpen, S.J.; Anania, F.A. Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J. Nutr. Biochem. 2015, 26, 285–292. [Google Scholar] [CrossRef]
- Stephenson, K.; Kennedy, L.; Hargrove, L.; Demieville, J.; Thomson, J.; Alpini, G.; Francis, H. Updates on Dietary Models of Nonalcoholic Fatty Liver Disease: Current Studies and Insights. Gene Expr. 2018, 18, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.S.; Hvid, H.; Damgaard, J.; Nygaard, H.; Ingvorsen, C.; Wulff, E.M.; Lykkesfeldt, J.; Fledelius, C. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague–Dawley rats. Diabetol. Metabol. Syndr. 2018, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Armstrong, M.J. Lean NAFLD: A not so benign condition? Hepatol. Commun. 2018, 2, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Marques-Vidal, P.; Cortez-Pinto, H. Hepatic histology in obese patients undergoing bariatric surgery. J. Hepatol. 2006, 45, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Subichin, M.; Clanton, J.; Makuszewski, M.; Bohon, A.; Zografakis, J.G.; Dan, A. Liver disease in the morbidly obese: A review of 1000 consecutive patients undergoing weight loss surgery. Surg. Obes. Relat. Dis. 2015, 11, 137–141. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jorgensen, S.M.D.; Thomsen, K.L.; Moller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.M.; Brunt, E.M. Pathology of nonalcoholic fatty liver disease. Am. J. Clin. Pathol. 2007, 128, 837–847. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Jin, D.; Tashiro, K.; Masubuchi, S.; Ozeki, M.; Hirokawa, F.; Hayashi, M.; Takai, S.; Uchiyama, K. A novel hamster nonalcoholic steatohepatitis model induced by a high-fat and high-cholesterol diet. Exp. Anim. 2018, 67, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Denk, H.; Abuja, P.M.; Zatloukal, K. Animal models of NAFLD from the pathologist’s point of view. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 929–942. [Google Scholar] [CrossRef]
- Suppli, M.P.; Rigbolt, K.T.; Veidal, S.S.; Heebøll, S.; Eriksen, P.L.; Demant, M.; Bagger, J.I.; Nielsen, J.C.; Oró, D.; Thrane, S.W.; et al. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal-weight individuals. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G462–G472. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Bagger, J.I.; Nielsen, J.C.; Oró, D.; Thrane, S.W.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Gambino, R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies. Nat. Rev. Drug Discov. 2016, 15, 249. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Yang, L.; Xie, S.; Zhu, H. IMM-H007, a new therapeutic candidate for nonalcoholic fatty liver disease, improves hepatic steatosis in hamsters fed a high-fat diet. FEBS Open Biol. 2017, 7, 1379–1391. [Google Scholar] [CrossRef]
- Van de Giessen, E.; La Fleur, S.E.; de Bruin, K.; van den Brink, W.; Booij, J. Free-choice and no-choice high-fat diets affect striatal dopamine D2/3 receptor availability, caloric intake, and adiposity. Obesity 2012, 20, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Sundborn, G.; Thornley, S.; Merriman, T.R.; Lang, B.; King, C.; Lanaspa, M.A.; Johnson, R.J. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity 2019, 27, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Togo, J.; Hu, S.; Li, M.; Niu, C.; Speakman, J.R. Impact of dietary sucrose on adiposity and glucose homeostasis in C57BL/6J mice depends on mode of ingestion: Liquid or solid. Mol. Metab. 2019, 27, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Dietschy, J. Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: Human and animal studies. Am. J. Clin. Nutr. 1997, 65, 1590S–1596S. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Jiao, R.; Peng, C.; Wong, Y.M.; Yeung, V.S.; Huang, Y.; Chen, Z.-Y. Choosing hamsters but not rats as a model for studying plasma cholesterol-lowering activity of functional foods. Mol. Nutr. Food Res. 2009, 53, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Ke, J.Y.; Pellizzon, M.A. Targeted Nutrient Modifications in Purified Diets Differentially Affect Nonalcoholic Fatty Liver Disease and Metabolic Disease Development in Rodent Models. Curr. Dev. Nutr. 2020, 4, nzaa078. [Google Scholar] [CrossRef] [PubMed]
- Pellizzon, M. Choice of laboratory animal diet influences intestinal health. Lab. Anim. 2016, 45, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Setchell, K.D. Animal models impacted by phytoestrogens in commercial chow: Implications for pathways influenced by hormones. Lab. Investig. 2001, 81, 735–747. [Google Scholar] [CrossRef]
- Kozul, C.D.; Nomikos, A.P.; Hampton, T.H.; Warnke, L.A.; Gosse, J.A.; Davey, J.C.; Thorpe, J.E.; Jackson, B.P.; Ihnat, M.A.; Hamilton, J.W. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem. Biol. Interact. 2008, 173, 129–140. [Google Scholar] [CrossRef]




| Macronutrient Contribution | Altromin 1324 (Chow) | D16010104 (CTRL) | D16010102 (NASH) |
|---|---|---|---|
| Fat (kcal%) | 11 | 10 | 40 |
| Protein (kcal%) | 24 | 20 | 20 |
| Carbohydrates (kcal%) | 65 | 70 | 40 |
| Total | 100 | 100 | 100 |
| Metabolizable energy (kcal/g) | ~3.3 | 3.7 | 4.3 |
| Sampling Time Point | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma parameters | 2 Weeks | 1 Month | 4 Months | 8 Months | 12 Months | ||||||||||
| Chow (Mean ± SD) | CTRL (Mean ± SD) | NASH (Mean ± SD) | Chow (Mean ± SD) | CTRL (Mean ± SD) | NASH (Mean ± SD) | Chow (Mean ± SD) | CTRL (Mean ± SD) | NASH (Mean ± SD) | Chow (Mean ± SD) | CTRL (Mean ± SD) | NASH (Mean ± SD) | Chow (Mean ± SD) | CTRL (Mean ± SD) | NASH (Mean ± SD) | |
| Glucose † (mmol/L) | 3.0 ± 0.4 | 3.0 ± 0.7 | 3.0 ± 0.7 | 3.0 ± 0.3 | 3.1 ± 0.5 | 3.4 ± 0.7 | 4.4 ±0.6 | 4.9 ± 1.6 | 4.0 ± 0.8 | 3.9 ± 0.7 | 4.1 ± 0.6 | 4.1 ± 0.5 | 5.0 ± 0.6 | 6.1 ± 1.3 * | 5.0 ± 0.7 # |
| Insulin † (RIIE) | - | - | - | - | - | - | 183.4 ± 62.5 | 205.9 ± 127.7 | 194.5 ± 120.3 | 201.4 ± 136.7 | 249.1 ± 159.6 | 248.9 ± 195.7 | 245.6 ± 79.6 | 258.8 ± 89.2 | 296.9 ± 242.8 |
| HOMA-IR index † | - | - | - | - | - | - | 5.51 ± 2.55 | 8.31 ± 5.09 | 7.83 ± 6.55 | 6.53 ± 3.59 | 6.17 ± 4.29 | 8.70 ± 7.95 | 9.09 ± 3.40 | 11.52 ± 3.94 | 11.15 ± 9.63 |
| 3-β-hydroxybutyrate † (µmol/L) | 88.8 ± 29.7 | 67.8 ± 26.8 | 117.3 ± 41.1 ## | 84 ± 22.8 | 73.8 ± 26.0 | 174.6 ± 40.9 ****,#### | 227.5 ± 75.8 | 180.1 ± 44.0 | 304.9 ± 109.2 *,#### | 161.6 ± 49.75 | 148.1 ± 43.6 | 273.5 ± 62.7 ****,#### | 172.2 ± 42.7 | 162.6 ± 61.7 | 265.4 ± 135.8 |
| Haptoglobin † (g/L) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 **## | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.6 ± 0.1 ## | 0.8 ± 0.2 | 0.7 ± 0.3 | 0.9 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.8 ± 0.2 **### | 0.7 ± 0.3 | 0.5 ± 0.2 | 1.0 ± 0.4 ### |
| ALT † (U/L) | 32.3 ± 5.3 | 33.1 ± 13.1 | 36.1 ± 0.5 | 41.0 ± 10.8 | 39.7 ± 7.7 | 59.0 ± 16.6 **,## | 75.6 ± 68.5 | 63.1 ± 26.8 | 114.2 ± 72.3 *,## | 48.1 ± 13.8 | 43.5 ± 14.4 | 86.8 ± 34.1 ****,#### | 79.5 ± 22.0 | 82.0 ± 29.1 | 101.8 ± 39.0 |
| AST † (U/L) | 27.6 ± 11.7 | 48.9 ± 34.1 | 32.3 ± 15.8 | 27.7 ± 5.6 | 32.3 ± 8.7 | 46.8 ± 44.9 | 45.5 ± 22.0 | 43.4 ± 14.6 | 56.0 ± 35.3 | 27.2 ± 5.5 | 25.0 ± 3.5 | 31.7 ± 6.8 *,### | 37.8 ± 5.7 | 47.6 ± 14.0 | 41.1 ± 11.8 |
| Non-HDL-C † (mmol/L) | 0.8 ± 0.2 | 1.0 ± 0.2 * | 1.8 ± 0.3 ****,#### | 1.2 ± 0.2 | 1.3 ± 0.3 | 2.3 ± 0.4 ****,#### | 1.1 ± 0.2 | 1.5 ± 0.3 * | 3.2 ± 0.6 ****,#### | 0.7 ± 0.2 | 0.9 ± 0.3 | 2.8 ± 1.1 ****,#### | 0.8 ± 0.2 | 0.8 ± 0.3 | 3.1 ± 1.3 ****,#### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svop Jensen, V.; Fledelius, C.; Max Wulff, E.; Lykkesfeldt, J.; Hvid, H. Temporal Development of Dyslipidemia and Nonalcoholic Fatty Liver Disease (NAFLD) in Syrian Hamsters Fed a High-Fat, High-Fructose, High-Cholesterol Diet. Nutrients 2021, 13, 604. https://doi.org/10.3390/nu13020604
Svop Jensen V, Fledelius C, Max Wulff E, Lykkesfeldt J, Hvid H. Temporal Development of Dyslipidemia and Nonalcoholic Fatty Liver Disease (NAFLD) in Syrian Hamsters Fed a High-Fat, High-Fructose, High-Cholesterol Diet. Nutrients. 2021; 13(2):604. https://doi.org/10.3390/nu13020604
Chicago/Turabian StyleSvop Jensen, Victoria, Christian Fledelius, Erik Max Wulff, Jens Lykkesfeldt, and Henning Hvid. 2021. "Temporal Development of Dyslipidemia and Nonalcoholic Fatty Liver Disease (NAFLD) in Syrian Hamsters Fed a High-Fat, High-Fructose, High-Cholesterol Diet" Nutrients 13, no. 2: 604. https://doi.org/10.3390/nu13020604
APA StyleSvop Jensen, V., Fledelius, C., Max Wulff, E., Lykkesfeldt, J., & Hvid, H. (2021). Temporal Development of Dyslipidemia and Nonalcoholic Fatty Liver Disease (NAFLD) in Syrian Hamsters Fed a High-Fat, High-Fructose, High-Cholesterol Diet. Nutrients, 13(2), 604. https://doi.org/10.3390/nu13020604







