The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders
Abstract
1. Introduction
2. Methods
3. Mechanisms of Hypothalamic Inflammation and the Effects of Diet and Obesity
3.1. Mechanisms Involved in Hypothalamic Inflammation
3.2. Effects of Diet and Obesity on the Inflammatory Pathways
4. Association of Diet and Obesity with Cognitive and Mood Disorders
4.1. Association of Diet and Obesity with Cognitive Disorders
4.2. Association of Diet and Obesity with Mood Disorders
4.3. Common Pathophysiologic Mechanisms Associating Obesity with Cognitive and Mood Disorders
5. Presumed Role of Hypothalamic Inflammation in Cognitive and Mood Disorders
6. Potential Prevention of Mood and Cognitive Disorders by Treating Hypothalamic Inflammation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO|Obesity. Available online: https://www.who.int/topics/obesity/en/ (accessed on 2 May 2020).
- Solomons, N.W.; Gross, R. Urban Nutrition in Developing Countries. Nutr. Rev. 1995, 53, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kvist, H.; Sjostrom, L.; Tylen, U. Adipose Tissue Volume Determinations in Women by Computed Tomography: Technical Considerations. Int. J. Obes. 1986, 10, 53–67. [Google Scholar] [PubMed]
- Thomas, E.L.; Saeed, N.; Hajnal, J.V.; Brynes, A.; Goldstone, A.P.; Frost, G.; Bell, J.D. Magnetic Resonance Imaging of Total Body Fat. J. Appl. Physiol. 1998, 85, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Rood, J.; Janderova, L.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; Smith, S.R. Relationship between Serum Resistin Concentrations and Insulin Resistance in Nonobese, Obese, and Obese Diabetic Subjects. J. Clin. Endocrinol. Metab. 2004, 89, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.-P.; Lemieux, I.; Bergeron, J.; Pibarot, P.; Mathieu, P.; Larose, E.; Rodes-Cabau, J.; Bertrand, O.F.; Poirier, P. Abdominal Obesity and the Metabolic Syndrome: Contribution to Global Cardiometabolic Risk. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1039–1049. [Google Scholar] [CrossRef]
- Piche, M.-E.; Poirier, P. Obesity, Ectopic Fat and Cardiac Metabolism. Expert Rev. Endocrinol. Metab. 2018, 13, 213–221. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Eguchi, K.; Manabe, I.; Oishi-Tanaka, Y.; Ohsugi, M.; Kono, N.; Ogata, F.; Yagi, N.; Ohto, U.; Kimoto, M.; Miyake, K.; et al. Saturated Fatty Acid and TLR Signaling Link Beta Cell Dysfunction and Islet Inflammation. Cell Metab. 2012, 15, 518–533. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.-Y.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bhusal, A.; Lee, W.-H.; Lee, I.-K.; Suk, K. Hypothalamic Inflammation and Malfunctioning Glia in the Pathophysiology of Obesity and Diabetes: Translational Significance. Biochem. Pharmacol. 2018, 153, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol Attenuates Obesity-Associated Peripheral and Central Inflammation and Improves Memory Deficit in Mice Fed a High-Fat Diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Castanon, N.; Luheshi, G.; Laye, S. Role of Neuroinflammation in the Emotional and Cognitive Alterations Displayed by Animal Models of Obesity. Front. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Kaprinis, G. Clinical Psychiatry; Medical School of Aristotle University of Thessaloniki: Thessaloniki, Greece.
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 2 May 2020).
- Sonnen, J.A.; Larson, E.B.; Crane, P.K.; Haneuse, S.; Li, G.; Schellenberg, G.D.; Craft, S.; Leverenz, J.B.; Montine, T.J. Pathological Correlates of Dementia in a Longitudinal, Population-Based Sample of Aging. Ann. Neurol. 2007, 62, 406–413. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Petrovitch, H.; Hardman, J.; Nelson, J.; Davis, D.G.; Ross, G.W.; Masaki, K.; Launer, L.; Markesbery, W.R. Cerebrovascular Pathology and Dementia in Autopsied Honolulu-Asia Aging Study Participants. Ann. N. Y. Acad. Sci. 2002, 977, 9–23. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Arvanitakis, Z.; Kelly, J.F.; Aggarwal, N.T.; Shah, R.C.; Wilson, R.S. Neuropathology of Older Persons without Cognitive Impairment from Two Community-Based Studies. Neurology 2006, 66, 1837–1844. [Google Scholar] [CrossRef]
- Knopman, D.S.; Parisi, J.E.; Salviati, A.; Floriach-Robert, M.; Boeve, B.F.; Ivnik, R.J.; Smith, G.E.; Dickson, D.W.; Johnson, K.A.; Petersen, L.E.; et al. Neuropathology of Cognitively Normal Elderly. J. Neuropathol. Exp. Neurol. 2003, 62, 1087–1095. [Google Scholar] [CrossRef]
- Rocchi, A.; Orsucci, D.; Tognoni, G.; Ceravolo, R.; Siciliano, G. The Role of Vascular Factors in Late-Onset Sporadic Alzheimer’s Disease. Genetic and Molecular Aspects. Curr. Alzheimer Res. 2009, 6, 224–237. [Google Scholar] [CrossRef]
- Roriz-Filho, J.S.; Sa-Roriz, T.M.; Rosset, I.; Camozzato, A.L.; Santos, A.C.; Chaves, M.L.F.; Moriguti, J.C.; Roriz-Cruz, M. (Pre)Diabetes, Brain Aging, and Cognition. Biochim. Biophys. Acta 2009, 1792, 432–443. [Google Scholar] [CrossRef]
- Naderali, E.K.; Ratcliffe, S.H.; Dale, M.C. Obesity and Alzheimer’s Disease: A Link between Body Weight and Cognitive Function in Old Age. Am. J. Alzheimers Dis. Other Demen. 2009, 24, 445–449. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M. Brain Insulin Resistance and Deficiency as Therapeutic Targets in Alzheimer’s Disease. Curr. Alzheimer Res. 2012, 9, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The Pathology and Pathophysiology of Vascular Dementia. Neuropharmacology 2018, 134, 226–239. [Google Scholar] [CrossRef] [PubMed]
- WHO/World Health Organisation/Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 2 May 2020).
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.L.; Vos, T.; Whiteford, H.A. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Lohoff, F.W. Overview of the Genetics of Major Depressive Disorder. Curr. Psychiatry Rep. 2010, 12, 539–546. [Google Scholar] [CrossRef]
- Krishnan, K.R.R. Biological Risk Factors in Late Life Depression. Biol. Psychiatry 2002, 52, 185–192. [Google Scholar] [CrossRef]
- Bruce, M.L. Psychosocial Risk Factors for Depressive Disorders in Late Life. Biol. Psychiatry 2002, 52, 175–184. [Google Scholar] [CrossRef]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-Fat Diet Impairs Hippocampal Neurogenesis in Male Rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]
- Pipatpiboon, N.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. PPARgamma Agonist Improves Neuronal Insulin Receptor Function in Hippocampus and Brain Mitochondria Function in Rats with Insulin Resistance Induced by Long Term High-Fat Diets. Endocrinology 2012, 153, 329–338. [Google Scholar] [CrossRef]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef]
- Kreutzer, C.; Peters, S.; Schulte, D.M.; Fangmann, D.; Turk, K.; Wolff, S.; Van Eimeren, T.; Ahrens, M.; Beckmann, J.; Schafmayer, C.; et al. Hypothalamic Inflammation in Human Obesity Is Mediated by Environmental and Genetic Factors. Diabetes 2017, 66, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Kalin, S.; Heppner, F.L.; Bechmann, I.; Prinz, M.; Tschop, M.H.; Yi, C.-X. Hypothalamic Innate Immune Reaction in Obesity. Nat. Rev. Endocrinol. 2015, 11, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic Inflammation and Obesity: A Mechanistic Review. Arch. Pharm. Res. 2019, 42, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-KappaB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197. [Google Scholar] [CrossRef]
- Williams, L.M. Hypothalamic Dysfunction in Obesity. Proc. Nutr. Soc. 2012, 71, 521–533. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Shi, Y.-C.; Zhang, Y.; Lin, S. The Role of Inflammation and Endoplasmic Reticulum Stress in Obesity-Related Cognitive Impairment. Life Sci. 2019, 233, 116707. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Thaler, J.P. Hypothalamic Inflammation and Gliosis in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 325–330. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER Stress and the Unfolded Protein Response in Neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From Endoplasmic-Reticulum Stress to the Inflammatory Response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Zhao, Z.; Li, S.; Yin, J. The Dynamic Changes of Endoplasmic Reticulum Stress Pathway Markers GRP78 and CHOP in the Hippocampus of Diabetic Mice. Brain Res. Bull. 2015, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-KappaB and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, P.; McGavern, D. The Anatomy and Immunology of Vasculature in the Central Nervous System. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.L. The Hardship of Obesity: A Soft-Wired Hypothalamus. Nat. Neurosci. 2005, 8, 561–565. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, D. Defective Hypothalamic Autophagy Directs the Central Pathogenesis of Obesity via the IkappaB Kinase Beta (IKKbeta)/NF-KappaB Pathway. J. Biol. Chem. 2011, 286, 32324–32332. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated Fatty Acids Activate TLR-Mediated Proinflammatory Signaling Pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated Long-Chain Fatty Acids Activate Inflammatory Signaling in Astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G.; Li, Y.; Wang, Y.; Liu, Z. Knockdown of Tlr4 in the Arcuate Nucleus Improves Obesity Related Metabolic Disorders. Sci. Rep. 2017, 7, 7441. [Google Scholar] [CrossRef]
- Gorina, R.; Font-Nieves, M.; Marquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 Activation Induces a Proinflammatory Environment through the Interplay between MyD88-Dependent NFkappaB Signaling, MAPK, and Jak1/Stat1 Pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef]
- Carmo-Silva, S.; Cavadas, C. Hypothalamic Dysfunction in Obesity and Metabolic Disorders. Adv. Neurobiol. 2017, 19, 73–116. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, H.; Li, J.-J.; Zhang, Y.-L.; Xin, L.; Li, F.; Lou, S.-J. The Signaling Mechanisms of Hippocampal Endoplasmic Reticulum Stress Affecting Neuronal Plasticity-Related Protein Levels in High Fat Diet-Induced Obese Rats and the Regulation of Aerobic Exercise. Brain. Behav. Immun. 2016, 57, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, S.L.; Davidson, T.L.; Zheng, W.; Kinzig, K.P. Western Diets Induce Blood-Brain Barrier Leakage and Alter Spatial Strategies in Rats. Behav. Neurosci. 2016, 130, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Spencer, S.J. Obesity and Neuroinflammation: A Pathway to Cognitive Impairment. Brain. Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Fuente-Martin, E.; Finan, B.; Kim, M.; Frank, A.; Garcia-Caceres, C.; Navas, C.R.; Gordillo, R.; Neinast, M.; Kalainayakan, S.P.; et al. Hypothalamic PGC-1α Protects against High-Fat Diet Exposure by Regulating ERα. Cell Rep. 2014, 9, 633–645. [Google Scholar] [CrossRef]
- Portovedo, M.; Ignacio-Souza, L.M.; Bombassaro, B.; Coope, A.; Reginato, A.; Razolli, D.S.; Torsoni, M.A.; Torsoni, A.S.; Leal, R.F.; Velloso, L.A.; et al. Saturated Fatty Acids Modulate Autophagy’s Proteins in the Hypothalamus. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Ávalos, Y.; Kerr, B.; Maliqueo, M.; Dorfman, M. Cell and Molecular Mechanisms behind Diet-Induced Hypothalamic Inflammation and Obesity. J. Neuroendocrinol. 2018, 30, e12598. [Google Scholar] [CrossRef]
- Hou, Q.; Guan, Y.; Yu, W.; Liu, X.; Wu, L.; Xiao, M.; Lü, Y. Associations between Obesity and Cognitive Impairment in the Chinese Elderly: An Observational Study. Clin. Interv. Aging 2019, 14, 367–373. [Google Scholar] [CrossRef]
- Noh, H.-M.; Han, J.; Kim, Y.J.; Jung, J.-H.; Roh, Y.K.; Song, H.J. Sex Differences in the Relationship between Cognitive Impairment and Overweight or Obesity in Late Life: A 3-Year Prospective Study. Medicine (Baltimore) 2019, 98, e14736. [Google Scholar] [CrossRef]
- Yau, P.L.; Castro, M.G.; Tagani, A.; Tsui, W.H.; Convit, A. Obesity and Metabolic Syndrome and Functional and Structural Brain Impairments in Adolescence. Pediatrics 2012, 130, e856–e864. [Google Scholar] [CrossRef]
- Lee, C.M.; Woodward, M.; Batty, G.D.; Beiser, A.S.; Bell, S.; Berr, C.; Bjertness, E.; Chalmers, J.; Clarke, R.; Dartigues, J.-F.; et al. Association of Anthropometry and Weight Change with Risk of Dementia and Its Major Subtypes: A Meta-Analysis Consisting 2.8 Million Adults with 57 294 Cases of Dementia. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12989. [Google Scholar] [CrossRef] [PubMed]
- Pedditzi, E.; Peters, R.; Beckett, N. The Risk of Overweight/Obesity in Mid-Life and Late Life for the Development of Dementia: A Systematic Review and Meta-Analysis of Longitudinal Studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Arnold, R.; Wells, J.C.K.; Tagliabue, A.; Colantuoni, A.; Albanese, E.; Brayne, C.; Stephan, B.C.M. Intentional Weight Loss in Overweight and Obese Individuals and Cognitive Function: A Systematic Review and Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-Y.; Li, M.; Han, L.; Tayie, F.; Yao, S.-S.; Huang, Z.; Ai, P.; Liu, Y.-Z.; Hu, Y.-H.; Xu, B. Dietary Fat Intake and Cognitive Function among Older Populations: A Systematic Review and Meta-Analysis. J. Prev. Alzheimers Dis. 2019, 6, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.Z.; Harty, B.; Mukamal, K.J.; Stoddard, A.M.; Vitolins, M.; Dunn, J.E. Monounsaturated, Trans, and Saturated Fatty Acids and Cognitive Decline in Women. J. Am. Geriatr. Soc. 2011, 59, 837–843. [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Layé, S.; Ferreira, G. Impairment of Hippocampal-Dependent Memory Induced by Juvenile High-Fat Diet Intake Is Associated with Enhanced Hippocampal Inflammation in Rats. Brain. Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef]
- Khazen, T.; Hatoum, O.A.; Ferreira, G.; Maroun, M. Acute Exposure to a High-Fat Diet in Juvenile Male Rats Disrupts Hippocampal-Dependent Memory and Plasticity through Glucocorticoids. Sci. Rep. 2019, 9, 12270. [Google Scholar] [CrossRef]
- Haagensen, A.M.J.; Klein, A.B.; Ettrup, A.; Matthews, L.R.; Sørensen, D.B. Cognitive Performance of Göttingen Minipigs Is Affected by Diet in a Spatial Hole-Board Discrimination Test. PLoS ONE 2013, 8, e79429. [Google Scholar] [CrossRef][Green Version]
- Gautier, Y.; Damien, B.; Serrand, Y.; Réthoré, N.; Mahérault, M.; Malbert, C.-H.; Meurice, P.; Coquery, N.; Moirand, R.; Val-Laillet, D. Western Diet, Obesity and Bariatric Surgery Sequentially Modulated Anxiety, Eating Patterns and Brain Responses to Sucrose in Adult Yucatan Minipigs. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Jung, S.J.; Woo, H.-T.; Cho, S.; Park, K.; Jeong, S.; Lee, Y.J.; Kang, D.; Shin, A. Association between Body Size, Weight Change and Depression: Systematic Review and Meta-Analysis. Br. J. Psychiatry J. Ment. Sci. 2017, 211, 14–21. [Google Scholar] [CrossRef]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Prospective Associations between Depression and Obesity for Adolescent Males and Females- A Systematic Review and Meta-Analysis of Longitudinal Studies. PLoS ONE 2016, 11, e0157240. [Google Scholar] [CrossRef] [PubMed]
- Quek, Y.-H.; Tam, W.W.S.; Zhang, M.W.B.; Ho, R.C.M. Exploring the Association between Childhood and Adolescent Obesity and Depression: A Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Anderson, D.; Lurie-Beck, J. The Relationship between Abdominal Obesity and Depression in the General Population: A Systematic Review and Meta-Analysis. Obes. Res. Clin. Pract. 2011, 5, e267–e360. [Google Scholar] [CrossRef] [PubMed]
- De Wit, L.; Luppino, F.; Van Straten, A.; Penninx, B.; Zitman, F.; Cuijpers, P. Depression and Obesity: A Meta-Analysis of Community-Based Studies. Psychiatry Res. 2010, 178, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Jokela, M.; Hamer, M.; Singh-Manoux, A.; Batty, G.D.; Kivimäki, M. Association of Metabolically Healthy Obesity with Depressive Symptoms: Pooled Analysis of Eight Studies. Mol. Psychiatry 2014, 19, 910–914. [Google Scholar] [CrossRef]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Is There a Bi-Directional Relationship between Depression and Obesity among Adult Men and Women? Systematic Review and Bias-Adjusted Meta Analysis. Asian J. Psychiatry 2016, 21, 51–66. [Google Scholar] [CrossRef]
- Serretti, A.; Mandelli, L. Antidepressants and Body Weight: A Comprehensive Review and Meta-Analysis. J. Clin. Psychiatry 2010, 71, 1259–1272. [Google Scholar] [CrossRef]
- Gibson-Smith, D.; Bot, M.; Milaneschi, Y.; Twisk, J.W.; Visser, M.; Brouwer, I.A.; Penninx, B.W.J.H. Major Depressive Disorder, Antidepressant Use, and Subsequent 2-Year Weight Change Patterns in the Netherlands Study of Depression and Anxiety. J. Clin. Psychiatry 2016, 77, e144–e151. [Google Scholar] [CrossRef]
- Bet, P.M.; Hugtenburg, J.G.; Penninx, B.W.J.H.; Hoogendijk, W.J.G. Side Effects of Antidepressants during Long-Term Use in a Naturalistic Setting. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013, 23, 1443–1451. [Google Scholar] [CrossRef]
- Nakajima, S.; Fukasawa, K.; Gotoh, M.; Murakami-Murofushi, K.; Kunugi, H. Saturated Fatty Acid Is a Principal Cause of Anxiety-like Behavior in Diet-Induced Obese Rats in Relation to Serum Lysophosphatidyl Choline Level. Int. J. Obes. 2020, 44, 727–738. [Google Scholar] [CrossRef]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Fröhlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-Fat Diet Induces Depression-like Behaviour in Mice Associated with Changes in Microbiome, Neuropeptide Y, and Brain Metabolome. Nutr. Neurosci. 2019, 22, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Mulder, P.; Macht, V.A.; Kaigler, K.F.; Wilson, S.P.; Wilson, M.A.; Reagan, L.P. Dietary restriction reverses obesity-induced anhedonia. Physiol. Behav. 2014, 128, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.; Dinel, A.-L.; Ferreira, G.; Layé, S.; Castanon, N. Diet-Induced Obesity Progressively Alters Cognition, Anxiety-like Behavior and Lipopolysaccharide-Induced Depressive-like Behavior: Focus on Brain Indoleamine 2,3-Dioxygenase Activation. Brain. Behav. Immun. 2014, 41. [Google Scholar] [CrossRef] [PubMed]
- Gautier, Y.; Luneau, I.; Coquery, N.; Meurice, P.; Malbert, C.-H.; Guerin, S.; Kemp, B.; Bolhuis, J.E.; Clouard, C.; Huërou-Luron, I.L.; et al. Maternal Western Diet during Gestation and Lactation Modifies Adult Offspring’s Cognitive and Hedonic Brain Processes, Behavior, and Metabolism in Yucatan Minipigs. FASEB J. 2018, 32, 6478–6794. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Seshadri, S.; Beiser, A.; Au, R.; Himali, J.J.; Palumbo, C.; Wolf, P.A.; DeCarli, C. Midlife Vascular Risk Factor Exposure Accelerates Structural Brain Aging and Cognitive Decline. Neurology 2011, 77, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Benedict, C.; Burgos, J.; Kempton, M.J.; Kullberg, J.; Nordenskjöld, R.; Kilander, L.; Nylander, R.; Larsson, E.-M.; Johansson, L.; et al. Late-Life Obesity Is Associated with Smaller Global and Regional Gray Matter Volumes: A Voxel-Based Morphometric Study. Int. J. Obes. 2013, 37, 230–236. [Google Scholar] [CrossRef]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain Structure and Obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef]
- Gazdzinski, S.; Kornak, J.; Weiner, M.W.; Meyerhoff, D.J. Body Mass Index and Magnetic Resonance Markers of Brain Integrity in Adults. Ann. Neurol. 2008, 63, 652–657. [Google Scholar] [CrossRef]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western Diet Is Associated with a Smaller Hippocampus: A Longitudinal Investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Emory, E. Executive Function and the Frontal Lobes: A Meta-Analytic Review. Neuropsychol. Rev. 2006, 16, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.M.; Kinzenbaw, D.A.; Chen, X.; Zhan, S.; Mezzetti, E.; Filosa, J.; Ergul, A.; Faulkner, J.L.; Faraci, F.M.; Didion, S.P. Nox2-Derived Superoxide Contributes to Cerebral Vascular Dysfunction in Diet-Induced Obesity. Stroke 2013, 44, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Prakash, R.; Chawla, D.; Du, W.; Didion, S.P.; Filosa, J.A.; Zhang, Q.; Brann, D.W.; Lima, V.V.; Tostes, R.C.; et al. Early Effects of High-Fat Diet on Neurovascular Function and Focal Ischemic Brain Injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1001–R1008. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Busquets, O.; Ettcheto, M.; Pallàs, M.; Beas-Zarate, C.; Verdaguer, E.; Auladell, C.; Folch, J.; Camins, A. Long-Term Exposition to a High Fat Diet Favors the Appearance of β-Amyloid Depositions in the Brain of C57BL/6J Mice. A Potential Model of Sporadic Alzheimer’s Disease. Mech. Ageing Dev. 2017, 162, 38–45. [Google Scholar] [CrossRef]
- Vagena, E.; Ryu, J.K.; Baeza-Raja, B.; Walsh, N.M.; Syme, C.; Day, J.P.; Houslay, M.D.; Baillie, G.S. A High-Fat Diet Promotes Depression-like Behavior in Mice by Suppressing Hypothalamic PKA Signaling. Transl. Psychiatry 2019, 9, 141. [Google Scholar] [CrossRef]
- Rasgon, N.L.; Kenna, H.A.; Wroolie, T.E.; Kelley, R.; Silverman, D.; Brooks, J.; Williams, K.E.; Powers, B.N.; Hallmayer, J.; Reiss, A. Insulin Resistance and Hippocampal Volume in Women at Risk for Alzheimer’s Disease. Neurobiol. Aging 2011, 32, 1942–1948. [Google Scholar] [CrossRef]
- Kan, C.; Silva, N.; Golden, S.H.; Rajala, U.; Timonen, M.; Stahl, D.; Ismail, K. A Systematic Review and Meta-Analysis of the Association Between Depression and Insulin Resistance. Diabetes Care 2013, 36, 480–489. [Google Scholar] [CrossRef]
- Grillo, C.A.; Piroli, G.G.; Kaigler, K.F.; Wilson, S.P.; Wilson, M.A.; Reagan, L.P. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav. Brain Res. 2011, 222, 230–235. [Google Scholar] [CrossRef]
- Rasgon, N.L.; McEwen, B.S. Insulin Resistance—A Missing Link No More. Mol. Psychiatry 2016, 21, 1648–1652. [Google Scholar] [CrossRef]
- Engin, A. Diet-Induced Obesity and the Mechanism of Leptin Resistance. Adv. Exp. Med. Biol. 2017, 960, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Lamers, F.; Bot, M.; Drent, M.L.; Penninx, B.W.J.H. Leptin Dysregulation Is Specifically Associated With Major Depression With Atypical Features: Evidence for a Mechanism Connecting Obesity and Depression. Biol. Psychiatry 2017, 81, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Papargyri, P.; Zapanti, E.; Salakos, N.; Papargyris, L.; Bargiota, A.; Mastorakos, G. Links between HPA Axis and Adipokines: Clinical Implications in Paradigms of Stress-Related Disorders. Expert Rev. Endocrinol. Metab. 2018, 13, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.-F.; Qi, C.-C.; Zhou, J.-N. Imbalance of Leptin Pathway and Hypothalamus Synaptic Plasticity Markers Are Associated with Stress-Induced Depression in Rats. Behav. Brain Res. 2013, 249, 38–43. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, D.X.; Jiang, H.; Pan, F.; Ho, C.S.; Ho, R.C. The Effects of High-Fat-Diet Combined with Chronic Unpredictable Mild Stress on Depression-like Behavior and Leptin/LepRb in Male Rats. Sci. Rep. 2016, 6, 35239. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Marked Alterations in the Distal Gut Microbiome Linked to Diet-Induced Obesity. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between Diet-Related Changes in the Gut Microbiome and Cognitive Flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.-R. Obese-Type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered Bile Acid Profile Associates with Cognitive Impairment in Alzheimer’s Disease-An Emerging Role for Gut Microbiome. Alzheimers Dement. J. Alzheimers Assoc. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. JAD 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Cizza, G. The Hypothalamic-Pituitary-Adrenal Axis, Obesity, and Chronic Stress Exposure: Sleep and the HPA Axis in Obesity. Curr. Obes. Rep. 2012, 1, 208–215. [Google Scholar] [CrossRef]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-Related and Basic Determinants of Hair Cortisol in Humans: A Meta-Analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA Axis in Major Depression: Cortisol, Clinical Symptomatology and Genetic Variation Predict Cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Gillespie, C.F.; Nemeroff, C.B. Hypercortisolemia and Depression. Psychosom. Med. 2005, 67 (Suppl. 1), S26–S28. [Google Scholar] [CrossRef]
- Holsboer, F. The Corticosteroid Receptor Hypothesis of Depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Pariante, C.M. Why Are Depressed Patients Inflamed? A Reflection on 20 Years of Research on Depression, Glucocorticoid Resistance and Inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Ising, M.; Horstmann, S.; Kloiber, S.; Lucae, S.; Binder, E.B.; Kern, N.; Künzel, H.E.; Pfennig, A.; Uhr, M.; Holsboer, F. Combined Dexamethasone/Corticotropin Releasing Hormone Test Predicts Treatment Response in Major Depression—A Potential Biomarker? Biol. Psychiatry 2007, 62, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tatomir, A.; Micu, C.; Crivii, C. The Impact of Stress and Glucocorticoids on Memory. Clujul Med. 2014, 87, 3–6. [Google Scholar] [CrossRef]
- Lara, V.P.; Caramelli, P.; Teixeira, A.L.; Barbosa, M.T.; Carmona, K.C.; Carvalho, M.G.; Fernandes, A.P.; Gomes, K.B. High Cortisol Levels Are Associated with Cognitive Impairment No-Dementia (CIND) and Dementia. Clin. Chim. Acta Int. J. Clin. Chem. 2013, 423, 18–22. [Google Scholar] [CrossRef]
- Valsamakis, G.; Papatheodorou, D.; Chalarakis, N.; Manolikaki, M.; Margeli, A.; Papassotiriou, I.; Barber, T.M.; Kumar, S.; Kalantaridou, S.; Mastorakos, G. Maternal Chronic Stress Correlates with Serum Levels of Cortisol, Glucose and C-Peptide in the Fetus, and Maternal Non Chronic Stress with Fetal Growth. Psychoneuroendocrinology 2020, 114, 104591. [Google Scholar] [CrossRef]
- Bowers, M.E.; Yehuda, R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 232–244. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; McGill, M.A.; Lehrner, A.; Yehuda, R. Endocrine Aspects of PTSD: Hypothalamic-Pituitary-Adrenal (HPA) Axis and Beyond. In Comprehensive Guide to Post-Traumatic Stress Disorder; Martin, C.R., Preedy, V.R., Patel, V.B., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–14. ISBN 978-3-319-08613-2. [Google Scholar]
- Pepping, J.K.; Freeman, L.R.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. NOX2 Deficiency Attenuates Markers of Adiposopathy and Brain Injury Induced by High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E392–E404. [Google Scholar] [CrossRef]
- Moraes, J.C.; Coope, A.; Morari, J.; Cintra, D.E.; Roman, E.A.; Pauli, J.R.; Romanatto, T.; Carvalheira, J.B.; Oliveira, A.L.R.; Saad, M.J.; et al. High-Fat Diet Induces Apoptosis of Hypothalamic Neurons. PLoS ONE 2009, 4, e5045. [Google Scholar] [CrossRef]
- Rivera, P.; Pérez-Martín, M.; Pavón, F.J.; Serrano, A.; Crespillo, A.; Cifuentes, M.; López-Ávalos, M.-D.; Grondona, J.M.; Vida, M.; Fernández-Llebrez, P.; et al. Pharmacological Administration of the Isoflavone Daidzein Enhances Cell Proliferation and Reduces High Fat Diet-Induced Apoptosis and Gliosis in the Rat Hippocampus. PLoS ONE 2013, 8, e64750. [Google Scholar] [CrossRef] [PubMed]
- Dinel, A.-L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Cognitive and Emotional Alterations Are Related to Hippocampal Inflammation in a Mouse Model of Metabolic Syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Lowell, J.A. Th17 Cells in Depression. Brain. Behav. Immun. 2018, 69, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, T.; Chen, P.; Ouyang, J.; Xu, G.; Zeng, Z.; Sun, Y. Emerging Tendency towards Autoimmune Process in Major Depressive Patients: A Novel Insight from Th17 Cells. Psychiatry Res. 2011, 188, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Davami, M.H.; Baharlou, R.; Ahmadi Vasmehjani, A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M.R. Elevated IL-17 and TGF-β Serum Levels: A Positive Correlation between T-Helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin. Neurosci. 2016, 7, 137–142. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Fardan, A.S.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Attia, S.M. IL-17A Causes Depression-like Symptoms via NFκB and P38MAPK Signaling Pathways in Mice: Implications for Psoriasis Associated Depression. Cytokine 2017, 97, 14–24. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, K.-F.; Liu, Z.; Qiu, Y.-H.; Peng, Y.-P. Th17 Cell-Mediated Neuroinflammation Is Involved in Neurodegeneration of Aβ1-42-Induced Alzheimer’s Disease Model Rats. PLoS ONE 2013, 8, e75786. [Google Scholar] [CrossRef]
- Capuron, L.; Gumnick, J.F.; Musselman, D.L.; Lawson, D.H.; Reemsnyder, A.; Nemeroff, C.B.; Miller, A.H. Neurobehavioral Effects of Interferon-Alpha in Cancer Patients: Phenomenology and Paroxetine Responsiveness of Symptom Dimensions. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2002, 26, 643–652. [Google Scholar] [CrossRef]
- Lotrich, F.E.; Rabinovitz, M.; Gironda, P.; Pollock, B.G. Depression Following Pegylated Interferon-Alpha: Characteristics and Vulnerability. J. Psychosom. Res. 2007, 63, 131–135. [Google Scholar] [CrossRef][Green Version]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Daly, M. The Relationship of C-Reactive Protein to Obesity-Related Depressive Symptoms: A Longitudinal Study. Obesity 2013, 21, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Weber, J.S.; Magiakou, M.A.; Gunn, H.; Chrousos, G.P. Hypothalamic-Pituitary-Adrenal Axis Activation and Stimulation of Systemic Vasopressin Secretion by Recombinant Interleukin-6 in Humans: Potential Implications for the Syndrome of Inappropriate Vasopressin Secretion. J. Clin. Endocrinol. Metab. 1994, 79, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Chrousos, G.P.; Weber, J.S. Recombinant Interleukin-6 Activates the Hypothalamic-Pituitary-Adrenal Axis in Humans. J. Clin. Endocrinol. Metab. 1993, 77, 1690–1694. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Schéle, E.; Benrick, A.; Grahnemo, L.; Egecioglu, E.; Anesten, F.; Pálsdóttir, V.; Jansson, J.-O. Inter-Relation between Interleukin (IL)-1, IL-6 and Body Fat Regulating Circuits of the Hypothalamic Arcuate Nucleus. J. Neuroendocrinol. 2013, 25, 580–589. [Google Scholar] [CrossRef]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H. Immune Modulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis during Viral Infection. Viral Immunol. 2005, 18, 41–78. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Hu, F.; Miller, A.H. Cytokine-Effects on Glucocorticoid Receptor Function: Relevance to Glucocorticoid Resistance and the Pathophysiology and Treatment of Major Depression. Brain. Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef]
- Webster, J.C.; Oakley, R.H.; Jewell, C.M.; Cidlowski, J.A. Proinflammatory Cytokines Regulate Human Glucocorticoid Receptor Gene Expression and Lead to the Accumulation of the Dominant Negative β Isoform: A Mechanism for the Generation of Glucocorticoid Resistance. Proc. Natl. Acad. Sci. USA 2001, 98, 6865–6870. [Google Scholar] [CrossRef]
- Escoll, P.; Ranz, I.; Muñoz-Antón, N.; Van-den-Rym, A.; Alvarez-Mon, M.; Martínez-Alonso, C.; Sanz, E.; De-la-Hera, A. Sustained Interleukin-1β Exposure Modulates Multiple Steps in Glucocorticoid Receptor Signaling, Promoting Split-Resistance to the Transactivation of Prominent Anti-Inflammatory Genes by Glucocorticoids. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, H.; Miller, A.H. Interleukin 1alpha (IL-1alpha) Induced Activation of P38 Mitogen-Activated Protein Kinase Inhibits Glucocorticoid Receptor Function. Mol. Psychiatry 2004, 9, 65–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johansen-Berg, H.; Gutman, D.A.; Behrens, T.E.J.; Matthews, P.M.; Rushworth, M.F.S.; Katz, E.; Lozano, A.M.; Mayberg, H.S. Anatomical Connectivity of the Subgenual Cingulate Region Targeted with Deep Brain Stimulation for Treatment-Resistant Depression. Cereb. Cortex 2008, 18, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal Limbic-Cortical Function and Negative Mood: Converging PET Findings in Depression and Normal Sadness. Am. J. Psychiatry 1999, 156, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D.; Lopez, J.F.; Lyons, D.M.; Burke, S.; Wallace, M.; Schatzberg, A.F. Glucocorticoid and Mineralocorticoid Receptor MRNA Expression in Squirrel Monkey Brain. J. Psychiatr. Res. 2000, 34, 383–392. [Google Scholar] [CrossRef]
- Sudheimer, K.D.; Abelson, J.L.; Taylor, S.F.; Martis, B.; Welsh, R.C.; Warner, C.; Samet, M.; Manduzzi, A.; Liberzon, I. Exogenous Glucocorticoids Decrease Subgenual Cingulate Activity Evoked by Sadness. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 826–845. [Google Scholar] [CrossRef]
- Sudheimer, K.; Keller, J.; Gomez, R.; Tennakoon, L.; Reiss, A.; Garrett, A.; Kenna, H.; O’Hara, R.; Schatzberg, A.F. Decreased Hypothalamic Functional Connectivity with Subgenual Cortex in Psychotic Major Depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 849–860. [Google Scholar] [CrossRef]
- Shin, A.C.; Zheng, H.; Berthoud, H.-R. An Expanded View of Energy Homeostasis: Neural Integration of Metabolic, Cognitive, and Emotional Drives to Eat. Physiol. Behav. 2009, 97, 572–580. [Google Scholar] [CrossRef]
- Berthoud, H.-R. Multiple Neural Systems Controlling Food Intake and Body Weight. Neurosci. Biobehav. Rev. 2002, 26, 393–428. [Google Scholar] [CrossRef]
- Shintani, F.; Kanba, S.; Nakaki, T.; Nibuya, M.; Kinoshita, N.; Suzuki, E.; Yagi, G.; Kato, R.; Asai, M. Interleukin-1 Beta Augments Release of Norepinephrine, Dopamine, and Serotonin in the Rat Anterior Hypothalamus. J. Neurosci. 1993, 13, 3574–3581. [Google Scholar] [CrossRef]
- Song, C.; Merali, Z.; Anisman, H. Variations of Nucleus Accumbens Dopamine and Serotonin Following Systemic Interleukin-1, Interleukin-2 or Interleukin-6 Treatment. Neuroscience 1999, 88, 823–836. [Google Scholar] [CrossRef]
- Bai, S.; Guo, W.; Feng, Y.; Deng, H.; Li, G.; Nie, H.; Guo, G.; Yu, H.; Ma, Y.; Wang, J.; et al. Efficacy and Safety of Anti-Inflammatory Agents for the Treatment of Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of Anti-Inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-Analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef] [PubMed]
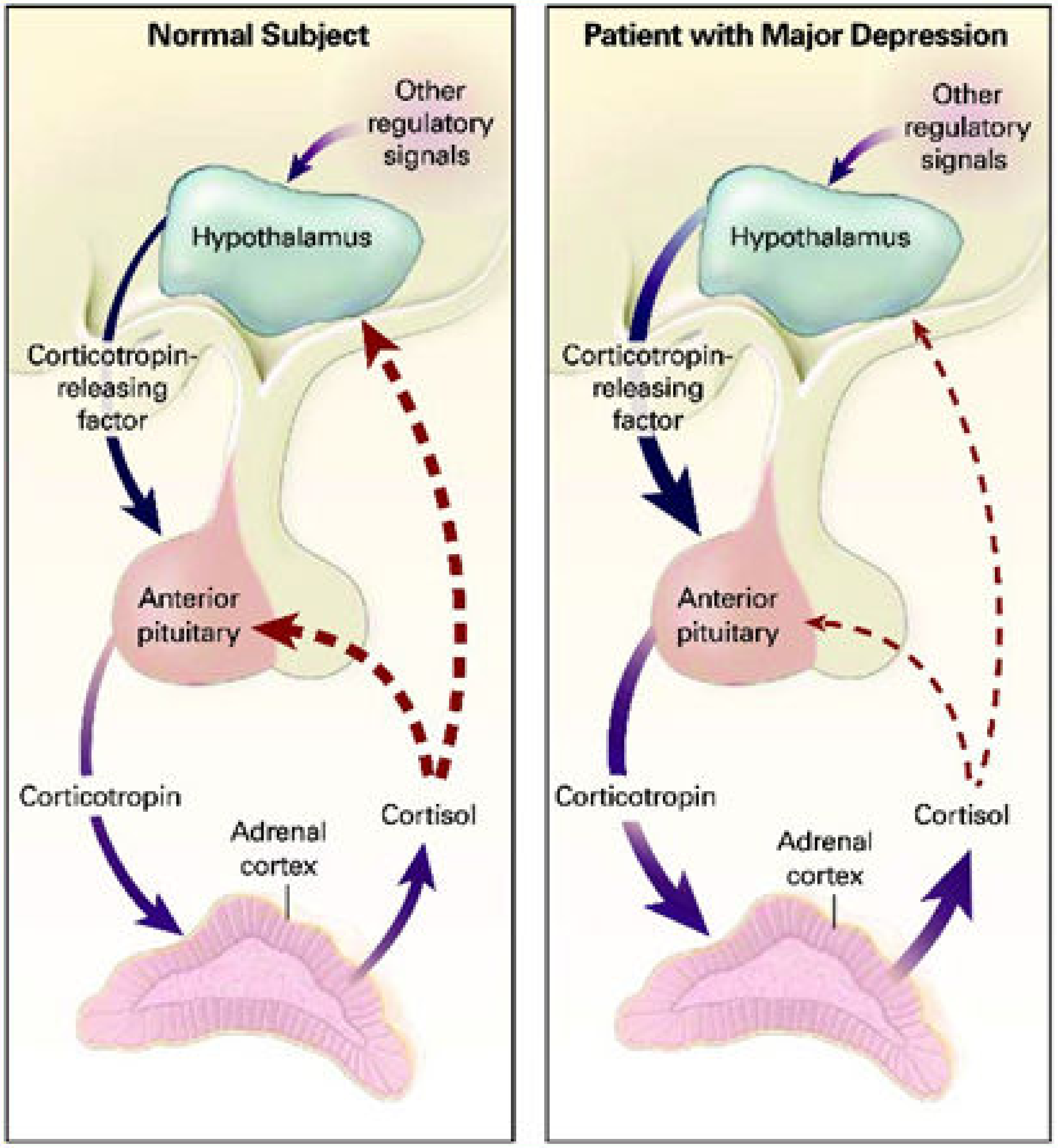
- Bao, A.-M.; Swaab, D.F. The Human Hypothalamus in Mood Disorders: The HPA Axis in the Center. IBRO Rep. 2018, 6, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Gray, T.S.; D’Souza, D.N.; Carrasco, G.A.; Damjanoska, K.J.; Dudas, B.; Garcia, F.; Zainelli, G.M.; Sullivan Hanley, N.R.; Battaglia, G.; et al. Desensitization of 5-HT1A Receptors by 5-HT2A Receptors in Neuroendocrine Neurons in Vivo. J. Pharmacol. Exp. Ther. 2004, 310, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Osei-Owusu, P.; James, A.; Crane, J.; Scrogin, K.E. 5-Hydroxytryptamine 1A Receptors in the Paraventricular Nucleus of the Hypothalamus Mediate Oxytocin and Adrenocorticotropin Hormone Release and Some Behavioral Components of the Serotonin Syndrome. J. Pharmacol. Exp. Ther. 2005, 313, 1324–1330. [Google Scholar] [CrossRef]
- Heisler, L.K.; Pronchuk, N.; Nonogaki, K.; Zhou, L.; Raber, J.; Tung, L.; Yeo, G.S.H.; O’Rahilly, S.; Colmers, W.F.; Elmquist, J.K.; et al. Serotonin Activates the Hypothalamic–Pituitary–Adrenal Axis via Serotonin 2C Receptor Stimulation. J. Neurosci. 2007, 27, 6956–6964. [Google Scholar] [CrossRef]
- Savitz, J. Role of Kynurenine Metabolism Pathway Activation in Major Depressive Disorders. Curr. Top. Behav. Neurosci. 2017, 31, 249–267. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The New “5-HT” Hypothesis of Depression: Cell-Mediated Immune Activation Induces Indoleamine 2,3-Dioxygenase, Which Leads to Lower Plasma Tryptophan and an Increased Synthesis of Detrimental Tryptophan Catabolites (TRYCATs), Both of Which Contribute to the Onset of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef]
- Czyrak, A.; Maćkowiak, M.; Chocyk, A.; Fijał, K.; Wedzony, K. Role of Glucocorticoids in the Regulation of Dopaminergic Neurotransmission. Pol. J. Pharmacol. 2003, 55, 667–674. [Google Scholar]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.-M.; Ruhé, H.G.; Gao, S.-F.; Swaab, D.F. Neurotransmitters and Neuropeptides in Depression. Handb. Clin. Neurol. 2012, 106, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Binder, E.B.; Künzel, H.E.; Schuld, A.; Lucae, S.; Uhr, M.; Pollmächer, T.; Holsboer, F.; Ising, M. Successful Antidepressant Therapy Restores the Disturbed Interplay between TNF-Alpha System and HPA Axis. Biol. Psychiatry 2006, 60, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Mueller, N.K.; Figueiredo, H. Role of GABA and Glutamate Circuitry in Hypothalamo-Pituitary-Adrenocortical Stress Integration. Ann. N. Y. Acad. Sci. 2004, 1018, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, J.-J.; Zhu, Y.; Kosten, T.; Li, D.-P. Chronic Unpredictable Mild Stress Induces Loss of GABA Inhibition in Corticotrophin-Releasing Hormone-Expressing Neurons through NKCC1 Upregulation. Neuroendocrinology 2017, 104, 194–208. [Google Scholar] [CrossRef]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The Effects of Stress and Stress Hormones on Human Cognition: Implications for the Field of Brain and Cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Lupien, S.J.; De Leon, M.; De Santi, S.; Convit, A.; Tarshish, C.; Nair, N.P.; Thakur, M.; McEwen, B.S.; Hauger, R.L.; Meaney, M.J. Cortisol Levels during Human Aging Predict Hippocampal Atrophy and Memory Deficits. Nat. Neurosci. 1998, 1, 69–73. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Craft, S.; Hershey, T.; Askins, K.; Bardgett, M.E. Glucocorticoid-Induced Impairment in Declarative Memory Performance in Adult Humans. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 2047–2053. [Google Scholar] [CrossRef]
- Buss, C.; Wolf, O.T.; Witt, J.; Hellhammer, D.H. Autobiographic Memory Impairment Following Acute Cortisol Administration. Psychoneuroendocrinology 2004, 29, 1093–1096. [Google Scholar] [CrossRef]
- Conrad, C.D.; Galea, L.A.; Kuroda, Y.; McEwen, B.S. Chronic Stress Impairs Rat Spatial Memory on the Y Maze, and This Effect Is Blocked by Tianeptine Pretreatment. Behav. Neurosci. 1996, 110, 1321–1334. [Google Scholar] [CrossRef]
- De Quervain, D.J.; Roozendaal, B.; McGaugh, J.L. Stress and Glucocorticoids Impair Retrieval of Long-Term Spatial Memory. Nature 1998, 394, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Starkman, M.N.; Gebarski, S.S.; Berent, S.; Schteingart, D.E. Hippocampal Formation Volume, Memory Dysfunction, and Cortisol Levels in Patients with Cushing’s Syndrome. Biol. Psychiatry 1992, 32, 756–765. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal Volume Reduction in Major Depression. Am. J. Psychiatry 2000, 157, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Elgh, E.; Lindqvist Astot, A.; Fagerlund, M.; Eriksson, S.; Olsson, T.; Näsman, B. Cognitive Dysfunction, Hippocampal Atrophy and Glucocorticoid Feedback in Alzheimer’s Disease. Biol. Psychiatry 2006, 59, 155–161. [Google Scholar] [CrossRef]
- Green, K.N.; Billings, L.M.; Roozendaal, B.; McGaugh, J.L.; LaFerla, F.M. Glucocorticoids Increase Amyloid-Beta and Tau Pathology in a Mouse Model of Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 9047–9056. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, I.; Catania, C.; Riedemann, T.; Fry, J.P.; Breen, K.C.; Michaelidis, T.M.; Almeida, O.F.X. Glucocorticoids Trigger Alzheimer Disease-like Pathobiochemistry in Rat Neuronal Cells Expressing Human Tau. J. Neurochem. 2008, 107, 385–397. [Google Scholar] [CrossRef]
- Le Bras, A. Adiposity and Inflammation: A Pathway to Cognitive Dysfunction. Lab Anim. 2020, 49, 148. [Google Scholar] [CrossRef]
- Bondan, E.F.; Cardoso, C.V.; de Fátima Martins, M.; Otton, R. Memory Impairments and Increased GFAP Expression in Hippocampal Astrocytes Following Hypercaloric Diet in Rats. Arq. Neuropsiquiatr. 2019, 77, 601–608. [Google Scholar] [CrossRef]
- Opel, N.; Thalamuthu, A.; Milaneschi, Y.; Grotegerd, D.; Flint, C.; Leenings, R.; Goltermann, J.; Richter, M.; Hahn, T.; Woditsch, G.; et al. Brain Structural Abnormalities in Obesity: Relation to Age, Genetic Risk, and Common Psychiatric Disorders. Mol. Psychiatry 2020, 1–14. [Google Scholar] [CrossRef]
- Schmaal, L.; Hibar, D.P.; Sämann, P.G.; Hall, G.B.; Baune, B.T.; Jahanshad, N.; Cheung, J.W.; Van Erp, T.G.M.; Bos, D.; Ikram, M.A.; et al. Cortical Abnormalities in Adults and Adolescents with Major Depression Based on Brain Scans from 20 Cohorts Worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 2017, 22, 900–909. [Google Scholar] [CrossRef]
- Puig, J.; Blasco, G.; Daunis-I-Estadella, J.; Molina, X.; Xifra, G.; Ricart, W.; Pedraza, S.; Fernández-Aranda, F.; Fernández-Real, J.M. Hypothalamic Damage Is Associated with Inflammatory Markers and Worse Cognitive Performance in Obese Subjects. J. Clin. Endocrinol. Metab. 2015, 100, E276–E281. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J. Alzheimer’s Disease Neuroimaging Initiative (ADNI); Sukoff Rizzo, S.J.; Howell, G.R. Exercise Prevents Obesity-Induced Cognitive Decline and White Matter Damage in Mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Lu, T.; Zhan, Z.; Wei, W.; Lyu, X.; Jiang, Y.; Xue, X. The Effect of Swimming Exercise and Diet on the Hypothalamic Inflammation of ApoE-/- Mice Based on SIRT1-NF-ΚB-GnRH Expression. Aging 2020, 12, 11085–11099. [Google Scholar] [CrossRef] [PubMed]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; De Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated Fatty Acids Revert Diet-Induced Hypothalamic Inflammation in Obesity. PLoS ONE 2012, 7, e30571. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Hochstetter, D.; Yao, L.; Zhao, Y.; Zhou, J.; Wang, Y.; Xu, P. Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 5081. [Google Scholar] [CrossRef]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 Receptors Exist in the Parietal Cortex, Hypothalamus and Medulla of Human Brains and the GLP-1 Analogue Liraglutide Alters Brain Activity Related to Highly Desirable Food Cues in Individuals with Diabetes: A Crossover, Randomised, Placebo-Controlled Trial. Diabetologia 2016, 59, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Vianna, A.R.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Effects of Liraglutide in Hypothalamic Arcuate Nucleus of Obese Mice. Obesity 2016, 24, 626–633. [Google Scholar] [CrossRef]
- McClean, P.L.; Hölscher, C. Liraglutide Can Reverse Memory Impairment, Synaptic Loss and Reduce Plaque Load in Aged APP/PS1 Mice, a Model of Alzheimer’s Disease. Neuropharmacology 2014, 76 Pt A, 57–67. [Google Scholar] [CrossRef]

| Stimuli of Inflammatory Mechanisms | Mechanisms |
|---|---|
| Saturated fatty acids | Activation of TLR-4 pathway, Increased proinflammatory cytokines, Microglia activation |
| Saturated fatty acids, Hyperlipidemia Hyperglycemia | ER stress response |
| Western diet | Increased BBB permeability |
| High-fat diet | Disrupted autophagy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionysopoulou, S.; Charmandari, E.; Bargiota, A.; Vlahos, N.F.; Mastorakos, G.; Valsamakis, G. The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients 2021, 13, 498. https://doi.org/10.3390/nu13020498
Dionysopoulou S, Charmandari E, Bargiota A, Vlahos NF, Mastorakos G, Valsamakis G. The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients. 2021; 13(2):498. https://doi.org/10.3390/nu13020498
Chicago/Turabian StyleDionysopoulou, Sofia, Evangelia Charmandari, Alexandra Bargiota, Nikolaos F Vlahos, George Mastorakos, and Georgios Valsamakis. 2021. "The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders" Nutrients 13, no. 2: 498. https://doi.org/10.3390/nu13020498
APA StyleDionysopoulou, S., Charmandari, E., Bargiota, A., Vlahos, N. F., Mastorakos, G., & Valsamakis, G. (2021). The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients, 13(2), 498. https://doi.org/10.3390/nu13020498









