Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objective
2.2. Study Inclusion and Exclusion
2.3. Search Strategy
2.4. Data Extraction
2.5. Quality and Risk of Bias Assessment
2.6. Data Analysis
3. Results
3.1. Search Results
3.2. Quality Assessment
3.3. Effects of Nutrient Supplementation and Dietary Interventions
3.3.1. Vitamin A
3.3.2. Vitamin C and/or E
3.3.3. Vitamin D
3.3.4. Vitamin D and Calcium
3.3.5. Calcium
3.3.6. Iron and/or Folic Acid
3.3.7. Zinc
3.3.8. Multiple Micronutrients, with or without Lipid-Based Nutrient Supplementation
3.3.9. Polyunsaturated Omega-3 Fatty Acids
3.3.10. Dietary Interventions
3.3.11. Other Nutrient Factors
3.4. Evidence Map
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020. [Google Scholar]
- Mason, J.B.; Shrimpton, R.; Saldanha, L.S.; Ramakrishnan, U.; Victora, C.G.; Girard, A.W.; McFarland, D.A.; Martorell, R. The first 500 days of life: Policies to support maternal nutrition. Glob. Health Action 2015, 8, 23623. [Google Scholar] [CrossRef] [Green Version]
- King, J.C. A Summary of Pathways or Mechanisms Linking Preconception Maternal Nutrition with Birth Outcomes. J. Nutr. 2016, 146, 1437S–1444S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal Undernutrition Influences Placental-Fetal Development. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumey, L.H. Compensatory placental growth after restricted maternal nutrition in early pregnancy. Placenta 1998, 19, 105–111. [Google Scholar] [PubMed]
- Susser, M.; Stein, Z. Timing in Prenatal Nutrition: A Reprise of the Dutch Famine Study. Nutr. Rev. 2009, 52, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Von Dadelszen, P.; Magee, L.A. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 36, 83–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Dadelszen, P.; Ayres de Campos, D.; Barivalala, W. Classification of the hypertensive disorders of pregnancy. In The FIGO Textbook of Pregnancy Hypertension; Magee, L., von Dadelszen, P., Stones, W., Mathai, M., Eds.; The Global Library of Women’s Medicine: London, UK, 2016; pp. 33–61. [Google Scholar]
- Salam, R.A.; Qureshi, R.N.; Sheikh, S.; Khowaja, A.R.; Sawchuck, D.; Vidler, M.; von Dadelszen, P.; Zaidi, S.; Bhutta, Z. Potential for task-sharing to Lady Health Workers for identification and emergency management of pre-eclampsia at community level in Pakistan. Reprod. Health 2016, 13, 107. [Google Scholar] [CrossRef] [Green Version]
- Achamrah, N.; Ditisheim, A. Nutritional approach to preeclampsia prevention. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 168–173. [Google Scholar] [CrossRef]
- Mateussi, M.V.; Latorraca, C.D.O.C.; Daou, J.P.; Martimbianco, A.L.C.; Riera, R.; Pacheco, R.L.; Pachito, D.V. What do Cochrane systematic reviews say about interventions for vitamin D supplementation? Sao Paulo Med. J. 2017, 135, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Medley, N.; Vogel, J.P.; Care, A.; Alfirevic, Z. Interventions during pregnancy to prevent preterm birth: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2018, 11, CD012505. [Google Scholar] [CrossRef]
- Merialdi, M.; Carroli, G.; Villar, J.; Abalos, E.; Gulmezoglu, A.M.; Kulier, R.; de Onis, M. Nutritional interventions during pregnancy for the prevention or treatment of impaired fetal growth: An overview of randomized controlled trials. J. Nutr. 2003, 133, 1626S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Lopes, K.; Ota, E.; Shakya, P.; Dagvadorj, A.; Balogun, O.O.; Pena-Rosas, J.P.; De-Regil, L.M.; Mori, R. Effects of nutrition interventions during pregnancy on low birth weight: An overview of systematic reviews. BMJ Glob. Health 2017, 2, e000389. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleinjen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Pieper, D.; Puljak, L.; Gonzalez, L.M.; Minozzi, S. Comparison of AMSTAR 2 with ROBIS in systematic reviews including randomized and non-randomized studies. In Oral Presentation at Cochrane Colloquium; Cochrane: Edinburgh, UK, 2018. [Google Scholar]
- Khan, S.U.; Khan, M.S.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; Riaz, I.B.; Khan, M.B.; Kaluski, E.; et al. Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes. Ann. Intern. Med. 2019, 171, 1–10. [Google Scholar] [CrossRef]
- Iqbal, S.; Ekmekcioglu, C. Maternal and neonatal outcomes related to iron supplementation or iron status: A summary of meta-analyses. J. Matern. Neonatal. Med. 2019, 32, 1528–1540. [Google Scholar] [CrossRef]
- Kiely, M.; Hemmingway, A.; O’Callaghan, K.M. Vitamin D in pregnancy: Current perspectives and future directions. Ther. Adv. Musculoskelet. Dis. 2017, 9, 145–154. [Google Scholar] [CrossRef]
- Matei, A.; Saccone, G.; Vogel, J.P.; Armson, A.B. Primary and secondary prevention of preterm birth: A review of systematic reviews and ongoing randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 224–239. [Google Scholar] [CrossRef]
- Villar, J.; Merialdi, M.; Gulmezoglu, A.M.; Abalos, E.; Carroli, G.; Kulier, R.; De Onis, M. Characteristics of randomized controlled trials included in systematic reviews of nutritional interventions reporting maternal morbidity, mortality, preterm delivery, intrauterine growth restriction and small for gestational age and birth weight outcomes. J. Nutr. 2003, 133, 1632S. [Google Scholar] [CrossRef]
- Moutquin, J.M.; Garner, P.R.; Burrows, R.F.; Rey, E.; Helewa, M.E.; Lange, I.R.; Rabkin, S.W. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. CMAJ 1997, 157, 907–919. [Google Scholar] [PubMed]
- Mwangi, M.N.; Prentice, A.M.; Verhoef, H. Safety and benefits of antenatal oral iron supplementation in low-income countries: A review. Br. J. Haematol. 2017, 177, 884–895. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.M.; Kiely, M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients 2018, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Secher, N.J. Does fish oil prevent preterm birth? J. Perinat. Med. 2007, 35, S25–S27. [Google Scholar] [CrossRef]
- Villar, J.; De Onis, M.; Gulmezoglu, A.M. Nutritional and antimicrobial interventions to prevent preterm birth: An overview of randomized controlled trials. Obstet. Gynecol. Surv. 1998, 53, 575. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Osendarp, S.J.M.; Adu-Afarwuah, S.; Ahmed, S.; Ajello, C.; Bergeron, G.; Black, R.; Christian, P.; Cousens, S.; De Pee, S.; et al. Review of the evidence regarding the use of antenatal multiple micronutrient supplementation in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1444, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Eddib, A.; Yeh, J. Prevention of preeclampsia: Is it still a disappointment? Clin. Med. Women’s Health 2009, 2, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2014, 7, 153–178. [Google Scholar] [CrossRef] [Green Version]
- Gulmezoglu, M.; Villar, J.; De Onis, M. Effectiveness of Interventions to Prevent or Treat Impaired Fetal Growth. Obstet. Gynecol. Surv. 1997, 52, 139–148. [Google Scholar] [CrossRef]
- Hosli, I.; Zanetti-Daellenbach, R.; Holzgreve, W.; Lapaire, O. Role of omega 3-fatty acids and multivitamins in gestation. J. Perinat. Med. 2007, 35, S19–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.; Simkhada, P.; Smith, W.C.S. Evaluating effectiveness of complex interventions aimed at reducing maternal mortality in developing countries. J. Public Health 2005, 27, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Sakwinska, O.; Makinen, K.; Burdge, G.C.; Godfrey, K.M.; Silva-Zolezzi, I. Preterm Birth: A Narrative Review of the Current Evidence on Nutritional and Bioactive Solutions for Risk Reduction. Nutrients 2019, 11, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, D.K.; Allen, L.H. Interventions with Vitamins B6, B12 and C in Pregnancy. Paediatr. Perinat. Epidemiol. 2012, 26, 55–74. [Google Scholar] [CrossRef]
- Swaney, P.; Thorp, J.; Allen, I. Vitamin C Supplementation in Pregnancy--Does It Decrease Rates of Preterm Birth? A Systematic Review. Am. J. Perinatol. 2014, 31, 91–97. [Google Scholar]
- Agarwal, S.; Kovilam, O.; Agrawal, D.K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 755–769. [Google Scholar] [CrossRef]
- Zerfu, T.A.; Ayele, H.T. Micronutrients and pregnancy; effect of supplementation on pregnancy and pregnancy outcomes: A systematic review. Nutr. J. 2013, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Soltani, H.; Duxbury, A.M.S.; Rundle, R.; Chan, L.-N. A systematic review of the effects of dietary interventions on neonatal outcomes in adolescent pregnancy. Evid. Based Midwifery 2015, 13, 29–34. [Google Scholar]
- Hodgetts, V.; Morris, R.; Francis, A.; Gardosi, J.; Ismail, K. Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: A population study, systematic review and meta-analysis. BJOG An. Int. J. Obstet. Gynaecol. 2015, 122, 478–490. [Google Scholar]
- Imdad, A.; Bhutta, Z.A. Routine iron/folate supplementation during pregnancy: Effect on maternal anaemia and birth outcomes. Paediatr. Perinat. Epidemiol. 2012, 26, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaffee, B.W.; King, J.C. Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26, 118–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015, CD000230. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.H.; Fang, M.L.; Harari, O.; Dron, L.; Siden, E.G.; Majzoub, R.; Jeziorska, V.; Thorlund, K.; Mills, E.D.; Bhutta, Z.A. Association of Early Interventions With Birth Outcomes and Child Linear Growth in Low-Income and Middle-Income Countries: Bayesian Network Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open. 2019, 2, e197871. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of Women’s Nutrition Before and During Early Pregnancy on Maternal and Infant Outcomes: A Systematic review; Paediatric and Perinatal Epidemiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; Volume 26, pp. 285–301. [Google Scholar]
- Suchdev, P.S.; Pena-Rosas, J.P.; De-Regil, L.M.; PenaRosas, P.J.; DeRegil, M.L. Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women. Cochrane database Syst. Rev. 2015, CD011158. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.T.; Hegaard, H.K.; Huusom, L.D.; Pinborg, A.B. Multivitamin use and adverse birth outcomes in high-income countries: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 217, e1–e404. [Google Scholar] [CrossRef]
- Imdad, A.; Bhutta, Z.A. Maternal nutrition and birth outcomes: Effect of balanced protein-energy supplementation. Paediatr. Perinat. Epidemiol. 2012, 26, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Liberato, S.C.; Singh, G.; Mulholland, K. Effects of protein energy supplementation during pregnancy on fetal growth: A review of the literature focusing on contextual factors. Food. Nutr. Res. 2013, 57, 20499. [Google Scholar]
- Dodd, J.M.; Crowther, C.A.; Robinson, J.S. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: A systematic review. Acta Obstet. Gynecol. Scand. 2008, 87, 702–706. [Google Scholar] [CrossRef]
- Gui, S.; Jia, J.; Niu, X.; Bai, Y.; Zou, H.; Deng, J.; Zhou, R. Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: A systematic review. J. Renin. Angiotensin Aldosterone Syst. 2014, 15, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Dorniak-Wall, T.; Grivell, R.M.; Dekker, G.A.; Hague, W.; Dodd, J.M. The role of L-Arginine in the Prevention and Treatment of Pre-eclampsia: A Systematic Review of Randomised Trials. J Hum Hypertens. 2014, 28, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2008, CD004227. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Salles, A.M.; Galvao, T.F.; Silva, M.T.; Domingues Motta, L.C.; Pereira, M.G. Antioxidants for Preventing Preeclampsia: A Systematic Review. Sci. World J. 2012, 243476. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Gresham, E.; Bisquera, A.; Byles, J.E.; Hure, A.J. Effects of dietary interventions on pregnancy outcomes: A systematic review and meta-analysis. Matern. Child Nutr. 2016, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Vadillo-Ortega, F.; Perichart-Perera, O.; Espino, S.; Avila-Vergara, M.A.; Ibarra, I.; Ahued, R.; Godines, M.; Parry, S.; George Macones, G.; Jerome, F.; et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: Randomised controlled trial. BMJ 2011, 342, d2901. [Google Scholar] [CrossRef] [Green Version]
- Kongnyuy, E.J.; Wiysonge, C.S.; Shey, M.S. A systematic review of randomized controlled trials of prenatal and postnatal vitamin A supplementation of HIV-infected women. Int. J. Gynecol. Obstet. 2009, 104, 5–8. [Google Scholar] [CrossRef]
- McCauley, M.E.; van den Broek, N.; Dou, L.; Othman, M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015, CD008666. [Google Scholar] [CrossRef]
- Thorne-Lyman, A.L.; Fawzi, W.W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 36–54. [Google Scholar] [CrossRef]
- Coutsoudis, A.; Pillay, K.; Spooner, E.; Kuhn, L.; Coovadia, H.M. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. AIDS. 1999, 13, 1517–1524. [Google Scholar] [CrossRef]
- Cox, S.E.; Staalsoe, T.; Arthur, P.; Bulmer, J.N.; Tagbor, H.; Hviid, L.; Frost, C.; Riley, E.M.; Kirkwood, B.R. Maternal vitamin A supplementation and immunity to malaria in pregnancy in Ghanaian primigravids. Trop. Med. Int. Health 2005, 10, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Dijkhuizen, M.A.; Wieringa, F.T.; West, C.E. Zinc plus β-carotene supplementation of pregnant women is superior to β-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am. J. Clin. Nutr. 2004, 80, 1299–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawzi, W.W.; Msamanga, G.I.; Spiegelman, D.; Urassa, E.J.N.; McGrath, N.; Mwakagile, D.; Antelman, G.; Mbise, R.; Hererra, G.; Kapiga, S.; et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998, 351, 1477–1482. [Google Scholar] [CrossRef]
- Kirkwood, B.R.; Hurt, L.; Amenga-Etego, S.; Tawiah, C.; Zandoh, C.; Danso, S.; Hurt, C.; Edmond, K.; Hill, Z.; Asbroek, G.T.; et al. Effect of vitamin A supplementation in women of reproductive age on maternal survival in Ghana (ObaapaVitA): A cluster-randomised, placebo-controlled trial. Lancet 2010, 375, 1640–1649. [Google Scholar] [CrossRef]
- Kumwenda, N.; Miotti, P.G.; Taha, T.E.; Broadhead, R.; Biggar, R.J.; Jackson, J.B.; Melikian, G.; Richard, D.; Semba, R.D. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus-infected women in Malawi. Clin. Infect. Dis. 2002, 35, 618–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhika, M.S.; Bhaskaram, P.; Balakrishna, N.; Ramalakshmi, B.A. Red palm oil supplementation: A feasible diet-based approach to improve the vitamin A status of pregnant women and their infants. Food Nutr. Bull. 2003, 24, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, N.; White, S.; Flowers, C.; Cook, J.; Letsky, E.; Tanumihardjo, S.; Mhango, C.; Molyneux, M.; Neilson, J.P. Randomised trial of vitamin A supplementation in pregnant women in rural Malawi found to be anaemic on screening by HemoCue. BJOG An. Int. J. Obstet. Gynaecol. 2006, 113, 569–576. [Google Scholar] [CrossRef]
- West, K.P.; Katz, J.; Khatry, S.K.; Leclerq, S.C.; Pradhan, E.K.; Shrestha, S.R.; Connor, P.B.; Dali, S.M.; Christian, P.; Pokhrel, R.P.; et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or βcarotene on mortality related to pregnancy in Nepal. BMJ 1999, 318, 570–575. [Google Scholar] [CrossRef] [Green Version]
- West, K.P.; Christian, P.; Labrique, A.B.; Rashid, M.; Shamim, A.A.; Klemm, R.D.W.; Massie, A.B.; Mehdra, S.; Schulze, K.J.; Ali, H.; et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: A cluster randomized trial. JAMA J Am Med Assoc. 2011, 305, 1986–1995. [Google Scholar] [CrossRef]
- Christian, P.; Klemm, R.; Shamim, A.A.; Ali, H.; Rashid, M.; Shaikh, S.; Wu, L.; Mehra, S.; Labrique, A.; Katz, J.; et al. Effects of vitamin A and β-carotene supplementation on birth size and length of gestation in rural Bangladesh: A cluster-randomized trial. Am. J. Clin. Nutr. 2013, 97, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Rumbold, A.; Ota, E.; Nagata, C.; Shahrook, S.; Crowther, C.A. Vitamin C supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, CD004072. [Google Scholar] [CrossRef]
- Basaran, A.; Basaran, M.; Topatan, B. Combined vitamin C and E supplementation for the prevention of preeclampsia: A systematic review and meta-analysis. Obstet. Gynecol. Surv. 2010, 65, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Kusanovic, J.P.; Hassan, S.S. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 503.e1–503.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzos, N.; Mauri, D.; Tsappi, M.; Tzioras, S.; Kamposioras, K.; Cortinovis, I.; Casazza, G. Combined vitamin C and E supplementation during pregnancy for preeclampsia prevention: A systematic review. Obstet. Gynecol. Surv. 2007, 62, 202–206. [Google Scholar] [PubMed]
- Rahimi, R.; Nikfar, S.; Rezaie, A.; Abdollahi, M. A Meta-Analysis on the Efficacy and Safety of Combined Vitamin C and E Supplementation in Preeclamptic Women. Hypertens Pregnancy 2009, 28, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.C.; Mullin, P.M. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: A systematic review with meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ma, Z.; Liu, G.; Wang, L.; Guo, Y. Vitamins supplementation affects the onset of preeclampsia. J. Formos. Med. Assoc. 2018, 117, 6–13. [Google Scholar] [CrossRef]
- Rumbold, A.; Ota, E.; Hori, H.; Miyazaki, C.; Crowther, C.A. Vitamin E supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, CD004069. [Google Scholar] [CrossRef]
- Casanueva, E.; Ripoll, C.; Tolentino, M.; Morales, R.M.; Pfeffer, F.; Vilchis, P.; Vadillo-Ortega, F. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: A randomized trial. Am. J. Clin. Nutr. 2005, 81, 859–863. [Google Scholar] [CrossRef] [Green Version]
- Kiondo, P.; Wamuyu-Maina, G.; Wandabwa, J.; Bimenya, G.S.; Tumwesigye, N.M.; Okong, P. The effects of vitamin C supplementation on pre-eclampsia in Mulago Hospital, Kampala, Uganda: A randomized placebo controlled clinical trial. BMC Pregnancy Childbirth 2014, 14, 283. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.M.; Myatt, L.; Spong, C.Y.; Thom, E.A.; Hauth, J.C.; Leveno, K.J.; Pearson, G.D.; Wapner, R.J.; Varner, M.W.; Thorp Jr, J.M.; et al. Vitamins C and E to Prevent Complications of Pregnancy-Associated Hypertension. N. Engl. J. Med. 2010, 362, 1282–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumbold, A.R.; Crowther, C.A.; Haslam, R.R.; Dekker, G.A.; Robinson, J.S. Vitamins C and E and the Risks of Preeclampsia and Perinatal Complications. N. Engl. J. Med. 2006, 354, 1796–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinnato, J.A.; Freire, S.; Pinto e Silva, J.L.; Cunha Rudge, M.V.; Martins-Costa, S.; Koch, M.A.; Goco, N.; de Barros Santos, C.; Cecatti, J.G.; Costa, R.; et al. Antioxidant Therapy to Prevent Preeclampsia. Obstet. Gynecol. 2007, 110, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Purwar, M.; Merialdi, M.; Zavaleta, N.; Thi Nhu Ngoc, N.; Anthony, J.; De Greeff, A.; Poston, L.; Shennan, A. WHO Vitamin C and Vitamin E trial group. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG An. Int. J. Obstet. Gynaecol. 2009, 116, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Perez-Cuevas, R.; Xiong, X.; Reyes, H.; Roy, C.; Julien, P.; Smith, G.; von Dadelszen, P.; Leduc, L.; Audibert, F.; et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am. J. Obstet. Gynecol. 2010, 202, 239.e1–239.e10. [Google Scholar]
- Taghriri, A.; Danesh, A. Effects of vitamins E and C in reduction of preeclampsia blood pressure incidence in primigravids. J. Shahrekord Univ. Med. Sci. 2007, 9, 50–54. [Google Scholar]
- McCance, D.R.; Holmes, V.A.; Maresh, M.J.A.; Patterson, C.C.; Walker, J.D.; Pearson, D.W.M.; Young, I.S. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): A randomised placebo-controlled trial. Lancet 2010, 376, 259–266. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, C.T.; Schilling, D.; Clay, N.; Jackson, K.; Go, M.D.; Spitale, P.; Bunten, C.; Leiva, M.; Gonzales, D.; Hollister-Smith, J.; et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: A randomized clinical trial. JAMA 2014, 311, 2074–2082. [Google Scholar] [CrossRef] [Green Version]
- Steyn, P.S.; Odendaal, H.J.; Schoeman, J.; Stander, C.; Fanie, N.; Grové, D. A randomised, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labour. J. Obstet. Gynaecol. 2003, 23, 150–155. [Google Scholar] [CrossRef]
- Beazley, D.; Ahokas, R.; Livingston, J.; Griggs, M.; Sibai, B.M. Vitamin C and E supplementation in women at high risk for preeclampsia: A double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2005, 192, 520–521. [Google Scholar] [CrossRef]
- Chappell, L.C.; Seed, P.T.; Briley, A.L.; Kelly, F.J.; Lee, R.; Hunt, B.J.; Parmar, K.; Bewley, S.J.; Shennan, A.H.; Steer, P.J.; et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: A randomised trial. Lancet 1999, 354, 810–816. [Google Scholar] [CrossRef]
- Huria, A.; Gupta, P.; Kumar, D.; Sharma, M. Vitamin C and Vitamin E Supplementation in Pregnant Women at Risk for Pre Eclampsia: A Randomized Controlled Trial. Internet J. Health. 2009, 10. [Google Scholar] [CrossRef]
- Kalpdev, A.; Saha, S.C.; Dhawan, V. Vitamin C and e supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens Pregnancy. 2011, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Nasrolahi, S.; Alimohammady, S.; Zamani, M. The effect of antioxidants (vitamin C and E) on preeclampsia in primiparious women. J. Gorgan. Univ. Med. Sci. 2006, 8, 17–21. [Google Scholar]
- Poston, L.; Briley, A.; Seed, P.; Kelly, F.; Shennan, A. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Abramovici, A.; Gandley, R.E.; Clifton, R.G.; Leveno, K.J.; Myatt, L.; Wapner, R.J.; Thorp, J.M., Jr.; Mercer, B.M.; Peaceman, A.M.; Samuels, P.; et al. Prenatal vitamin C and E supplementation in smokers is associated with reduced placental abruption and preterm birth: A secondary analysis. BJOG An. Int. J. Obstet. Gynaecol. 2015, 122, 1740–1747. [Google Scholar] [CrossRef] [Green Version]
- Bastani, P.; Hamdi, K.; Abasalizadeh, F.; Navali, N. Effects of vitamin E supplementation on some pregnancy health indices: A randomized clinical trial. Int. J. Gen. Med. 2011, 4, 461. [Google Scholar] [CrossRef] [Green Version]
- Mahdy, Z.A.; Siraj, H.H.; Khaza’ai, H.; Mutalib, M.S.A.; Azwar, M.H.; Wahab, M.A.; Dali, A.Z.H.M.; Jaafar, R.; Ismail, N.A.M.; Jamil, M.A.; et al. Does palm oil vitamin E reduce the risk of pregnancy induced hypertension? Acta Medica 2013, 56, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Roth, D.E.; Leung, M.; Mesfin, E.; Qamar, H.; Watterworth, J.; Papp, E. Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ 2017, 359, j5237. [Google Scholar] [CrossRef] [Green Version]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 75–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Tao, Y.; Huang, K.; Zhu, B.; Tao, F. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J. Obstet. Gynaecol. Res. 2017, 43, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2019, 39, 1742–1752. [Google Scholar] [CrossRef]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2019, 120, 898–924. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Cavadino, A.; Williams, D.; Hypponen, E.; Williams, D.C.A. Vitamin D and Pre-Eclampsia: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2012, 60, 137–138. [Google Scholar]
- Vallibhakara, S.A.-O.; Rattanasiri, S.; Thakkinstian, A.; Khaing, W.; Tantrakul, V.; Vallibhakara, O.; Attia, J.; Thakkinstian, A. Calcium and Vitamin D Supplementation for Prevention of Preeclampsia: A Systematic Review and Network Meta-Analysis. Nutrients 2017, 9, 1141. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Blanco, I.; Agodi, A. Effects of Vitamin D Supplementation during Pregnancy on Birth Size: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, C.; De-Regil, L.M.; Lombardo, L.K.; Pena-Rosas, J.P. Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 2016, 164, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Palacios, C.; Kostiuk, L.K.; Pena-Rosas, P.J. Vitamin D supplementation for women during pregnancy. 2019, 1, CD008873. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015, 103, 1278–1288.e4. [Google Scholar]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Shakeri, H.; Esmaillzadeh, A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J. Nutr. 2013, 143, 1432–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke, O.G.; Carter, N.D.; Brown, I.R.F.; Cleeve, H.J.W.; Robinson, V.P.; Bone, C.D.M.; Robinson, V.P.; Winder, S.M. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br. Med. J. 1980, 280, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamali, M.; Beihaghi, E.; Mohammadi, A.A.; Asemi, Z. Effects of high-dose Vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm. Metab. Res. 2015, 47, 867–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.R.; Ahmad, T.; Hussain, R.; Bhutta, Z.A. A Randomized Controlled Trial of Oral Vitamin D Supplementation in Pregnancy to Improve Maternal Periodontal Health and Birth Weight. J. Int. Oral. Health 2016, 8, 657–665. [Google Scholar]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of prenatal supplementation with Vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA J. Am. Med. Assoc. 2016, 315, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Marya, R.K.; Ratjee, S.; Dua, V.; Sangwan, K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian. J. Med. Res. 1988, 88, 488–492. [Google Scholar] [PubMed]
- Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M.; Mansouri, A.; Najafi, M.; Khodabande, F. The Effect of Vitamin D and Calcium plus Vitamin D during Pregnancy on Pregnancy and Birth Outcomes: A Randomized Controlled Trial. J Caring Sci. 2015, 4, 35–44. [Google Scholar]
- Naghshineh, E.; Sheikhaliyan, S. Effect of vitamin D supplementation in the reduce risk of preeclampsia in nulliparous women. Adv. Biomed. Res. 2016, 5, 7. [Google Scholar]
- Razavi, M.; Jamilian, M.; Samimi, M.; Ebrahimi, F.A.; Taghizadeh, M.; Bekhradi, R.; Hosseini, E.S.; Kashani, H.H.; Karamali, M.; Asemi, Z. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab. 2017, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Roth, D.E.; Al Mahmud, A.; Raqib, R.; Akhtar, E.; Perumal, N.; Pezzack, B.; Baqui, A.H. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: The AViDD trial. Nutr. J. 2013, 12. [Google Scholar] [CrossRef] [Green Version]
- Sablok, A.; Batra, A.; Thariani, K.; Batra, A.; Bharti, R.; Aggarwal, A.R.; Kabi, B.C.; Chellani, H. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. 2015, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Sasan, S.B.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Effect of Vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2016, 315, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Hariharan, C.; Bhaumik, D. Role of vitamin D in reducing the risk of preterm labour. Int. J. Reprod. Contracept. Obs. Gynecol. 2015, 1, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, M.; Piri, Z.; Mohammadian, F.; Kamali, K.; Reza, H.; Moghadami, A. The Impact of Vitamin D Supplementation on Post-Partum Glucose Tolerance and Insulin Resistance in Gestational Diabetes: A Randomized Controlled Trial. Int. J. Endocrinol. Metab. 2016, 14, 34312. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.; Cheung, N.W.; Gunton, J.E.; Athayde, N.; Munns, C.F.; Duke, A.; McLean, M. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: A randomized controlled trial. Diabetes Care 2014, 37, 1837–1844. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.K.H.; Sykes, L.; Sethi, M.; Teoh, T.G.; Robinson, S. Vitamin D deficiency and supplementation during pregnancy. Clin. Endocrinol. 2009, 70, 685–690. [Google Scholar] [CrossRef]
- Zerofsky, M.S.; Jacoby, B.N.; Pedersen, T.L.; Stephensen, C.B. Daily Cholecalciferol Supplementation during Pregnancy Alters Markers of Regulatory Immunity, Inflammation, and Clinical Outcomes in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2388–2397. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Harvey, N.C.; Bishop, N.J.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Dawodu, A.; Saadi, H.F.; Bekdache, G.; Javed, Y.; Altaye, M.; Hollis, B.W. Randomized Controlled Trial (RCT) of Vitamin D Supplementation in Pregnancy in a Population With Endemic Vitamin D Deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2337–2346. [Google Scholar] [CrossRef] [Green Version]
- Delvin, E.E.; Salle, B.L.; Glorieux, F.H.; Adeleine, P.; David, L.S. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J. Pediatr. 1986, 109, 328–334. [Google Scholar] [CrossRef]
- Grant, C.C.; Stewart, A.W.; Scragg, R.; Milne, T.; Rowden, J.; Ekeroma, A.; Wall, C.; Mitchell, E.A.; Crengle, S.; Trenholme, A.; et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics 2014, 133, e143–e153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemipour, S.; Ziaee, A.; Javadi, A.; Movahed, F.; Elmizadeh, K.; Javadi, E.H.; Lalooha, F. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 172, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Kanani, F.H.; Ramzan, S.; Kausar, R.; Ayaz, S.; Khanani, R.; Pal, L. Obstetric and neonatal outcomes of maternal vitamin D supplementation: Results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J. Clin. Endocrinol. Metab. 2014, 99, 2448–2455. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Duley, L.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018, 10, CD001059. [Google Scholar] [CrossRef]
- Ponzetto, A.; Figura, N.; Riva, P. Prepregnancy Calcium Supplementation and Pre-eclampsia; Department of Medical Sciences, University of Turin: Turin, Italy, 2019; Volume 394, p. e6. [Google Scholar]
- Asemi, Z.; Tabassi, Z.; Heidarzadeh, Z.; Khorammian, H.; Sabihi, S.S.; Samimi, M. Effect of calcium-vitamin D supplementation on metabolic profiles in pregnant women at risk for pre-eclampsia: A randomized placebo-controlled trial. Pakistan J. Biol. Sci. 2012, 15, 316–324. [Google Scholar]
- Asemi, Z.; Samimi, M.; Siavashani, M.; Mazloomi, M.; Tabassi, Z.; Karamali, M.; Jamilian, M.; Esmaillzadeh, A. Calcium-Vitamin D co-supplementation affects metabolic profiles, but not pregnancy outcomes, in healthy pregnant women. Int. J. Prev. Med. 2016, 7, 49. [Google Scholar]
- Diogenes, M.E.L.; Bezerra, F.F.; Rezende, E.P.; Taveira, M.F.; Pinhal, I.; Donangelo, C.M. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2013, 98, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gou, W. Study on prevention of pregnancy induced hypertension and effect of platelet intracellular free ca(2+) by calcium supplementation. J. Xi’an Med. Univ. 2000, 21, 46–48. [Google Scholar]
- Samimi, M.; Kashi, M.; Foroozanfard, F.; Karamali, M.; Bahmani, F.; Asemi, Z.; Hamidian, Y.; Talari, H.R.; Esmaillzadeh, A. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J. Hum. Nutr. Diet. 2016, 29, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.-A.; Taherian, A.; Shirvani, A. Prevention of Preeclampsia with low-dose aspirin or calcium supplementation. Arch. Iran Med. 2002, 5, 151–156. [Google Scholar]
- Hofmeyr, J.G.; Manyame, S.; Medley, N.; Williams, M.J. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst. Rev. 2019, 9, CD011192. [Google Scholar] [CrossRef]
- Imdad, A.; Bhutta, Z.A. Effects of calcium supplementation during pregnancy on maternal, fetal and birth outcomes. Paediatr. Perinat. Epidemiol. 2012, 26, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Jabeen, A.; Bhutta, Z.A. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: A meta-analysis of studies from developing countries. BMC Public Health 2011, 11, S18. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, M.; Yakoob, M.Y.; Imdad, A.; Bhutta, Z.A. Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths. BMC Public Health. 2011, 11, S6. [Google Scholar] [CrossRef] [Green Version]
- Kulier, R.; de Onis, M.; Gulmezoglu, A.M.; Villar, J. Nutritional interventions for the prevention of maternal morbidity. Int. J. Obstet. Gynecol. 1998, 63, 231–246. [Google Scholar] [CrossRef]
- Patrelli, T.; Dall’Asta, A.; Gizzo, S.; Pedrazzi, G.; Piantelli, G.; Jasonni, V.M.; Modena, A.B. Calcium supplementation and prevention of preeclampsia: A meta-analysis. J. Matern. Neonatal Med. 2012, 25, 2570–2574. [Google Scholar]
- Sun, X.; Li, H.; He, X.; Li, M.; Yan, P.; Xun, Y.; Lu, C.; Yang, K.; Zhang, X. The association between calcium supplement and preeclampsia and gestational hypertension: A systematic review and meta-analysis of randomized trials. Hypertens Pregnancy 2019, 38, 129–139. [Google Scholar] [CrossRef]
- Tang, R.; Tang, I.C.; Henry, A.; Welsh, A. Limited evidence for calcium supplementation in preeclampsia prevention: A meta-analysis and systematic review. Hypertens Pregnancy 2015, 34, 181–203. [Google Scholar] [CrossRef]
- Villar, J.B.J.M.; Villar, J.; Belizan, J.M.; Villar, J.B.J.M. Same nutrient, different hypotheses: Disparities in trials of calcium supplementation during pregnancy. Am. J. Clin. Nutr. 2000, 71, 1375S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, L.; Li, W.; Xie, T.; Peng, X.; Li, B.; Xie, S.; Xu, J.; Zhou, X.H.; Guo, S.N. Calcium supplementation reducing the risk of hypertensive disorders of pregnancy and related problems: A meta-analysis of multicentre randomized controlled trials. Int. J. Nurs. Pract. 2015, 21, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bucher, H.; Guyatt, G.H.; Cook, R.; Hatala, R.; Cook, D.; Lang, J.; Hunt, D. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: A meta-analysis of randomized controlled trials. JAMA. 1996, 275, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Buppasiri, P.; Lumbiganon, P.; Thinkhamrop, J.; Ngamjarus, C.; Laopaiboon, M. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database Syst. Rev. 2011, CD007079. [Google Scholar] [CrossRef]
- Carroli, G.; Duley, L.; Belizan, J.M.; Villar, J. Calcium supplementation during pregnancy: A systematic review of randomised controlled trials. Br. J. Obstet. Gynaecol. 1994, 101, 753–758. [Google Scholar] [CrossRef]
- Hofmeyr GJAtallah, A.N.; Duley, L.R.A. Calcium supplementation to prevent pre-eclampsia-A systematic review. South Afr. Med. J. 2003, 93, 224. [Google Scholar]
- Hofmeyr, G.; Duley, L.; Atallah, A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: A systematic review and commentary. BJOG. 2007, 114, 933–943. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Belizan, J.M.; von Dadelszen, P. Study CPCAP. Low-dose calcium supplementation for preventing pre-eclampsia: A systematic review and commentary. BJOG AN Int. J. Obstet. Gynaecol. 2014, 121, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Aghamohammadi, A.; Zafari, M. Calcium supplementation in pregnancy and prevention of hypertensive disorders in elderly women. ScienceAsia. 2015, 41, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Almirante, C. Calcium supplementation during pregnancy in the prevention of EPH gestosis. Prenat. Neonatal Med. 1998, 3, 24. [Google Scholar]
- Levine, R.J.; Hauth, J.C.; Curet, L.B.; Sibai, B.M.; Catalano, P.M.; Morris, C.D.; DerSimonian, R.; Esterlitz, J.R.; Raymond, E.G.; Bild, D.E.; et al. Trial of Calcium to Prevent Preeclampsia. N. Engl. J. Med. 1997, 337, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jaramillo, P.; Narvaez, M.; Wetgel, R.; Yepez, R. Calcium supplementation reduces the risk of pregnancy-induced hypertension in an Andes population. BJOG 1989, 96, 648–655. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P.; Narvaez, M.; Felix, C.; Lopez, A. Dietary calcium supplementation and prevention of pregnancy hypertension. Lancet 1990, 335, 293. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Delgado, F.; Jácome, P.; Terán, E.; Ruano, C.; Rivera, J. Calcium supplementation and the risk of preeclampsia in Ecuadorian pregnant teenagers. Obstet. Gynecol. 1997, 90, 162–167. [Google Scholar] [CrossRef]
- Nenad, S.; Olivera, K.-V.; Goran, R.; Ljiljana, S. Did calcium management prevent preeclampsia? Pregnancy Hypertens. An. Int. J. Women’s Cardiovasc. Health 2011, 1, 287. [Google Scholar]
- Niromanesh, S.; Laghaii, S.; Mosavi-Jarrahi, A. Supplementary calcium in prevention of pre-eclampsia. Int. J. Gynecol. Obstet. 2001, 74, 17–21. [Google Scholar] [CrossRef]
- Purwar, M.; Kulkarni, H.; Motghare, V.; Dhole, S. Calcium Supplementation and Prevention of Pregnancy Induced Hypertension. J. Obstet. Gynaecol. Res. 1996, 22, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Fung, H.Y.M.; Hung, C.Y. Calcium and low-dose aspirin prophylaxis in women at high risk of pregnancy-induced hypertension. Hypertens. Pregnancy. 1999, 18, 165–172. [Google Scholar] [CrossRef]
- Sanchez-Ramos, L.; Briones, D.; Kaunitz, A.; Delvalle, G.; Gaudier, F.; Walker, C. Prevention of pregnancy-induced hypertension by calcium supplementation in angiotensin II-sensitive patients. Obs. Gynecol. 1994, 84, 349–353. [Google Scholar]
- Villar, J.; Repke, J.; Belizan, J.; Pareja, G. Calcium supplementation reduces blood pressure during pregnancy: Results of a randomized controlled clinical trial. Obstet. Gynecol. 1987, 70, 317–322. [Google Scholar]
- Bassaw, B.; Roopnarinesingh, S.; Roopnarinesingh, A.; Homer, H. Prevention of hypertensive disorders of pregnancy. J. Obstet. Gynaecol. 1998, 18, 123–126. [Google Scholar]
- Villar, J.; Repke, J.T. Calcium supplementation during pregnancy may reduce preterm delivery in high-risk populations. Am. J. Obstet. Gynecol. 1990, 163, 1124–1131. [Google Scholar] [CrossRef]
- Villar, J.; Abdel-Aleem, H.; Merialdi, M.; Mathai, M.; Ali, M.M.; Zavaleta, N.; Purwar, M.; Hofmeyr, J.; Campódonico, L.; Landoulsi, S.; et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am. J. Obstet. Gynecol. 2006, 194, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Wanchu, M.; Malhotra, S.; Khulla, M. Calcium supplementation in pre-eclampsia. J. Assoc. Physicians India 2001, 49, 795–798. [Google Scholar] [PubMed]
- Belizán, J.M.; Villar, J.; Gonzalez, L.; Campodonico, L.; Bergel, E. Calcium Supplementation to Prevent Hypertensive Disorders of Pregnancy. N. Engl. J. Med. 1991, 325, 1399–1405. [Google Scholar] [CrossRef]
- Boggess, K.A.; Samuel, L.; Schmucker, B.C.; Waters, J.; Easterling, T.R. A randomized controlled trial of the effect of third-trimester calcium supplementation on maternal hemodynamic function. Obstet. Gynecol. 1997, 90, 157–161. [Google Scholar] [CrossRef]
- Cong, K.; Chi, S.; Liu, G. Calcium supplementation during pregnancy for reducing pregnancy induced hypertension. Chin. Med. J. 1995, 108, 57–59. [Google Scholar]
- Crowther, C.A.; Hiller, J.E.; Pridmore, B.; Bryce, R.; Duggan, P.; Hague, W.M.; Robinson, J.S. Calcium Supplementation In Nulliparous Women for the Prevention of Pregnancy-Induced Hypertension, Preeclampsia and Preterm Birth: An Australian Randomized Trial. Aust. New Zeal. J. Obstet. Gynaecol. 1999, 39, 12–18. [Google Scholar] [CrossRef]
- Goldberg, G.R.; Jarjou, M.A.; Cole, T.J.; Prentice, A. Randomized, Placebo-Controlled, Calcium Supplementation Trial in Pregnant Gambian Women Accustomed to a Low Calcium Intake: Effects On maternal Blood Pressure and Infant Growth 1–4. Available online: https://academic.oup.com/ajcn/article/98/4/972/4577249 (accessed on 30 August 2020).
- Hofmeyr, G.J.; Betrán, A.P.; Singata-Madliki, M.; Cormick, G.; Munjanja, S.P.; Fawcus, S.S.; Mose, S.; Hall, D.; Ciganda, A.; Seuc, A.H.; et al. Prepregnancy and early pregnancy calcium supplementation among women at high risk of pre-eclampsia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 330–339. [Google Scholar] [CrossRef]
- Kumar, A.; Devi, S.G.; Batra, S.; Singh, C.; Shukla, D.K. Calcium supplementation for the prevention of pre-eclampsia. Int. J. Gynecol. Obstet. 2009, 104, 32–36. [Google Scholar]
- Cantor, A.G.; Bougatsos, C.; Dana, T.; Blazina, I.; McDonagh, M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: A systematic review for the U. S. Preventive Services Task Force. Ann. Intern. Med. 2015, 162, 566–576. [Google Scholar] [PubMed] [Green Version]
- Haider, B.A.; Olofin, I.; Wang, M.; Spiegelman, D.; Ezzati, M.; Fawzi, W.W. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2013, 346, f3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PenaRosas, P.J.; DeRegil, M.L.; GarciaCasal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 7, CD004736. [Google Scholar] [CrossRef] [Green Version]
- De-Regil, L.M.; Pena-Rosas, J.P.; Fernandez-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, CD007950. [Google Scholar] [CrossRef]
- Hua, X.; Zhang, J.; Guo, Y.; Shen, M.; Gaudet, L.; Janoudi, G.; Walker, M.; Wen, S.W. Effect of folic acid supplementation during pregnancy on gestational hypertension/preeclampsia: A systematic review and meta-analysis. Hypertens. Pregnancy 2016, 35, 447–460. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Salam, R.A.; Haider, B.A.; Bhutta, Z.A. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst. Rev. 2013, CD006896. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V. Folic acid supplementation in pregnancy to prevent preterm birth: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.K.L.; Chan, B.C.P.; Lam, K.F.; Tam, S.; Lao, T.T. Iron supplement in pregnancy and development of gestational diabetes-A randomised placebo-controlled trial. BJOG. 2009, 116, 789–798. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Parvanta, I.; Ickes, L.; Yip, R.; Brittenham, G.M. Iron supplementation during pregnancy, anemia, and birth weight: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Christian, P.; Khatry, S.K.; Katz, J.; Pradhan, E.K.; LeClerq, S.C.; Shrestha, S.R.; Adhikari, R.K.; Sommer, A.; Keith, P.W. Effects of alternative maternal micronutrient supplements on low birth weight in rural nepal: Double blind randomised community trial. BMJ 2003, 326, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.F.; De, J.P.; Allan, N.C. The prevention of megaloblastic anaemia in pregnancy in Nigeria. BJOG 1968, 75, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kirke, P.N.; Daly, L.E.; Elwood, J.H. A randomised trial of low dose folic acid to prevent neural tube defects the Irish Vitamin Study Group. Arch. Dis. Child. 1992, 67, 1442–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, N.; Sneddon, J.D.; Frost, C.; Stone, R.; MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Lee, J.I.; Lee, J.A.; Lim, H.S. Effect of time of initiation and dose of prenatal iron and folic acid supplementation on iron and folate nutriture of Korean women during pregnancy. Am. J. Clin. Nutr. 2005, 82, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.R.; Nickerson, H.J.; Olson, K.A.; Berg, R.L.; Meyer, J.A. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin. Med. Res. 2003, 1, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Ndyomugyenyi, R.; Magnussen, P. Chloroquine prophylaxis, iron-folie acid supplementation or case management of malaria attacks in primigravidae in western Uganda: Effects on maternal parasitaemia and haemoglobin levels and on birthweight. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 413–418. [Google Scholar] [CrossRef]
- Taylor, D.; Mallen, C.; McDougall, N.; Lind, T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. BJOG 1982, 89, 1011–1017. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, Y.; Dang, S.; Yan, H.; Dibley, M.J.; Chang, S.; Kong, L. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: Double blind cluster randomised controlled trial. BMJ 2008, 337, 1211–1215. [Google Scholar] [CrossRef] [Green Version]
- Ziaei, S.; Norrozi, M.; Faghihzadeh, S.; Jafarbegloo, E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥ 13.2 g/dL. BJOG 2007, 114, 684–688. [Google Scholar] [CrossRef]
- Eskeland, B.; Malterud, K.; Ulvik, R.J.; Hunskaar, S. Iron supplementation in pregnancy: Is less enough? Acta Obstet. Gynecol. Scand. 1997, 76, 822–828. [Google Scholar] [CrossRef]
- Menendez, C.; Todd, J.; Alonso, P.L.L.; Francis, N.; Lulat, S.; Ceesay, S.; M’boge, B.; Greenwood, B.M. The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 590–593. [Google Scholar] [CrossRef]
- Falahi, E.; Akbari, S.; Ebrahimzade, F.; Gargari, B.P. Impact of prophylactic iron supplementation in healthy pregnant women on maternal iron status and birth outcome. Food Nutr. Bull. 2011, 32, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, L.J.; Dainty, J.R.; Hollands, W.J.; Bull, V.J.; Hoogewerff, J.A.; Foxall, R.J.; McAnena, L.; Strain, J.J.; Fairweather-Tait, S.J. Effect of high-dose iron supplements on fractional zinc absorption and status in pregnant women. Am. J. Clin. Nutr. 2007, 85, 131–136. [PubMed]
- Liu, J.M.; Mei, Z.; Ye, R.; Serdula, M.K.; Ren, A.; Cogswell, M.E. Micronutrient supplementation and pregnancy outcomes: Double-blind randomized controlled trial in China. JAMA Intern. Med. 2013, 173, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Crowther, C.A.; Gibson, R.A.; Gibson, R.S.; Skeaff, C.M. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ouladsahebmadarek, E.; Sayyah-Melli, M.; Taghavi, S.; Abbasalizadeh, S.; Seyedhejazie, M. The effect of supplemental iron elimination on pregnancy outcome. Pakistan J. Med. Sci. 2011, 27, 641–645. [Google Scholar]
- Siega-Riz, A.M.; Hartzema, A.G.; Turnbull, C.; Thorp, J.; McDonald, T.; Cogswell, M.E. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: A randomized controlled trial. Am. J. Obstet. Gynecol. 2006, 194, 512–519. [Google Scholar] [CrossRef]
- Charles, D.H.M.; Ness, A.R.; Campbell, D.; Smith, G.D.; Whitley, E.; Hall, M.H. Folic acid supplements in pregnancy and birth outcome: Re-analysis of a large randomised controlled trial and update of Cochrane review. Paediatr. Perinat. Epidemiol. 2005, 19, 112–124. [Google Scholar] [CrossRef]
- Merialdi, M.; Caulfield, L.E.; Zavaleta, N.; Figueroa, A.; Dominici, F.; Dipietro, J.A. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. Am. J. Obstet. Gynecol. 2004, 190, 1106–1112. [Google Scholar] [CrossRef]
- Mahomed, K.; James, D.K.; Golding, J.; McCabe, R. Zinc supplementation during pregnancy: A double blind randomised controlled trial. Br. Med. J. 1989, 299, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Osendarp, S.J.M.; Van Raaij, J.M.A.; Darmstadt, G.L.; Baqui, A.H.; Hautvast, J.G.A.J.; Fuchs, G.J. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: A randomised placebo controlled trial. Lancet 2001, 357, 1080–1085. [Google Scholar] [CrossRef]
- Robertson, J.S.; Heywood, B.; Atkinson, S.M. Zinc supplementation during pregnancy. J. Public Health 1991, 13, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Saaka, M.; Oosthuizen, J.; Beatty, S. Effect of prenatal zinc supplementation on birthweight. J. Health Popul. Nutr. 2009, 27, 619–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmer, K.; Lort-Phillips, L.; James, C.; Thompson, R.P.H. A double-blind trial of zinc supplementation in pregnancy. Eur. J. Clin. Nutr. 1991, 45, 139–144. [Google Scholar] [PubMed]
- Xie, L.; Chen, X.; Pan, J. The effects of zinc supplementation to Chinese rural pregnant women and their pregnancy outcome. J. Shanghai Second. Med. Univ. 2001, 13, 119–124. [Google Scholar]
- Castillo-Durán, C.; Marín, V.B.; Alcázar, L.S.; Iturralde, H.; Ruz, M.O. Controlled trial of zinc supplementation in Chilean pregnant adolescents. Nutr. Res. 2001, 21, 715–724. [Google Scholar] [CrossRef]
- Caulfield, L.E.; Zavaleta, N.; Figueroa, A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am. J. Clin. Nutr. 1999, 69, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Cherry, F.F.; Sandstead, H.H.; Rojas, P.; Johnson, L.A.K.; Batson, H.K.; Wang, X.B. Adolescent pregnancy: Associations among body weight, zinc nutriture, and pregnancy outcome. Am. J. Clin. Nutr. 1989, 50, 945–954. [Google Scholar] [CrossRef]
- Danesh, A.; Janghorbani, M.; Mohammadi, B. Effects of zinc supplementation during pregnancy on pregnancy outcome in women with history of preterm delivery: A double-blind randomized, placebo-controlled trial. J. Matern. Neonatal. Med. 2010, 23, 403–408. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Copper, R.L.; Dubard, M.B.; Hauth, J.C.; Tamura, T.; Johnston, K.E.; Hauth, J.C. The Effect of Zinc Supplementation on Pregnancy Outcome. JAMA J. Am. Med. Assoc. 1995, 274, 463–468. [Google Scholar] [CrossRef]
- Hafeez, A.; Mehmood, G.; Mazhar, F. Oral zinc supplementation in pregnant women and its effect on birth weight: A randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 2005, 90, F170–F171. [Google Scholar] [CrossRef] [PubMed]
- Hunt, I.F.; Murphy, N.J.; Cleaver, A.C. Zinc supplementation during pregnancy: Effects on selected blood constituents and on progress and outcome of pregnancy in low-income women of Mexican descent. Am J Clin Nutr. 1984, 40, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Jønsson, B.; Hauge, B.; Larsen, M.F.; Hald, F. Zinc supplementation during pregnancy: A double blind randomised controlled trial. Acta Obstet. Gynecol. Scand. 1996, 75, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H.D.; Fisher, D.J.; Osmond, C.; Margetts, B.M. Multiple micronutrient supplementation during pregnancy in low-income countries: A meta-analysis of effects on birth size and length of gestation. Food Nutr. Bull. 2009, 30, S533–S546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, B.A.; Yakoob, M.Y.; Bhutta, Z.A. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health. 2011, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Spiegelman, D.; Shankar, A.H.; Fawzi, W.W. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: Meta-analysis and meta-regression. Bull. World Health Organ. 2011, 89, 402B–411B. [Google Scholar] [CrossRef] [Green Version]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane database Syst Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- Ronsmans, C.; Fisher, D.J.; Osmond, C.; Margetts, B.M.; Fall, C.H. Multiple micronutrient supplementation during pregnancy in low-income countries: A meta-analysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr. Bull. 2009, 30, S547. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.S.; Ohlsson, A. Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: A meta-analysis. CMAJ 2009, 180, E99–E108. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.R.; Shankar, A.H.; Wu, L.S.-F.; Aboud, S.; Adu-Afarwuah, S.; Ali, H.; Agustina, R.; Arifeen, S.; Ashorn, P.; Bhutta, Z.A.; et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: A meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob. Health 2017, 5, E1090–E1100. [Google Scholar] [CrossRef] [Green Version]
- Fawzi, W.W.; Msamanga, G.I.; Urassa, W.; Hertzmark, E.; Petraro, P.; Willett, W.C.; Spiegelman, D. Vitamins and Perinatal Outcomes among HIV-Negative Women in Tanzania. N. Engl. J. Med. 2007, 356, 1423–1431. [Google Scholar] [CrossRef] [Green Version]
- Friis, H.; Gomo, E.; Nyazema, N.; Ndhlovu, P.; Krarup, H.; Kæstel, P.; Michaelsen, K.F. Effect of multimicronutrient supplementation on gestational length and birth size: A randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am. J. Clin. Nutr. 2004, 80, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Ray, M.; Dua, T.; Radhakrishnan, G.; Kumar, R.; Sachdev, H.P.S. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring: A double-blind, randomized, placebo-controlled trial. Arch Pediatr. Adolesc. Med. 2007, 161, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Delhi, I.N. Multicentric study of efficacy of periconceptional folic acid containing vitamin supplementation in prevention of open neural tube defects in India. Indian, J. Med. Res. 2000, 112, 206–211. [Google Scholar]
- Johnson, W.; Darboe, M.K.; Sosseh, F.; Nshe, P.; Prentice, A.M.; Moore, S.E. Association of prenatal lipid-based nutritional supplementation with fetal growth in rural Gambia. Matern. Child Nutr. 2017, 13, e12367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kæstel, P.; Michaelsen, K.F.; Aaby, P.; Friis, H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: A randomised, controlled trial in Guinea-Bissau. Eur. J. Clin. Nutr. 2005, 59, 1081–1089. [Google Scholar] [CrossRef] [Green Version]
- Osrin, D.; Vaidya, A.; Shrestha, Y.; Baniya, R.B.; Manandhar, D.S.; Adhikari, R.K.; Filteau, S.; Tomkins, A.; Anthony, M.D.L. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: Double-blind, randomised controlled trial. Lancet 2005, 365, 955–962. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; González-Cossío, T.; Neufeld, L.M.; Rivera, J.; Martorell, R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: A randomized controlled trial in a semirural community in Mexico. Am. J. Clin. Nutr. 2003, 77, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, D.; Huybregts, L.; Lanou, H.; Henry, M.C.; Meda, N.; Menten, J.; Kolsteren, P.; The MISAME Study Group. Effects of maternal multiple micronutrient supplementation on fetal growth: A double-blind randomized controlled trial in rural Burkina Faso. Am. J. Clin. Nutr. 2008, 88, 1330–1340. [Google Scholar]
- Rumiris, D.; Purwosunu, Y.; Wibowo, N.; Farina, A.; Sekizawa, A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens Pregnancy 2006, 25, 241–253. [Google Scholar] [CrossRef]
- Sunawang Utomo, B.; Hidayat, A.; Kusharisupeni, S. Preventing low birthweight through maternal multiple micronutrient supplementation: A cluster-randomized, controlled trial in lndramayu, West Java. Food Nutr. Bull. 2009, 30, S488–S495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofail, F.; Persson, L.Å.; Arifeen SEl Hamadani, J.D.; Mehrin, F.; Ridout, D.; Ekström, E.C.; Huda, S.N.; Grantham-McGregor, S.M. Effects of prenatal food and micronutrient supplementation on infant development: A randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am. J. Clin. Nutr. 2008, 87, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, K.P.; Shamim, A.A.; Mehra, S.; Labrique, A.B.; Ali, H.; Shaikh, S.; Klemm, R.D.; Wu, L.S.; Mitra, M.; Haque, R.; et al. Effect of Maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: The JiVitA-3 randomized trial. JAMA 2014, 312, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Zagré, N.M.; Desplats, G.; Adou, P.; Mamadoultaibou, A.; Aguayo, V.M. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: A cluster-randomized, double-blind, controlled programmatic study in rural Niger. Food Nutr. Bull. 2007, 28, 317–327. [Google Scholar] [CrossRef]
- Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: A double-blind cluster-randomised trial. Lancet 2008, 371, 215–227. [Google Scholar] [CrossRef]
- Adu-Afarwuah, S.; Lartey, A.; Okronipa, H.; Ashorn, P.; Zeilani, M.; Peerson, J.M.; Arimond, M.; Vosti, S.; Dewey, K.G. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am. J. Clin. Nutr. 2015, 101, 835–846. [Google Scholar] [CrossRef]
- Ashorn, P.; Alho, L.; Ashorn, U.; Cheung, Y.B.; Dewey, K.G.; Harjunmaa, U.; Lartey, A.; Nkhoma, M.; Phiri, N.; Phuka, J.; et al. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Bhutta, Z.A.; Rizvi, A.; Raza, F.; Hotwani, S.; Zaidi, S.; Hossain, S.M.; Soofi, S.; Bhutta, S. A comparative evaluation of multiple micronutrient and iron-folic acid supplementation during pregnancy in Pakistan: Impact on pregnancy outcomes. Food Nutr. Bull. 2009, 30, S496–S505. [Google Scholar] [CrossRef]
- Brough, L.; Rees, G.A.; Crawford, M.A.; Morton, R.H.; Dorman, E.K. Effect of multiple-micronutrient supplementation on maternal nutrient status, infant birth weight and gestational age at birth in a low-income, multi-ethnic population. Br J Nutr. 2010, 104, 437–445. [Google Scholar] [CrossRef]
- Merchant, A.T.; Msamanga, G.; Villamor, E.; Saathoff, E.; O’Brien, M.; Hertzmark, E.; Hunter, D.J.; Fawzi, W.W. Multivitamin supplementation of HIV-positive women during pregnancy reduces hypertension. J. Nutr. 2005, 135, 1776–1781. [Google Scholar] [CrossRef] [Green Version]
- Persson, L.Å.; Arifeen, S.; Ekström, E.C.; Rasmussen, K.M.; Frongillo, E.A.; Yunus, M. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: The MINIMat randomized trial. JAMA 2012, 307, 2050–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, P.; Khatry, S.K.; LeClerq, S.C.; Dali, S.M. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. Int. J. Gynecol. Obstet. 2009, 106, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Hoodbhoy, Z.; Salam, R.A.; Bhutta, A.Z.; Valenzuela-Rubio, N.G.; Weise Prinzo, Z.; Bhutta, Z.A. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst. Rev. 2018, CD012610. [Google Scholar] [CrossRef] [PubMed]
- Goto, E. Effectiveness of Prenatal Lipid-Based Nutrient Supplementation to Improve Birth Outcomes: A Meta-analysis. Am. J. Trop. Med. Hyg. 2019, 101, 994–999. [Google Scholar] [CrossRef]
- Huybregts, L.; Roberfroid, D.; Lanou, H.; Menten, J.; Meda, N.; Van Camp, J.; Kolsteren, P. Prenatal food supplementation fortified with multiple micronutrients increases birth length: A randomized controlled trial in rural Burkina Faso. Am. J. Clin. Nutr. 2009, 90, 1593–1600. [Google Scholar]
- Mridha, M.K.; Matias, S.L.; Chaparro, C.M.; Paul, R.R.; Hussain, S.; Vosti, S.A.; Harding, K.L.; Cummins, J.R.; Day, L.T.; Saha, S.L.; et al. Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am. J. Clin. Nutr. 2016, 103, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Graft-Johnson, J.; de Vesel, L.; Rosen, H.E.; Rawlins, B.; Abwao, S.; Mazia, G.; Bozsa, R.; Mwebesa, W.; Khadka, N.; Kamunya, R.; et al. Cross-sectional observational assessment of quality of newborn care immediately after birth in health facilities across six sub-Saharan African countries. BMJ Open 2017, 7, e014680. [Google Scholar] [CrossRef]
- Allen, R.; Rogozinska, E.; Sivarajasingam, P.; Khan, K.S.; Thangaratinam, S. Effect of diet- And lifestyle-based metabolic risk-modifying interventions on preeclampsia: A meta-analysis. Acta Obstet. Gynecol. Scand. 2014, 93, 973–985. [Google Scholar] [CrossRef]
- Chen, B.; Ji, X.; Zhang, L.; Hou, Z.; Li, C.; Tong, Y. Fish Oil Supplementation does not Reduce Risks of Gestational Diabetes Mellitus, Pregnancy-Induced Hypertension, or Pre-Eclampsia: A Meta-Analysis of Randomized Controlled Trials. Med. Sci. Monit. 2015, 21, 2322–2330. [Google Scholar] [CrossRef] [Green Version]
- Saccone, G.; Saccone, I.; Berghella, V. Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: Which evidence? J. Matern. Neonatal. Med. 2016, 29, 2389–2397. [Google Scholar] [CrossRef] [Green Version]
- Szajewska, H.; Horvath, A.; Koletzko, B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006, 83, 1337–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol Assess 2016, 224, 1–826. [Google Scholar]
- Salvig, J.D.; Lamont, R.F. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: A systematic review and meta-analysis. ACTA Obstet. Gynecol. Scand. 2011, 90, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ji, X.; Zhang, L.; Hou, Z.; Li, C.; Tong, Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: A meta-analysis of 21 randomized controlled trials. J. Matern Neonatal Med. 2016, 29, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Koletzko, B.; Szajewska, H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2007, 98, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Imhoff-Kunsch, B.; Briggs, V.; Goldenberg, T.; Ramakrishnan, U. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26, 91–107. [Google Scholar] [CrossRef]
- Kar, S.; Wong, M.; Rogozinska, E.; Thangaratinam, S. Effects of omega-3 fatty acids in prevention of early preterm delivery: A systematic review and meta-analysis of randomized studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 40–46. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V.; Maruotti, G.M.; Sarno, L.; Martinelli, P. Omega-3 supplementation during pregnancy to prevent recurrent intrauterine growth restriction: Systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet. Gynecol. 2015, 46, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Saccone, G.; Berghella, V. Omega-3 long chain polyunsaturated fatty acids to prevent preterm birth: A systematic review and meta-analysis. Obstet Gynecol. 2015, 125, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Saccone, G.; Berghella, V. Omega-3 supplementation to prevent recurrent preterm birth: A systematic review and metaanalysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2015, 213, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucia Bergmann, R.; Bergmann, K.E.; Haschke-Becher, E.; Richter, R.; Dudenhausen, J.W.; Barclay, D.; Haschke, F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J. Perinat. Med. 2007, 35, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Much, D.; Vollhardt, C.; Brunner, S.; Schmid, D.; Sedlmeier, E.M.; Heimberg, E.; Schuster, T.; Zimmermann, A.; Schneider, K.T.M.; et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open-label randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helland, I.B.; Saugstad, O.D.; Smith, L.; Saarem, K.; Solvoll, K.; Ganes, T.; Drevon, C.A. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001, 108, e82. [Google Scholar] [CrossRef] [Green Version]
- Horvaticek, M.; Djelmis, J.; Ivanisevic, M.; Oreskovic, S.; Herman, M. Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C-peptide preservation in pregnant women with type-1 diabetes: Randomized placebo controlled clinical trial. Eur. J. Clin. Nutr. 2017, 71, 968–972. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Kolahdooz, F.; Khalaji, F.; Razavi, M.; Asemi, Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Matern Neonatal Med. 2016, 29, 669–675. [Google Scholar] [CrossRef]
- Jamilian, M.; Hashemi Dizaji, S.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Karamali, M.; Akbari, M.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Biomarkers of Oxidative Stress, Inflammation and Pregnancy Outcomes in Gestational Diabetes. Can. J. Diabetes 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Lalooha, F. Evaluation of the effect of omega-3 supplements in the prevention of preeclampsia among high risk women. Afr. J. Pharm. Pharmacol. 2012, 6, 2580–2583. [Google Scholar] [CrossRef] [Green Version]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, C.A.; Hamilton, R.; McCulloch, D.L.; Montgomery, C.; Weaver, L.T. Scotopic electroretinogram in term infants born of mothers supplemented with docosahexaenoic acid during pregnancy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3685–3691. [Google Scholar] [CrossRef] [Green Version]
- Mardones, F.; Urrutia, M.T.; Villarroel, L.; Rioseco, A.; Castillo, O.; Rozowski, J.; Tapia, J.L.; Bastias, G.; Bacallao, J.; Rojas, I. Effects of a dairy product fortified with multiple micronutrients and omega-3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutr. 2008, 11, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Miller, S.; Harris, M.; Baker, S.; Davalos, D.; Clark, A.; McGirr, K.A. Intake of Total Omega-3 Docosahexaenoic Acid Associated with Increased Gestational Length and Improved Cognitive Performance at 1 Year of Age. J. Nutr. Health Food. Eng. 2016, 5, 642–651. [Google Scholar] [CrossRef]
- Bisgaard, H.; Stokholm, J.; Chawes, B.; Vissing, N.; Bjarnadóttir, E.; Schoos, A.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. Acta Pediatr. Esp. 2016, 375, 25. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Djahanbakhch, O.; Hutchinson, J.; Bhullar, A.S.; Raveendran, M.; Hallot, A.; Eram, S.; Namugere, I.; Nateghian, S.; Ghebremeskel, K. Effect of docosahexaenoic acid-enriched fish oil supplementation in pregnant women with type 2 diabetes on membrane fatty acids and fetal body composition-double-blinded randomized placebo-controlled trial. Diabet Med. 2014, 31, 1331–1340. [Google Scholar] [CrossRef]
- Min, Y.; Djahanbakhch, O.; Hutchinson, J.; Eram, S.; Bhullar, A.S.; Namugere, I.; Ghebremeskel, K. Efficacy of docosahexaenoic acid-enriched formula to enhance maternal and fetal blood docosahexaenoic acid levels: Randomized double-blinded placebo-controlled trial of pregnant women with gestational diabetes mellitus. Clin. Nutr. 2016, 35, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Mozurkewich, E.L.; Clinton, C.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Berman, D.R.; Marcus, S.M.; Romero, V.C.; Treadwell, M.C.; Keeton, K.L.; et al. The Mothers, Omega-3, and Mental Health Study: A double-blind, randomized controlled trial. Am. J. Obstet. Gynecol. 2013, 208, 313.e1–313.e9. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.F.; Dalby Sorensen, J.; Secher, N.J.; Hedegaard, M.; Brink Henriksen, T.; Hansen, H.S.; Grant, A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992, 339, 1003–1007. [Google Scholar] [CrossRef]
- Olsen, S.F.; Secher, N.J.; Tabor, A.; Weber, T.; Walker, J.J.; Gluud, C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. BJOG An. Int. J. Obstet. Gynaecol. 2000, 107, 382–395. [Google Scholar] [CrossRef]
- Onwude, J.L.; Lilford, R.J.; Hjartardottir, H.; Staines, A.; Tuffnell, D. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. BJOG 1995, 102, 95–100. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Mohammad-Alizadeh, S.; Mirghafourvand, M.; Farshbaf-Khalili, S.; Jafarilar-Agdam, N.; Farshbaf-Khalili, A. The effect of fish oil supplementation on maternal and neonatal outcomes: A triple-blind, randomized controlled trial. J. Perinat. Med. 2017, 45, 1069–1077. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Stein, A.D.; Parra-Cabrera, S.; Wang, M.; Imhoff-Kunsch, B.; Juárez-Márquez, S.; Rivera, J.; Martorell, R. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: Randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr. Bull. 2010, 31, S108–S116. [Google Scholar] [CrossRef] [PubMed]
- Smuts, C.M.; Borod, E.; Peeples, J.M.; Carlson, S.E. High-DHA eggs: Feasibility as a means to enhance circulating DHA in mother and infant. Lipids. 2003, 38, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Smuts, C.M.; Huang, M.; Mundy, D.; Plasse, T.; Major, S.; Carlson, S.E. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet. Gynecol. 2003, 101, 469–479. [Google Scholar] [PubMed]
- Bulstra-Ramakers, M.T.E.W.; Huisjes, H.J.; Visser, G.H.A. The effects of 3g eicosapentaenoic acid daily on recurrence of intrauterine growth retardation and pregnancy induced hypertension. BJOG 1995, 102, 123–126. [Google Scholar] [CrossRef]
- Tofail, F.; Kabir, I.; Hamadani, J.D.; Chowdhury, F.; Yesmin, S.; Mehreen, F.; Huda, S.N. Supplement of fish-oil and soy-oil during pregnancy and psychomotor development of infants. J. Health Popul. Nutr. 2006, 24, 48–56. [Google Scholar]
- Van Goor, S.A.; Dijck-Brouwer, D.A.J.; Hadders-Algra, M.; Doornbos, B.; Erwich, J.J.H.M.; Schaafsma, A.; Muskiet, F.A. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 65–69. [Google Scholar] [CrossRef]
- D’Almeida, A.; Carter, J.P.; Anatol, A.; Prost, C. Effects of a combination of evening primrose oil (Gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) versus magnesium, and versus placebo in preventing pre-eclampsia. Women Health 1992, 19, 117–131. [Google Scholar] [CrossRef]
- De Groot, R.H.M.; Hornstra, G.; Van Houwelingen, A.C.; Roumen, F. Effect of α-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am. J. Clin. Nutr. 2004, 79, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Dilli, D.; Doğan, N.N.; İpek, M.Ş.; Çavuş, Y.; Ceylaner, S.; Doğan, H.; Dursun, A.; Küçüközkan, T.; Zenciroğlu, A. MaFOS-GDM trial: Maternal fish oil supplementation in women with gestational diabetes and cord blood DNA methylation at insulin like growth factor-1 (IGF-1) gene. Clin. Nutr. ESPEN 2018, 23, 73–78. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Yang, X.H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Hauguel-de Mouzon, S. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: A randomized double-blind controlled clinical trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, M.; Thom, E.; Klebanoff, M.A.; Thorp, J.; Sorokin, Y.; Varner, M.W.; Wapner, R.J.; Caritis, S.N.; Iams, J.D.; Carpenter, M.W.; et al. Omega-3 Fatty Acid Supplementation to Prevent Recurrent Preterm Birth. Obstet. Gynecol. 2010, 115, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, M.A.; Reece, M.S.; McGregor, J.A.; Wilson, J.W.; Burke, S.M.; Wheeler, M.; Anderson, J.E.; Auld, G.W.; French, J.I.; Allen, K.G. The Effect of Omega-3 Docosahexaenoic Acid Supplementation on Gestational Length: Randomized Trial of Supplementation Compared to Nutrition Education for Increasing n-3 Intake from Foods. Biomed. Res. Int. 2015, 2015, 123078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofail, F.; Hamadani, J.D.; Ahmed, A.Z.T.; Mehrin, F.; Hakim, M.; Huda, S.N. The mental development and behavior of low-birth-weight Bangladeshi infants from an urban low-income community. Eur. J. Clin. Nutr. 2012, 66, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.J.; Yelland, L.; Mcphee, A.J.; Quinlivan, J.; Gibson, R.A.; Makrides, M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia 1-3. Am. J. Clin. Nutr. 2012, 95, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- Duley, L.; Henderson‐Smart, D.J.; Meher, S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst. Rev. 2005, 4, CD005548. [Google Scholar] [CrossRef] [Green Version]
- Jahanfar, S.; Jaafar, H.S. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochrane Database Syst. Rev. 2015, CD006965. [Google Scholar] [CrossRef]
- Gresham, E.; Byles, J.E.; Bisquera, A.; Hure, A.J. Effects of dietary interventions on neonatal and infant outcomes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 1298–1321. [Google Scholar] [CrossRef] [Green Version]
- Ota, E.; Tobe-Gai, R.; Mori, R.; Farrar, D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst. Rev. 2015, CD000032. [Google Scholar] [CrossRef]
- Syngelaki, A.; Sequeira Campos, M.; Roberge, S.; Andrade, W.; Nicolaides, K.H. Diet and exercise for preeclampsia prevention in overweight and obese pregnant women: Systematic review and meta-analysis. J. Matern. Neonatal Med. 2019, 32, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogoziska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Han, S.; Chen, G.-C.; Li, Z.-N.; Silva-Zolezzi, I.; Parés, G.V.; Wang, Y.; Qin, L.Q. Effects of low-glycemic-index diets in pregnancy on maternal and newborn outcomes in pregnant women: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2018, 57, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.; Corica, D.; Mion, E.; Gonçalves, D.; Mottat, G.; Merati, R.; Ragusa, A.; Morabito, A. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: A randomized clinical trial. Diabet Med. 2005, 22, 1536–1541. [Google Scholar]
- Wolff, S.; Legarth, J.; Vangsgaard, K.; Toubro, S.; Astrup, A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int. J. Obes. 2008, 32, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, A.F.L.; Devlieger, R.; Nuyts, E.; Witters, I.; Gyselaers, W.; Van Den Bergh, B.R.H. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: A randomized controlled trial. Int. J. Obes. 2013, 37, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Guelinckx, I.; Devlieger, R.; Mullie, P.; Vansant, G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Luoto, R.; Kinnunen, T.I.; Aittasalo, M.; Kolu, P.; Raitanen, J.; Ojala, K.; Mansikkamäki, K.; Lamberg, S.; Vasankari, T.; Komulainen, T.; et al. Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial. PLoS Med. 2011, 8, e1001036. [Google Scholar] [CrossRef]
- Phelan, S.; Phipps, M.G.; Abrams, B.; Darroch, F.; Schaffner, A.; Wing, R.R. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for delivery study. Am. J. Clin. Nutr. 2011, 93, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Polley, B.A.; Wing, R.R.; Sims, C.J. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int. J. Obes. 2002, 26, 1494–1502. [Google Scholar] [CrossRef] [Green Version]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Renault, K.M.; Nørgaard, K.; Nilas, L.; Carlsen, E.M.; Cortes, D.; Pryds, O.; Secher, N.J. The Treatment of Obese Pregnant Women (TOP) study: A randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am. J. Obstet. Gynecol. 2014, 210, 134.e1–134.e9. [Google Scholar] [CrossRef]
- Bruno, R.; Petrella, E.; Bertarini, V.; Pedrielli, G.; Neri, I.; Facchinetti, F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern. Child Nutr. 2017, 13, e12333. [Google Scholar] [CrossRef] [Green Version]
- Dodd, J.M.; Newman, A.; Moran, L.J.; Deussen, A.R.; Grivell, R.M.; Yelland, L.N.; Crowther, C.A.; McPhee, A.J.; Wittert, G.; Owens, J.A.; et al. The effect of antenatal dietary and lifestyle advice for women who are overweight or obese on emotional well-being: The LIMIT randomized trial. Acta Obstet. Gynecol. Scand. 2016, 95, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Briley, C.; Flanagan, N.L.; Lewis, N.M. In-home prenatal nutrition intervention increased dietary iron intakes and reduced low birthweight in low-income African-American women. J. Am. Diet. Assoc. 2002, 102, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Kafatos, A.G.; Vlachonikolis, I.G.; Codrington, C.A. Nutrition during pregnancy: The effects of an educational intervention program in Greece. Am. J. Clin. Nutr. 1989, 50, 970–979. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef] [Green Version]
- Jahan, K.; Roy, S.K.; Mihrshahi, S.; Sultana, N.; Khatoon, S.; Roy, H.; Datta, L.R.; Roy, A.; Jahan, S.; Khatun, W.; et al. Short-Term nutrition education reduces low birthweight and improves pregnancy outcomes among urban poor women in Bangladesh. Food Nutr. Bull. 2014, 35, 414–421. [Google Scholar] [CrossRef]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp Jr, J.M.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef]
- Peccei, A.; Blake-Lamb, T.; Rahilly, D.; Hatoum, I.; Bryant, A. Intensive Prenatal Nutrition Counseling in a Community Health Setting. Obstet. Gynecol. 2017, 130, 423–432. [Google Scholar] [CrossRef]
- Thornton, Y.S.; Smarkola, C.; Kopacz, S.M.; Ishoof, S.B. Perinatal outcomes in nutritionally monitored obese pregnant women: A randomized clinical trial. J. Natl. Med. Assoc. 2009, 101, 569–577. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A.; Mahony, R.; Foley, M.E.; McAuliffe, F.M. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): Randomised control trial. BMJ 2012, 345, e5605. [Google Scholar] [CrossRef] [Green Version]
- Say, L.; Gülmezoglu, A.M.; Hofmeyr, G.J. Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst. Rev. 2003, CD000148. [Google Scholar] [CrossRef]
- Salam, R.A.; Zuberi, N.F.; Bhutta, Z.A. Pyridoxine (vitamin B6) supplementation during pregnancy or labour for maternal and neonatal outcomes. Cochrane database Syst Rev. 2015, CD000179. [Google Scholar] [CrossRef]
- Harding, K.B.; Pena-Rosas, J.P.; Webster, A.C.; Yap, C.M.; Payne, B.A.; Ota, E.; De-Regil, L.M. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst. Rev. 2017, 3, CD011761. [Google Scholar] [CrossRef]
- Makrides, M.; Crosby, D.D.; Shepherd, E.; Crowther, C.A. Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev. 2014, CD000937. [Google Scholar] [CrossRef]
- Meher, S.; Duley, L. Garlic for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2010, CD006065. [Google Scholar] [CrossRef]
- Palacios, C.; Trak-Fellermeier, M.A.; Martinez, R.X.; Lopez-Perez, L.; Lips, P.; Salisi, J.A.; John, J.C.; Peña-Rosas, J.P. Regimens of vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019, CD013446. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Díaz, L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.A.; Mantzioris, E.; Middleton, P.T.; Muhlhausler, B.S. Gestational age and maternal status of DHA and other polyunsaturated fatty acids in pregnancy: A systematic review. Prostaglandins Leukot. Essent. Fatty Acids 2019, 144, 16–31. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef]
- Kinshella, M.-L.W.; Moore, S.E.; Elango, R. The missing focus on women’s health in the First 1,000 Days approach to nutrition. Public Health Nutr. 2020, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Red and processed meat: More with less? Am. J. Clin. Nutr. 2020, 111, 252–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacagnella, R.C.; Cecatti, J.G.; Osis, M.J.; Souza, J.P. The role of delays in severe maternal morbidity and mortality: Expanding the conceptual framework. Reprod. Health Matters 2012, 20, 155–163. [Google Scholar] [CrossRef]
- Thaddeus, S.; Maine, D. Too far to walk: Maternal mortality in context. Soc. Sci. Med. 1994, 38, 1091–1110. [Google Scholar] [CrossRef]
- Ruel, M.T.; Alderman, H. Nutrition-Sensitive Interventions and Programmes: How can They Help to Cccelerate Progress in Improving Maternal and Child Nutrition? Lancet 2013, 382, 536–551. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.W.; White, R.R.; Rybak, N.; Gaudet, L.M.; Robson, S.; Hague, W.; Simms-Stewart, D.; Carroli, G.; Smith, G.; Fraser, W.D.; et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): Double blind, phase III, randomised controlled, international, multicentre trial. BMJ 2018, 362, 18. [Google Scholar] [CrossRef] [Green Version]
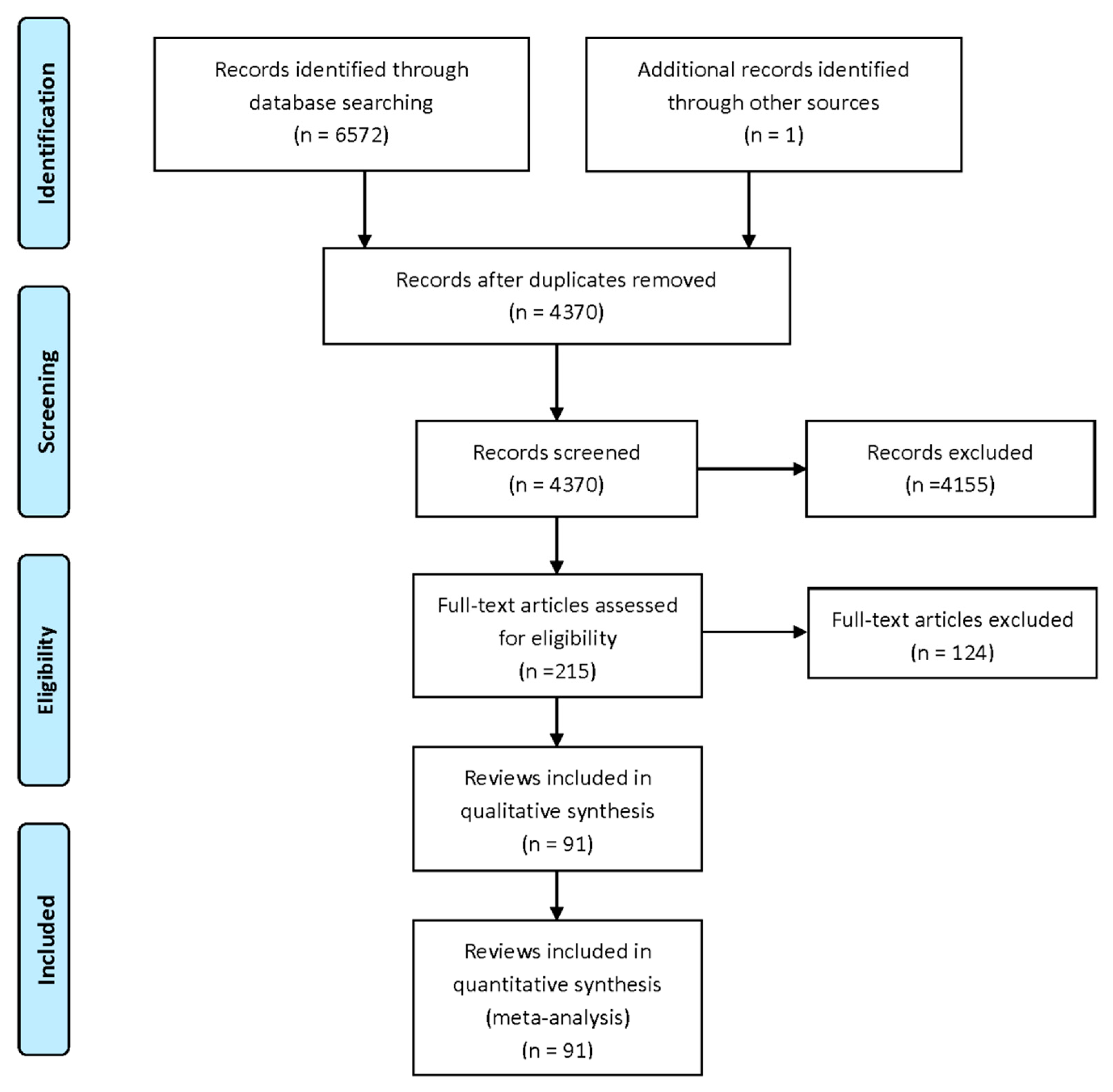
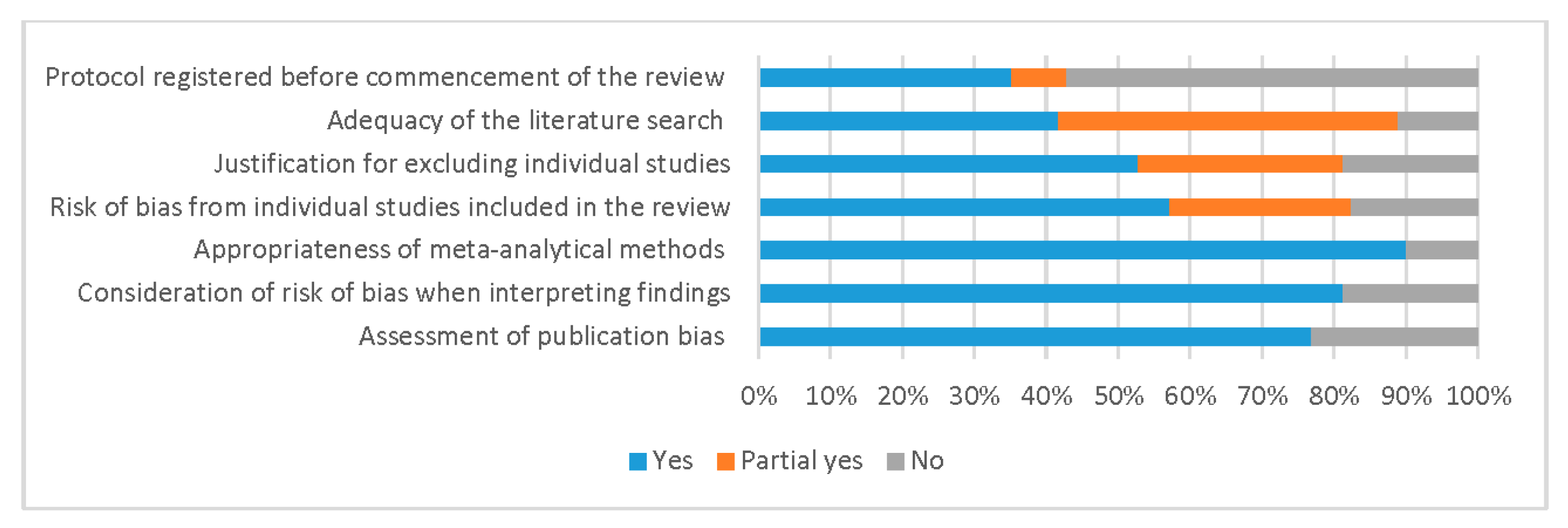
| PE | SGA | LBW | PTB | Stillbirth | Maternal Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutritional supplements | |||||||||||||
| Vitamin A | N/A | RR 1.00 (0.98–1.03, I2 = 0%, 3 studies, n = 14,694) ●●● | RR 0.92 (0.75–1.13, I2 = 51%, 6 studies, n = 16,214) ●● | RR 0.98 (0.94–1.01, I2 = 20%, 6 studies, n = 40,788) ●●● | RR 0.97 (0.92–1.03, I2 = 0%, 4 studies, n = 140,145) ●●● | RR 0.82 (0.56–1.19, I2 = 62%, 5 studies, n = 161,474) ●● | |||||||
| Vitamin B6 | RR 1.71 (0.85–3.45, I2 N/A, 2 studies, n = 1197) ●● | N/A | N/A | N/A | N/A | N/A | |||||||
| Vitamin C and/or E | RR 0.96 (0.89–1.04, I2 33%, 19 studies, n = 24,819) ●● | RR 0.96 (0.89–1.03, I2 24%, 13 studies, n = 21,964) ●●● | RR 0.93 (0.73–1.19, I2 86%, 7 studies, n = 14,356) ●● | RR 1.00 (0.90–1.11, I2 53%,17 studies, n = 23,687) ●● | RR 1.21 (0.92–1.58, I2 0%, 9 studies, n = 19,908) ●●● | RR 0.60 (0.14–2.52, I2 0%, 6 studies, n = 17,574) ●●● | |||||||
| Vitamin D | RR 0.62 (0.43–0.91, I2 0%, 12 studies, n = 1353) ●●●● | RR 0.59 (0.39–0.88, I2 0%, 5 studies, n = 726) ●● | RR 0.76 (0.54–1.06, I2 64%, 6 studies, n = 792) ●● | RR 0.70 (0.49–1.00, I2 39%, 17 studies, n = 4019) ●●● | RR 0.62 (0.19–2.00, I2 0%, 5 studies, n = 860) ●●● | N/A | |||||||
| Vitamin D and calcium | RR 0.49 (0.31–0.77, I2 0%, 3 studies, n = 1120) ●●●● | RR 0.90 (0.58–1.38, I2 NA, 1 study, n = 660) ●● | RR 0.63 (0.12–3.24, I2 16%, 2 studies, n = 107) ●● | RR 1.53 (1.02–2.30, I2 0%, 6 studies, n = 988) ●●● | N/A | N/A | |||||||
| Calcium | RR 0.52 (0.41–0.65, I2 67%, 24 studies, n = 27,442) ●● | RR 1.01 (0.83–1.23, I2 20%, 9 studies, n = 6407) ●●● | RR 0.84 (0.73–0.96, I2 43%, 11 studies, n =7800) ●●● | RR 0.53 (0.33–0.86, I2 95%, 18 studies, n = 14,078) ●● | RR 0.55 (0.24–1.23, I2 75%, 7 studies, n = 10,687) ●● | RR 0.59 (0.18–1.92, I2 40%, 5 studies, n = 10,057) ●●● | |||||||
| Iodine | N/A | RR 1.26 (0.77–2.05, I2 0%, 2 studies, n = 377) ●● | RR 0.56 (0.26–1.20, I2 0%, 2 studies, n = 377) ●● | RR 0.71 (0.30–1.66, I2 32%, 2 studies, n = 376) ●● | N/A | N/A | |||||||
| Iron and/or folic acid | RR 0.99 (0.67–1.47, I2 0%, 6 studies, n = 4454) ●● | RR 0.92 (0.80–1.06, I2 67%, 7 studies, n = 8507 ●● | RR 0.87 (0.77–0.98, I2 27%, 13 studies, n = 22,946) ●●● | RR 0.97 (0.89–1.06, I2 0%, 15 studies, n = 24,420) ●●● | RR 0.88 (0.66–1.17, I2 0%, 7 studies, n = 16,891) ●●● | N/A | |||||||
| Magnesium | RR 0.87 (0.58–1.32, I2 0%, 3 studies, n = 1042) ●●● | RR 0.76 (0.54–1.07, I2 7%, 3 studies, n = 1291) ●●● | RR 0.95 (0.83–1.09, I2 22%, 5 studies, n = 5577) ●●● | RR 0.89 (0.69–1.14, I2 37%, 7 studies, n = 5981) ●●● | RR 0.73 (0.43–1.25, I2 0%, 4 studies, n = 5526) ●● | N/A | |||||||
| Zinc | RR 1.31 (0.82–2.10, I2 39%, 5 studies, n = 2518) ●● | RR 1.03 (0.95–1.11, I2 28%, 8 studies, n =4334) ●●● | RR 1.05 (0.94–1.17, I2 26%, 12 studies, n = 5545) ●● | RR 0.86 (0.76–0.97, I2 17%, 16 studies, n = 7563) ●●● | RR 0.20 (0.01–4.12, I2 NA, 2 studies, n = 376) ●● | RR 0.31 (0.01–7.43, I2 NA, 1 study, n = 85) ●● | |||||||
| Garlic | RR 0.78 (0.31–1.93, I2 N/A, 1 study, n = 100) ●● | N/A | N/A | N/A | N/A | N/A | |||||||
| Multiple micronutrients | RR 0.40 (0.27–0.59, I2 0%, 2 studies, n = 510) ●● | RR 0.88 (0.82–0.95, I2 59%, 16 studies, n = 39,696) ●●● | RR 0.87 (0.85–0.90, I2 22%, 20 studies, n = 70,293) ●●●● | RR 0.93 (0.85–1.02, I2 74%, 17 studies, n = 87,731) ●● | RR 1.09 (0.89–1.34, I2 75%, 20 studies, n = 11,4385) ●● | RR 1.17 (0.82–1.67, I2 0%, 8 studies, n = 84,081) ●●● | |||||||
| Lipid-based nutrients | N/A | RR 0.93 (0.88–0.98, I2 0%, 4 studies, n = 4828) ●●●● | RR 0.89 (0.82–0.98, I2 0%, 5 studies, n = 5851) ●●●● | RR 0.99 (0.86–1.14, I2 0%, 5 studies, n = 6072) ●●● | RR 1.06 (0.60–1.87, I2 55%, 4 studies, n = 6778) ●● | RR 0.52 (0.12–2.28, I2 0%, 3 studies, n = 5628) ●●● | |||||||
| Balanced protein-energy | RR 1.48 (0.82–2.66, I2 N/A, 2 study, n = 463) ●● | RR 0.79 (0.69–0.90, I2 16%, 7 studies, n = 4408) ●●● | N/A | RR 0.96 (0.80–1.16, I2 0%, 5 studies, n = 3374) ●● | RR 0.60 (0.39–0.94, I2 10%, 5 studies, n = 3408) ●●● | N/A | |||||||
| High protein | N/A | RR 1.58 (1.03–2.41, I2 N/A, 1 study, n = 505) ●● | N/A | N/A | RR 0.81 (0.31–2.15, I2 NA, 1 study, n = 529) ●● | N/A | |||||||
| Calf blood extract | N/A | RR 0.54 (0.20–1.47, I2 N/A, 1 study, n = 31) | N/A | N/A | N/A | N/A | |||||||
| Glucose | N/A | RR 1.11 (0.64–1.92, I2 N/A, 1 study, n = 30) ● | N/A | N/A | N/A | N/A | |||||||
| Galactose | N/A | RR 0.78 (0.39–1.54, I2 N/A, 1 study, n = 30) ● | N/A | N/A | N/A | N/A | |||||||
| Omega-3 | RR 0.87 (0.71–1.07, I2 9%, 18 studies, n = 7166) ●●● | RR 1.01 (0.90–1.13, I2 0%, 8 studies, n = 6907) ●●● | RR 0.87 (0.79–0.96, I2 28%, 16 studies, n = 7940) ●●●● | RR 0.85 (0.76–0.95, I2 13%, 28 studies, n = 10,524) ●●●● | RR 0.89 (0.58–1.37, I2 32%, 13 studies, n = 7547) ●● | N/A | |||||||
| Dietary interventions | |||||||||||||
| Salt restriction | RR 1.11 (0.46–2.66, I2 0%, 2 studies, n = 603) ●● | RR 1.50 (0.73–3.07, I2 NA, 1 study, n = 242) ●● | N/A | RR 1.08 (0.46–2.56, I2 NA, 1 study, n = 242) ●● | N/A | N/A | |||||||
| Caffeine restriction | N/A | RR 0.97 (0.57–1.64, I2 NA, 1 study, n = 1150) ●● | N/A | RR 0.81 (0.48–1.37, I2 NA, 1 study, n = 1153) ●● | N/A | N/A | |||||||
| Antenatal dietary counselling | RR 0.97 (0.84–1.14, I2 6%, 15 studies, n = 8087) ●●● | RR 1.15 (0.94–1.41, I2 0%, 10 studies, n = 5529) ●●● | RR 0.54 (0.17–1.71, I2 84%, 4 studies, n = 2271) ●● | RR 0.72 (0.61–0.86 I2 21%, 14 studies, n = 7612) ●●●● | RR 0.63 (0.28–1.40, I2 0%, 6 studies, n = 5631) ●●● | RR 1.08 (0.07–17.32, I2 NA, 1 study, n = 2122) ●● | |||||||
| LEGEND Direction of effect and strength of association | |||||||||||||
| Benefit—Strong effect (RR < 0.40) | Benefit—Moderate effect (RR 0.40–0.69) | Benefit—Weak effect (RR 0.70–0.89) | Benefit—Not discernible (RR 0.90–0.99) | No significant effect (95% CI crosses 1.00) | |||||||||
| Harm—Not discernable (RR 1.01–1.09) | Harm—Weak effect (RR 1.10–1.49) | Harm—Moderate effect (RR 1.50–2.99) | Harm—Strong effect (RR ≥ 3.00) | Not available | |||||||||
| Certainty of the evidence | |||||||||||||
| High ●●●● | Moderate ●●● | Low ●● | Very low ● | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinshella, M.-L.W.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.A.; von Dadelszen, P.; Moore, S.E.; Elango, R.; The PRECISE Conceptual Framework Working Group. Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients 2021, 13, 472. https://doi.org/10.3390/nu13020472
Kinshella M-LW, Omar S, Scherbinsky K, Vidler M, Magee LA, von Dadelszen P, Moore SE, Elango R, The PRECISE Conceptual Framework Working Group. Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients. 2021; 13(2):472. https://doi.org/10.3390/nu13020472
Chicago/Turabian StyleKinshella, Mai-Lei Woo, Shazmeen Omar, Kerri Scherbinsky, Marianne Vidler, Laura A. Magee, Peter von Dadelszen, Sophie E. Moore, Rajavel Elango, and The PRECISE Conceptual Framework Working Group. 2021. "Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map" Nutrients 13, no. 2: 472. https://doi.org/10.3390/nu13020472
APA StyleKinshella, M.-L. W., Omar, S., Scherbinsky, K., Vidler, M., Magee, L. A., von Dadelszen, P., Moore, S. E., Elango, R., & The PRECISE Conceptual Framework Working Group. (2021). Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients, 13(2), 472. https://doi.org/10.3390/nu13020472






