Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes
Abstract
1. Introduction
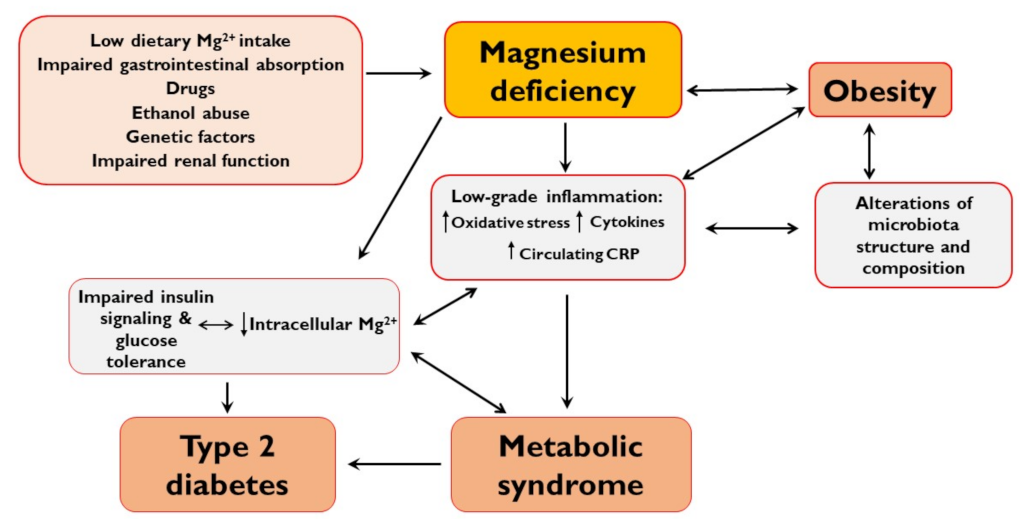
2. Mg2+ Deficiency
3. Mg2+ and Obesity
4. Mg2+ in Metabolic Syndrome
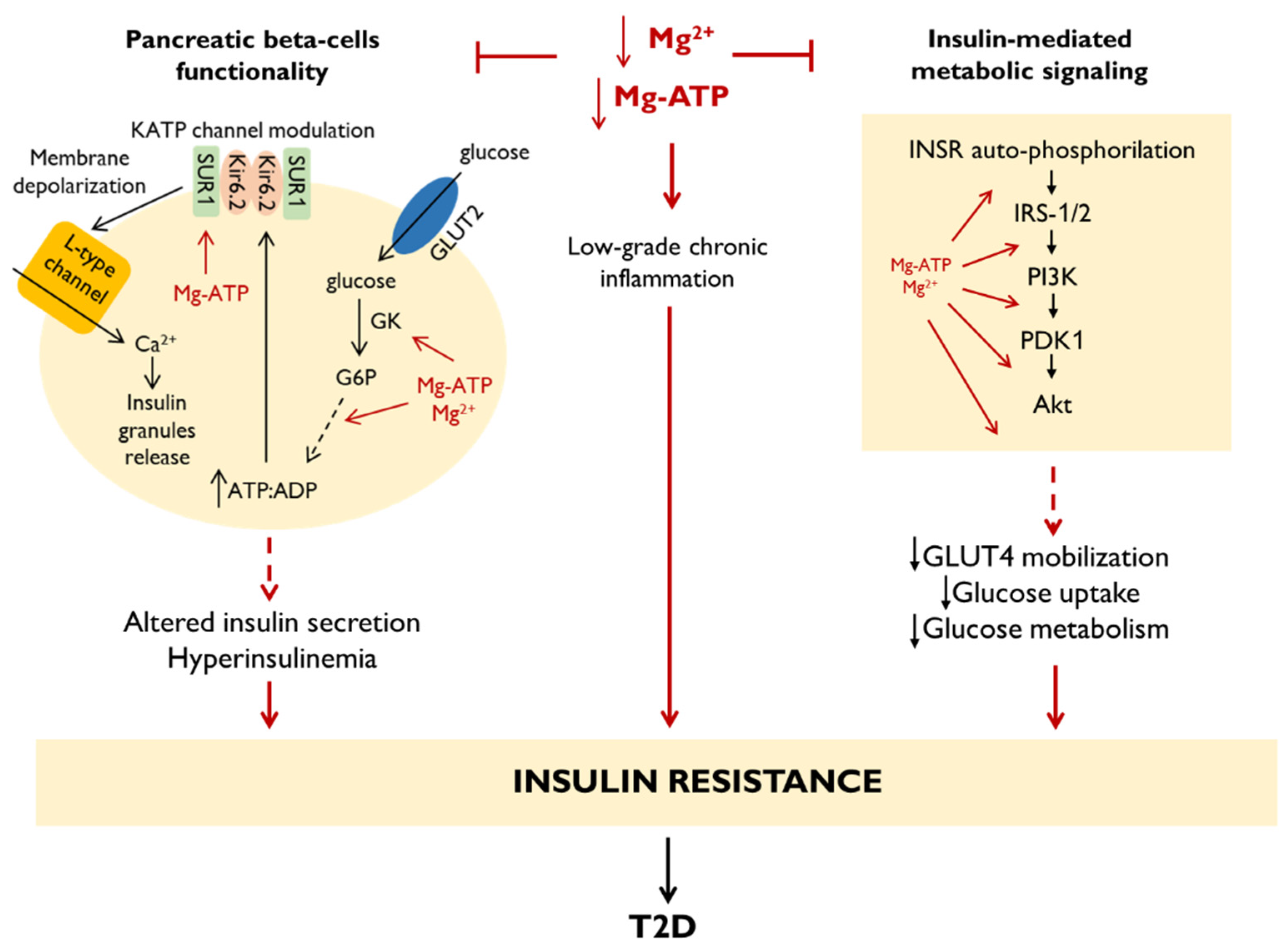
5. Mg2+ in Type 2 Diabetes
6. Mg2+ and Gut Microbiota
7. Dietary Mg2+
8. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for magnesium. Efsa J. 2015, 13, 4186. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. CKJ Clin. Kidney J. 2012, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Von Ehrlich, B.; Barbagallo, M.; Classen, H.G.; Guerrero-Romero, F.; Mooren, F.C.; Rodriguez-Moran, M.; Vierling, W.; Vormann, J.; Kisters, K. Significance of magnesium in insulin resistance, metabolic syndrome, and diabetes—Recommendations of the Association of Magnesium Research e.V. Trace Elem. Electrolytes 2017, 34, 124–129. [Google Scholar] [CrossRef]
- Nielsen, F.H. Effects of magnesium depletion on inflammation in chronic disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 525–530. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Banach, M. Effect of magnesium supplements on serum C-reactive protein: A systematic review and meta-analysis. Arch. Med. Sci. 2018, 14, 707–716. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Oh, H.E.; Deeth, H.C. Magnesium in milk. Int. Dairy J. 2017, 71, 89–97. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. The Problematic Use of Dietary Reference Intakes to Assess Magnesium Status and Clinical Importance. Biol. Trace Elem. Res. 2019, 188, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. Int. Rev. J. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Lowenstein, F.W.; Stanton, M.F. Serum Magnesium Levels in The United States, 1971–1974. J. Am. Coll. Nutr. 1986, 5, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Guidance for the determination of status indicators and dietary requirements for magnesium. Magnes. Res. 2016, 29, 154–160. [Google Scholar] [CrossRef]
- Razzaque, M.S. Magnesium: Are We Consuming Enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef]
- Topf, J.M.; Murray, P.T. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2003, 4, 195–206. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.Y.; Bruyère, O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef]
- Van Dronkelaar, C.; Van Velzen, A.; Abdelrazek, M.; Van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e3. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004, 15, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H. Dietary magnesium intake in a national sample of US adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported dietary intake, disparity between the reported consumption and the level needed for adequacy and food sources of calcium, phosphorus, magnesium and vitamin D in the Spanish population: Findings from the ANIBES study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K. Factors influencing magnesium consumption among adults in the United States. Nutr. Rev. 2018, 76, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: A review. Medicine 2019, 55, 337. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Magnesium and inflammatory bowel disease. Magnesium 1988, 7, 78–83. [Google Scholar]
- Kruis, W.; Phuong Nguyen, G. Iron Deficiency, Zinc, Magnesium, Vitamin Deficiencies in Crohn’s Disease: Substitute or Not? Dig. Dis. 2016, 34, 105–111. [Google Scholar] [CrossRef]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; O’keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis Coronary artery disease. Open Hear. 2018, 5, 668. [Google Scholar] [CrossRef]
- Bateman, S.W. A Quick Reference on Magnesium. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 235–239. [Google Scholar] [CrossRef]
- Chrysant, S.G. Proton pump inhibitor-induced hypomagnesemia complicated with serious cardiac arrhythmias. Expert Rev. Cardiovasc. 2019, 17, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwínska, A.; Brzozowska, K.; Forma, A.; MacIejewski, R. Serum iron, magnesium, copper, and manganese levels in alcoholism: A systematic review. Molecules 2019, 24, 1361. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.; Ross, D.P.; Talwar, D.; Forrest, E.; Naz Abbasi, H.; Leach, J.P.; Woods, M.; Zhu, L.Y.; Dickson, S.; Kwok, T.; et al. Low serum magnesium and 1-year mortality in alcohol withdrawal syndrome. Eur. J. Clin. Investig. 2019, 49, e13152. [Google Scholar] [CrossRef] [PubMed]
- Viering, D.H.H.M.; De Baaij, J.H.F.; Walsh, S.B.; Kleta, R.; Bockenhauer, D. Genetic causes of hypomagnesemia, a clinical overview. Pediatr. Nephrol. 2017, 32, 1123–1135. [Google Scholar] [CrossRef]
- López-González, B.; Molina-López, J.; Florea, D.I.; Quintero-Osso, B.; Pérez De La Cruz, A.; Ma, E.; Del Pozo, P. Association between magnesium-deficient status and anthropometric and clinical-nutritional parameters in posmenopausal women. Nutr Hosp. 2014, 29, 658–664. [Google Scholar] [CrossRef]
- Touitou, Y.; Godard, J.P.; Ferment, O.; Chastang, C.; Proust, J.; Bogdan, A.; Auzéby, A.; Touitou, C. Prevalence of magnesium and potassium deficiencies in the elderly. Clin. Chem. 1987, 33, 518–523. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium, inflammation, and obesity in chronic disease. Nutr. Rev. 2010, 68, 333–340. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Dos Santos, L.R.; De Sousa Melo, S.R.; De Oliveira Santos, R.; De Oliveira, A.R.S.; Cruz, K.J.C.; Do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, X.; Li, M.; Yan, S.; Zhao, H.; Pan, Y.; Wang, C.; Yao, Y.; Jin, L.; Li, B. Association between dietary mineral nutrient intake, body mass index, and waist circumference in U.S. Adults using quantile regression analysis NHANES 2007–2014. PeerJ 2020, 8, e9127. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Macronutrient Intake and Distribution in the Etiology, Prevention and Treatment of Osteosarcopenic Obesity. Curr. Aging Sci. 2016, 10, 83–105. [Google Scholar] [CrossRef]
- Galan, P.; Preziosi, P.; Durlach, V.; Valeix, P.; Ribas, L.; Bouzid, D.; Favier, A.; Hercberg, S. Dietary magnesium intake in a French adult population. Magnes. Res. 1997, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, C.; Yang, K.; Zhu, J.; Xun, P.; Shikany, J.M.; He, K. Magnesium intake is inversely associated with risk of obesity in a 30-year prospective follow-up study among American young adults. Eur. J. Nutr. 2020, 59, 3745–3753. [Google Scholar] [CrossRef] [PubMed]
- Devaux, S.; Adrian, M.; Laurant, P.; Berthelot, A.; Quignard-Boulangé, A. Dietary magnesium intake alters age-related changes in rat adipose tissue cellularity. Magnes. Res. 2016, 29, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Carriquiry, A.; Da Costa, T.H.M.; Ariza, A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018, 17, 114. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 113, 1675–1682. [Google Scholar] [CrossRef]
- Shamnani, G.; Rukadikar, C.; Gupta, V.; Singh, S.; Tiwari, S.; Bhartiy, S.; Sharma, P. Serum magnesium in relation with obesity. Natl. J. Physiol. Pharm. Pharm. 2018, 8, 1074–1077. [Google Scholar] [CrossRef]
- Maguire, D.; Talwar, D.; Shiels, P.G.; McMillan, D. The role of thiamine dependent enzymes in obesity and obesity related chronic disease states: A systematic review. Clin. Nutr. ESPEN 2018, 25, 8–17. [Google Scholar] [CrossRef]
- Mishra, S.; Padmanaban, P.; Deepti, G.N.; Sarkar, G.; Sumathi, S.; Toora, B.D. Serum magnesium and dyslipidemia in type-2 diabetes mellitus. Biomed. Res. 2012, 23, 295–300. [Google Scholar]
- Ansari, M.R.; Maheshwari, N.; Shaikh, M.A.; Laghari, M.S.; Darshana; Lal, K.; Ahmed, K. Correlation of serum magnesium with dyslipidemia in patients on maintenance hemodialysis. Saudi J. Kidney Dis. Transpl. 2012, 23, 21–25. [Google Scholar] [CrossRef]
- Deepti, R.; Nalini, G. Anbazhagan Relationship between hypomagnesemia and dyslipidemia in type 2 diabetes mellitus. Asian J. Pharm. Res. Health Care 2014, 6, 32–36. [Google Scholar]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Body mass index, vitamin d, and type 2 diabetes: A systematic review and meta-analysis. Nutrients 2018, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin d activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhu, X.; Manson, J.A.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence Vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalidi, B.; Kimball, S.M.; Rotondi, M.A.; Ardern, C.I. Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in U.S. adults: A cross-sectional analysis of NHANES, 2001–2010. Nutr. J. 2017, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Stokic, E.; Romani, A.; Ilincic, B.; Kupusinac, A.; Stosic, Z.; Isenovic, E.R. Chronic Latent Magnesium Deficiency in Obesity Decreases Positive Effects of Vitamin D on Cardiometabolic Risk Indicators. Curr. Vasc. Pharm. 2018, 16, 610–617. [Google Scholar] [CrossRef]
- Farhanghi, M.A.; Mahboob, S.; Ostadrahimi, A. Obesity induced Magnesium deficiency can be treated by vitamin D supplementation. J. Pak. Med. Assoc. 2009, 59, 258–261. [Google Scholar]
- Song, Y.; Ridker, P.M.; Manson, J.A.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef]
- McKeown, N.M.; Jacques, P.F.; Zhang, X.L.; Juan, W.; Sahyoun, N.R. Dietary magnesium intake is related to metabolic syndrome in older Americans. Eur. J. Nutr. 2008, 47, 210–216. [Google Scholar] [CrossRef]
- Mirmiran, P.; Shab-Bidar, S.; Hosseini-Esfahani, F.; Asghari, G.; Hosseinpour-Niazi, S.; Azizi, F. Magnesium intake and prevalence of metabolic syndrome in adults: Tehran lipid and glucose study. Public Health Nutr. 2012, 15, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Bae, Y.J. Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in Korean Adults: The Korea national health and nutrition examination survey (2007-2008). Biol. Trace Elem. Res. 2013, 156, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Xun, P.; Fly, A.D.; Yokota, K.; He, K. Dietary magnesium intake and risk of metabolic syndrome: A meta-analysis. Diabet. Med. 2014, 31, 1301–1309. [Google Scholar] [CrossRef]
- Sarrafzadegan, N.; Khosravi-Boroujeni, H.; Lotfizadeh, M.; Pourmogaddas, A.; Salehi-Abargouei, A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; He, L.; Li, Y.; Xu, L.; Ping, F.; Li, W.; Zhang, H. Reduced Insulin Resistance Partly Mediated the Association of High Dietary Magnesium Intake with Less Metabolic Syndrome in a Large Chinese Population. Diabetes. Metab. Syndr. Obes. 2020, 13, 2541–2550. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.; Perunicic-Pekovic, G.; Jovanovic, D.; Gluvic, Z.; Cankovic-Kadijevic, M. Association of blood pressure and metabolic syndrome components with magnesium levels in drinking water in some Serbian municipalities. J. Water Health 2012, 10, 161–169. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Ohira, T.; Date, C.; Tamakoshi, A. Associations of dietary magnesium intake with mortality from cardiovascular disease: The JACC study. Atherosclerosis 2012, 221, 587–595. [Google Scholar] [CrossRef]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary magnesium and cardiovascular disease: A review with emphasis in epidemiological studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Maria De Lourdes, L.; Cruz, T.; Rodrigues, L.E.; Bomfim, O.; Melo, J.; Correia, R.; Porto, M.; Cedro, A.; Vicente, E. Serum and intracellular magnesium deficiency in patients with metabolic syndrome-Evidences for its relation to insulin resistance. Diabetes Res. Clin. Pract. 2009, 83, 257–262. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S.; Syedmoradi, L.; Azizi, F. Low serum magnesium levels in elderly subjects with metabolic syndrome. Biol. Trace Elem. Res. 2010, 136, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, J.; Zeng, C.; Yang, T.; Li, H.; Cui, Y.; Xie, D.; Xu, B.; Liu, Z.; Li, J.; et al. Association between serum magnesium concentration and metabolic syndrome, diabetes, hypertension and hyperuricaemia in knee osteoarthritis: A cross-sectional study in Hunan Province, China. BMJ Open 2018, 8, e019159. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Fang, X.; Su, D.; Huang, L.; He, M.; Zhao, D.; Zou, Y.; Zhang, R. Dietary Calcium Intake and the Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 19046. [Google Scholar] [CrossRef] [PubMed]
- Moore-Schiltz, L.; Albert, J.M.; Singer, M.E.; Swain, J.; Nock, N.L. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001-2010 data. Br. J. Nutr. 2015, 114, 924–935. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.K.; Bae, Y.J. Relationship between serum calcium and magnesium concentrations and metabolic syndrome diagnostic components in middle-aged Korean men. Biol. Trace Elem. Res. 2012, 146, 35–41. [Google Scholar] [CrossRef]
- Dai, Q.; Shu, X.O.; Deng, X.; Xiang, Y.B.; Li, H.; Yang, G.; Shrubsole, M.J.; Ji, B.; Cai, H.; Chow, W.H.; et al. Modifying effect of calcium/magnesium intake ratio and mortality: A population based cohort study. BMJ Open 2013, 3, e002111. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Xun, P.; He, K.; Qin, L.-Q. Magnesium Intake and Risk of Type 2 Diabetes. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef]
- Bertinato, J.; Wang, K.C.; Hayward, S. Serum magnesium concentrations in the Canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients 2017, 9, 296. [Google Scholar] [CrossRef]
- Zhao, B.; Zeng, L.; Zhao, J.; Wu, Q.; Dong, Y.; Zou, F.; Gan, L.; Wei, Y.; Zhang, W. Association of magnesium intake with type 2 diabetes and total stroke: An updated systematic review and meta-analysis. BMJ Open 2020, 10, 32240. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Esmeralda, C.A.C.; Ibrahim, S.N.A.; David, P.E.; Maldonado, I.C.; David, A.S.; Escorza, M.A.Q.; Dealmy, D.G. Deranged fractional excretion of magnesium and serum magnesium levels in relation to retrograde glycaemic regulation in patients with type 2 diabetes mellitus. Curr. Diabetes Rev. 2020, 17, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, H.; Li, M.; Liang, C.; Fan, Z.; Aaseth, J.; He, J.; Montgomery, S.; Cao, Y. Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Sul, J.; Song, Y.; Li, X.; LeBlanc, J.; You, Y.; Butch, A.; Liu, S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: A randomized, double-blind, controlled, crossover trial in overweight individuals. Am. J. Clin. Nutr. 2011, 93, 463–473. [Google Scholar] [CrossRef]
- Mooren, F.C.; Krüger, K.; Völker, K.; Golf, S.W.; Wadepuhl, M.; Kraus, A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects—A double-blind, placebo-controlled, randomized trial. Diabetes Obes. Metab. 2011, 13, 281–284. [Google Scholar] [CrossRef]
- Hruby, A.; Guasch-Ferré, M.; Bhupathiraju, S.N.; Manson, J.E.; Willett, W.C.; McKeown, N.M.; Hu, F.B. Magnesium Intake, Quality of Carbohydrates, and Risk of Type 2 Diabetes: Results From Three U.S. Cohorts. Diabetes Care 2017, 40, 1695–1702. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Puljung, M.C.; Vedovato, N. Neonatal Diabetes and the KATP Channel: From Mutation to Therapy. Trends Endocrinol. Metab. 2017, 28, 377–387. [Google Scholar] [CrossRef]
- Kostov, K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: Focusing on the processes of insulin secretion and signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef]
- Günther, T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes. Res. 2010, 23, 5–18. [Google Scholar] [CrossRef]
- Gommers, L.M.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; De Baaij, J.H.F. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Apell, H.J.; Hitzler, T.; Schreiber, G. Modulation of the Na,K-ATPase by Magnesium Ions. Biochemistry 2017, 56, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Grycova, L.; Sklenovsky, P.; Lansky, Z.; Janovska, M.; Otyepka, M.; Amler, E.; Teisinger, J.; Kubala, M. ATP and magnesium drive conformational changes of the Na+/K+-ATPase cytoplasmic headpiece. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.V.; Hocherb, B.; Verkaart, S.; Van Zeeland, F.; Pfab, T.; Slowinski, T.; Chen, Y.P.; Schlingmann, K.P.; Schaller, A.; Gallati, S.; et al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 109, 11324–11329. [Google Scholar] [CrossRef]
- Hassan, S.A.U.; Ahmed, I.; Nasrullah, A.; Haq, S.; Ghazanfar, H.; Sheikh, A.B.; Zafar, R.; Askar, G.; Hamid, Z.; Khushdil, A.; et al. Comparison of Serum Magnesium Levels in Overweight and Obese Children and Normal Weight Children. Cureus 2017, 9, e1607. [Google Scholar] [CrossRef]
- Zaakouk, A.M.; Hassan, M.A.; Tolba, O.A. Serum magnesium status among obese children and adolescents. Egypt. Pediatr. Assoc. Gaz. 2016, 64, 32–37. [Google Scholar] [CrossRef]
- Huerta, M.G.; Roemmich, J.N.; Kington, M.L.; Bovbjerg, V.E.; Weltman, A.L.; Holmes, V.F.; Patrie, J.T.; Rogol, A.D.; Nadler, J.L. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 2005, 28, 1175–1181. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Wang, M.; Monaco, M.H.; Donovan, S.M. Impact of early gut microbiota on immune and metabolic development and function. Semin. Fetal Neonatal Med. 2016, 21, 380–387. [Google Scholar] [CrossRef]
- Parekh, P.J.; Balart, L.A.; Johnson, D.A. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin. Transl. Gastroenterol. 2015, 6, e91. [Google Scholar] [CrossRef]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; De Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Friedenberg, F. Obesity and GERD. Gastroenterol. Clin. N. Am. 2014, 43, 161–173. [Google Scholar] [CrossRef]
- Gommers, L.M.M.; Ederveen, T.H.A.; Van Der Wijst, J.; Overmars-Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; De Baaij, J.H.F.; Hoenderop, J.G.J. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef]
- Pachikian, B.D.; Neyrinck, A.M.; Deldicque, L.; De Backer, F.C.; Catry, E.; Dewulf, E.M.; Sohet, F.M.; Bindels, L.B.; Everard, A.; Francaux, M.; et al. Changes in intestinal bifidobacteria levels are associated with the inflammatory response in magnesium-deficient mice. J. Nutr. 2010, 140, 509–514. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Semenkovich, C.F.; Danska, J.; Darsow, T.; Dunne, J.L.; Huttenhower, C.; Insel, R.A.; McElvaine, A.T.; Ratner, R.E.; Shuldiner, A.R.; Blaser, M.J. American Diabetes Association and JDRF Research Symposium: Diabetes and the Microbiome. Diabetes 2015, 64, 3967–3977. [Google Scholar] [CrossRef]
- Caesar, R. Pharmacologic and Nonpharmacologic Therapies for the Gut Microbiota in Type 2 Diabetes. Can. J. Diabetes 2019, 43, 224–231. [Google Scholar] [CrossRef]
- Nuli, R.; Cai, J.; Kadeer, A.; Zhang, Y.; Mohemaiti, P. Integrative Analysis Toward Different Glucose Tolerance-Related Gut Microbiota and Diet. Front. Endocrinol. (Lausanne) 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Crowley, E.K.; Long-Smith, C.M.; Murphy, A.; Patterson, E.; Murphy, K.; O’Gorman, D.M.; Stanton, C.; Nolan, Y.M. Dietary supplementation with a magnesium-rich marine mineral blend enhances the diversity of gastrointestinal microbiota. Mar. Drugs 2018, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Helyon 2020, 6, e05390. [Google Scholar] [CrossRef] [PubMed]
- Elderawi, W.A.; Naser, I.A.; Taleb, M.H.; Abutair, A.S. The Effects of Oral Magnesium Supplementation on Glycemic Response among Type 2 Diabetes Patients. Nutrients 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Cortes, A.; Ble-Castillo, J.L.; Guerrero-Romero, F.; Cordova-Uscanga, R.; Juárez-Rojop, I.E.; Aguilar-Mariscal, H.; Tovilla-Zarate, C.A.; Del Rocio Lopez-Guevara, M. No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes. Res. 2014, 27, 48–56. [Google Scholar] [CrossRef]
- Razzaghi, R.; Pidar, F.; Momen-Heravi, M.; Bahmani, F.; Akbari, H.; Asemi, Z. Magnesium Supplementation and the Effects on Wound Healing and Metabolic Status in Patients with Diabetic Foot Ulcer: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2018, 181, 207–215. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Reyes-Romero, M.A.; Guerrero-Romero, F. No positive effect of oral magnesium supplementation in the decreases of inflammation in subjects with prediabetes: A pilot study. Magnes. Res. 2012, 25, 140–146. [Google Scholar] [CrossRef]
- Steward, C.J.; Zhou, Y.; Keane, G.; Cook, M.D.; Liu, Y.; Cullen, T. One week of magnesium supplementation lowers IL-6, muscle soreness and increases post-exercise blood glucose in response to downhill running. Eur. J. Appl. Physiol. 2019, 119, 2617–2627. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Banjanin, N.; Belojevic, G. Changes of blood pressure and hemodynamic parameters after oral magnesium supplementation in patients with essential hypertension—an intervention study. Nutrients 2018, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Hatzistavri, L.S.; Sarafidis, P.A.; Georgianos, P.I.; Tziolas, I.M.; Aroditis, C.P.; Zebekakis, P.E.; Pikilidou, M.I.; Lasaridis, A.N. Oral magnesium supplementation reduces ambulatory blood pressure in patients with mild hypertension. Am. J. Hypertens. 2009, 22, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramírez, M.; Rodríguez-Morán, M.; Reyes-Romero, M.A.; Guerrero-Romero, F. Effect of oral magnesium supplementation on the transcription of TRPM6, TRPM7, and SLC41A1 in individuals newly diagnosed of pre-hypertension. A randomized, double-blind, placebo-controlled trial. Magnes. Res. 2017, 30, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Moeinzadeh, F.; Saadatnia, M.; Shahidi, S.; McGee, J.C.; Minagar, A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: A double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013, 69, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Plat, J.; Bakker, S.J.L.; Mensink, R.P. Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: A randomized controlled trial in overweight/obese adults. Sci. Rep. 2017, 7, 106. [Google Scholar] [CrossRef]
- Rodríguez-Morán, M.; Simental-Mendía, L.E.; Gamboa-Gómez, C.I.; Guerrero-Romero, F. Oral Magnesium Supplementation and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Adv. Chronic Kidney Dis. 2018, 25, 261–266. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sahebkar, A.; Rodríguez-Morán, M.; Guerrero-Romero, F. Effect of magnesium supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2017, 73, 525–536. [Google Scholar] [CrossRef]
- Cosaro, E.; Bonafini, S.; Montagnana, M.; Danese, E.; Trettene, M.S.; Minuz, P.; Delva, P.; Fava, C. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1213–1220. [Google Scholar] [CrossRef]
- Firoz, M.; Graber, M. Bioavallability of US commercial magnesium preparations. Magnes. Res. 2001, 14, 257–262. [Google Scholar] [PubMed]
- Verhas, M.; De, V.; Guéronnière, L.; Grognet, J.-M.; Paternot, J.; Hermanne, A.; Van Den Winkel, P.; Gheldof, R.; Martin, P.; Fantino, M.; et al. Magnesium bioavailability from mineral water. A study in adult men. Eur. J. Clin. Nutr. 2002, 56, 442–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Gueux, E.; Tressol, J.C.; Mazur, A.; Rayssiguier, Y. Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes. Res. 2005, 18, 215–223. [Google Scholar] [PubMed]
- Uysal, N.; Kizildag, S.; Yuce, Z.; Guvendi, G.; Kandis, S.; Koc, B.; Karakilic, A.; Camsari, U.M.; Ates, M. Timeline (Bioavailability) of Magnesium Compounds in Hours: Which Magnesium Compound Works Best? Biol. Trace Elem. Res. 2019, 187, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.S.; Zobitz, M.M.; Poindexter, J.R.; Pak, C.Y.C. Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr. 1990, 9, 48–55. [Google Scholar] [CrossRef]
- Ates, M.; Kizildag, S.; Yuksel, O.; Hosgorler, F.; Yuce, Z.; Guvendi, G.; Kandis, S.; Karakilic, A.; Koc, B.; Uysal, N. Dose-Dependent Absorption Profile of Different Magnesium Compounds. Biol. Trace Elem. Res. 2019, 192, 244–251. [Google Scholar] [CrossRef]
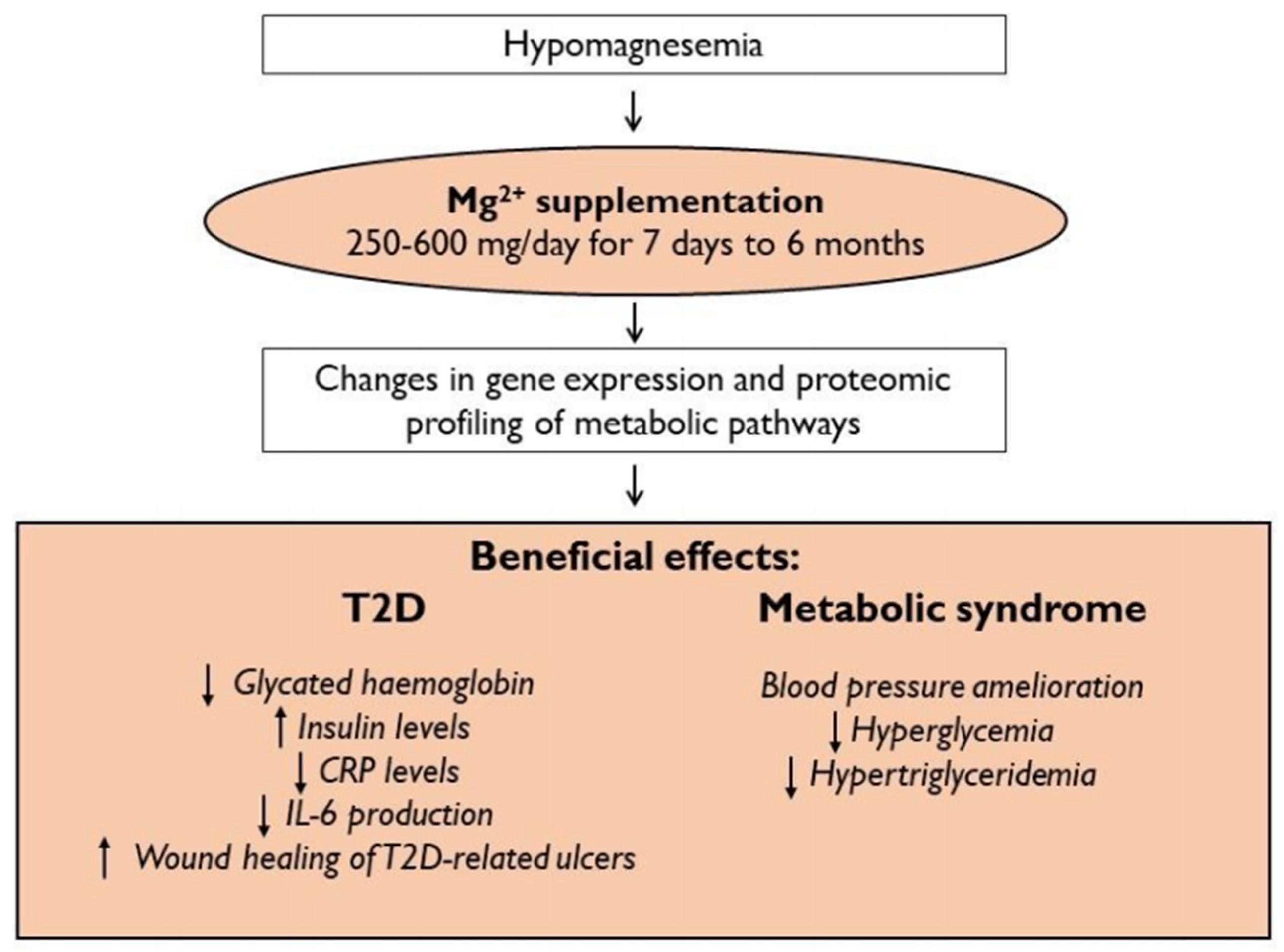
| Author(s) | Year | Dosage of Mg2+ Supplementation | Type of Salt | Timing of Mg2+ Supplementation | Effects of Mg2+ Supplementation | Ref. |
|---|---|---|---|---|---|---|
| Elderawi WA et al. | 2018 | 250 mg/day | Oxide, gluconate, lactate | 3 months | Improves glycemic control in T2D subjects with a reduction of glycated hemoglobin, insulin levels, C-peptide, and HOMA-IR. | [116] |
| Navarrete-Cortes A et al. | 2014 | 360 mg/day | Lactate | 3 months | No effects on insulin sensitivity. | [117] |
| Razzaghi R et al. | 2018 | 250 mg/day | Oxide | 12 weeks | Improves wound healing of diabetic foot ulcers, decreasing the lesion size, and ameliorating glucose metabolism. | [118] |
| Simental-Mendía LE et al. | 2012 | 380 mg/day | Chloride | 3 months | Reduces IL-6 plasmatic levels. | [119] |
| Steward CJ et al. | 2019 | 500 mg/day | Oxide, stearate | 7 days | Lowers IL-6 levels, reduces muscle soreness and increases post-exercise blood glucose. | [120] |
| Banjanin N et al. | 2018 | 300 mg/day | Oxide | 1 month | Decreases systolic and diastolic pressures, systemic vascular resistance, and left cardiac work. | [122] |
| Hatzistavri LS et al. | 2009 | 600 mg/day | Pidolate | 12 weeks | Reduces ambulatory blood pressure. | [123] |
| Rodríguez-Ramírez M et al. | 2017 | 360 mg/day | Lactate | 4 months | Increases TRPM6 mRNA relative expression. | [124] |
| Cunha AR et al. | 2017 | 600 mg twice a day | Chelate (not better specified) | 6 months | Improves endothelial function and subclinical atherosclerosis. | [125] |
| Mortazavi M et al. | 2013 | 440 mg 3 times per week | Oxide | 6 months | Decreases carotid intimate-media thickness, which is a marker of cardiovascular disease. | [126] |
| Joris PJ et al. | 2017 | 350 mg/day | Citrate | 24 weeks | No effect on endothelial function. | [127] |
| Rodríguez-Morán M et al. | 2018 | 380 mg/day | Chloride | 16 weeks | Improves MetS by reducing blood pressure, hyperglycemia, and hypertriglyceridemia. | [128] |
| Cosaro E et al. | 2014 | 370 mg twice a day | Pidolate | 8 weeks | Effects on blood pressure, vascular function, and glycolipid profile. | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. https://doi.org/10.3390/nu13020320
Piuri G, Zocchi M, Della Porta M, Ficara V, Manoni M, Zuccotti GV, Pinotti L, Maier JA, Cazzola R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients. 2021; 13(2):320. https://doi.org/10.3390/nu13020320
Chicago/Turabian StylePiuri, Gabriele, Monica Zocchi, Matteo Della Porta, Valentina Ficara, Michele Manoni, Gian Vincenzo Zuccotti, Luciano Pinotti, Jeanette A. Maier, and Roberta Cazzola. 2021. "Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes" Nutrients 13, no. 2: 320. https://doi.org/10.3390/nu13020320
APA StylePiuri, G., Zocchi, M., Della Porta, M., Ficara, V., Manoni, M., Zuccotti, G. V., Pinotti, L., Maier, J. A., & Cazzola, R. (2021). Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients, 13(2), 320. https://doi.org/10.3390/nu13020320










