Endoscopic Treatment of Obesity and Nutritional Aspects of Bariatric Endoscopy
Abstract
1. Introduction
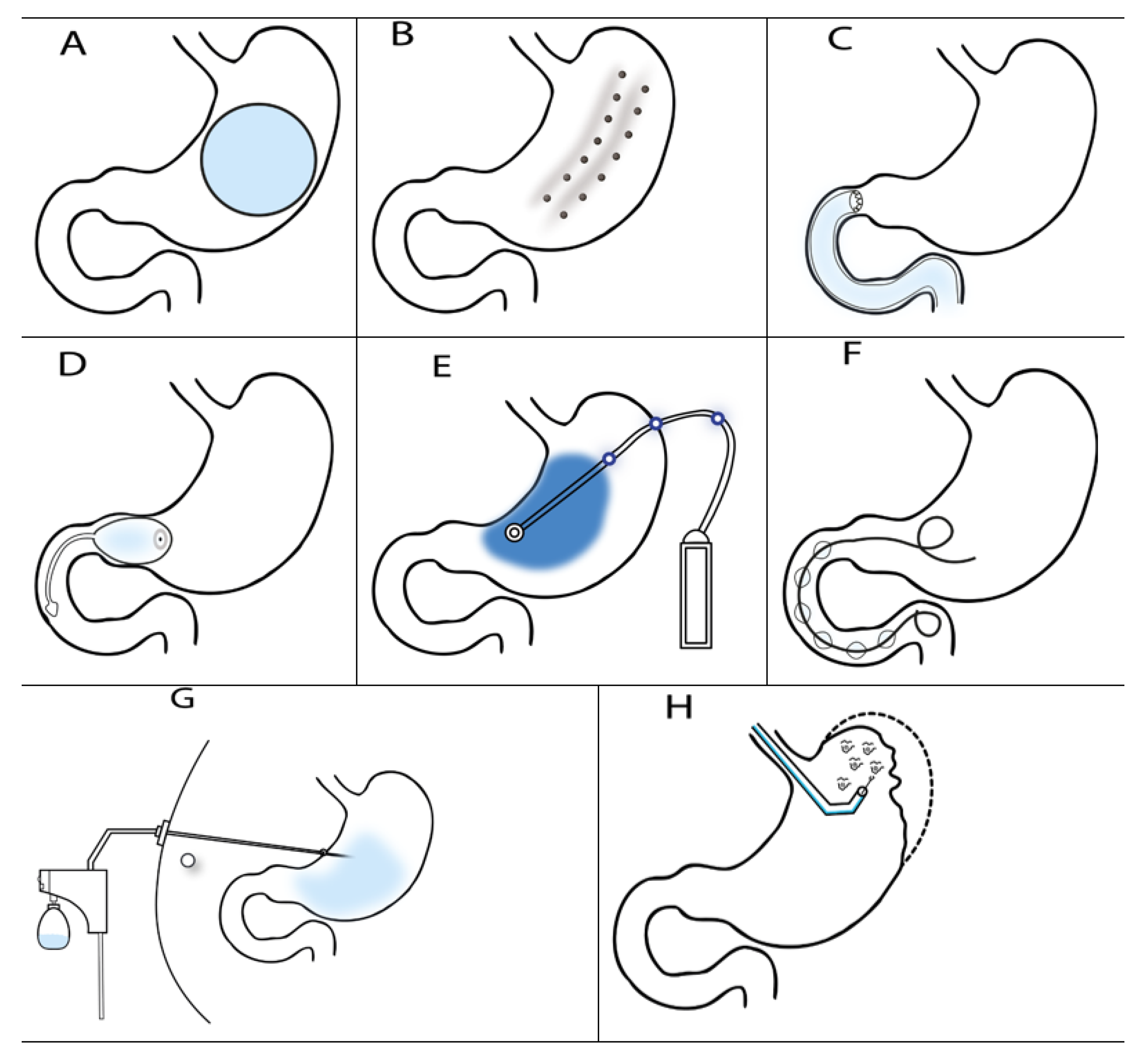
Endoscopy Bariatric Procedures
2. Procedures That Reduce Gastric Volume
2.1. Intragastric Balloons (IGB)
2.2. TransPyloric Shuttle (TPS)
2.3. SatiSphere
3. Restrictive Procedures
3.1. Endoscopic Sleeve Gastroplasty (ESG)
3.2. Primary Obesity Surgery Endoluminal (POSE)
3.3. Transoral Gastroplasty
4. Bypass Techniques
4.1. Duodenojejunal Bypass (DJB)
4.2. Gastroduodenojejunal Bypass
5. Aspiration Therapy
6. Gastric Electrical Stimulation (GES)
7. Other Methods
7.1. Duodenal Mucosa Resurfacing (DMR)
7.2. The Incisionless Magnetic Anastomotic System (IMAS)
7.3. Botulinum Toxin A
8. Nutrition
8.1. Nutrition and Dietary Recommendations for Bariatric Endoscopy
8.2. Specifics of Nutrition in Bariatric Endoscopic Surgery
8.3. Nutritional Deficiency
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- WHO Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 November 2021).
- Overweight and Obesity—BMI Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Overweight_and_obesity_-_BMI_statistics (accessed on 20 November 2021).
- Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 11 October 2021).
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e6. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Obesity. N. Engl. J. Med. 2002, 346, 591–602. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.E.; Mantzoros, C.S. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metab. Clin. Exp. 2019, 92, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 be a target for reward system related disorders? A Qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 2020, 14, 614884. [Google Scholar] [CrossRef] [PubMed]
- Eldar, S.; Heneghan, H.M.; Brethauer, S.A.; Schauer, P.R. Bariatric surgery for treatment of obesity. Int. J. Obes. 2011, 35, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Schauer, P.; Nguyen, N.T. Surgical approaches to the treatment of obesity: Bariatric surgery. Med. Clin. North Am. 2011, 95, 1009–1030. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Jung, S.H.; Yoon, J.H.; Choi, H.S.; Nam, S.J.; Kim, K.O.; Kim, D.H.; Kim, J.W.; Sohn, W.; Hyun, Y.S.; Park, C.H.; et al. Obesity, Comparative efficacy of bariatric endoscopic procedures in the treatment of morbid obesity: A systematic review and network meta-analysis. Endoscopy 2020, 52, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists—Executive summary. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [PubMed]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef]
- McCarty, T.R.; Thompson, C.C. The current state of bariatric endoscopy. Dig. Endosc. 2021, 33, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Tawadros, A.; Makar, M.; Kahaleh, M.; Sarkar, A. Overview of bariatric and metabolic endoscopy interventions. Ther. Adv. Gastrointest. Endosc. 2020, 13, 2631774520935239. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Edmundowicz, S.A.; Thompson, C.C. Endoscopic bariatric and metabolic therapies: New and emerging technologies. Gastroenterology 2017, 152, 1791–1801. [Google Scholar] [CrossRef]
- Rubino, F.; R′Bibo, S.L.; del Genio, F.; Mazumdar, M.; McGraw, T.E. Metabolic surgery: The role of the gastrointestinal tract in diabetes mellitus. Nat. Rev. Endocrinol 2010, 6, 102–109. [Google Scholar] [CrossRef][Green Version]
- Hadefi, A.; Huberty, V.; Lemmers, A.; Arvanitakis, M.; Maggs, D.; Costamagna, G.; Devière, J. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes. Dig. Dis. 2018, 36, 322–324. [Google Scholar] [CrossRef]
- Nieben, O.G.; Harboe, H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet 1982, 1, 198–199. [Google Scholar] [CrossRef]
- Velchik, M.G.; Kramer, F.M.; Stunkard, A.J.; Alavi, A. Effect of the Garren-Edwards gastric bubble on gastric emptying. J. Nucl. Med. 1989, 30, 692–696. [Google Scholar]
- Fittipaldi-Fernandez, R.J.; Zotarelli-Filho, I.J.; Diestel, C.F.; Klein, M.; de Santana, M.F.; de Lima, J.H.F.; Bastos, F.S.S.; Dos Santos, N.T. Intragastric Balloon: A Retrospective Evaluation of 5874 Patients on Tolerance, Complications, and Efficacy in Different Degrees of Overweight. Obes. Surg. 2020, 30, 4892–4898. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Asokkumar, R.; Rull, A.; Corbelle, F.; Beltran, L.; Bautista, I. Bariatric endoscopy procedure type or follow-up: What predicted success at 1 year in 962 obese patients? Endosc. Int. Open 2019, 7, E1691–W1698. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Rubio, M.A.; Prados, S.; Pastor, G.; Cruz, M.R.; Companioni, E.; Lopez, A. BioEnterics® intragastric balloon (BIB®). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes. Surg. 2011, 21, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Kotzampassi, K.; Grosomanidis, V.; Papakostas, P.; Penna, S.; Eleftheriadis, E. 500 intragastric balloons: What happens 5 years thereafter? Obes. Surg. 2012, 22, 896–903. [Google Scholar] [CrossRef]
- Suchartlikitwong, S.; Laoveeravat, P.; Mingbunjerdsuk, T.; Vutthikraivit, W.; Ismail, A.; Islam, S.; Islam, E. Usefulness of the ReShape intragastric balloon for obesity. Proceedings 2019, 32, 192–195. [Google Scholar] [CrossRef]
- Neylan, C.J.; Dempsey, D.T.; Tewksbury, C.M.; Williams, N.N.; Dumon, K.R. Endoscopic treatments of obesity: A comprehensive review. Surg. Obes. Relat. Dis. 2016, 12, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.M.; Geliebter, A. Intragastric balloon treatment for obesity: Review of recent studies. Adv. Ther. 2017, 34, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Pesta, C.M.; Bali, A.; Ibegbu, E.; Bazerbachi, F.; Moore, R.L.; Kumbhari, V.; Sharaiha, R.Z.; Curry, T.W.; DosSantos, G.; et al. Single fluid-filled intragastric balloon safe and effective for inducing weight loss in a real-world population. Clin. Gastroenterol. Hepatol. 2018, 16, 1073–1080.e1. [Google Scholar] [CrossRef]
- Fayad, L.; Cheskin, L.J.; Adam, A.; Badurdeen, D.S.; Hill, C.; Agnihotri, A.; Dunlap, M.; Simsek, C.; Khashab, M.A.; Kalloo, A.N.; et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: Efficacy, durability, and safety. Endoscopy 2019, 51, 532–539. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Edmundowicz, S.A.; Jonnalagadda, S.; Kumar, N.; Larsen, M.; Sullivan, S.; Thompson, C.C.; Banerjee, S.; Forc, A.B.E.T.; Comm, A.T. Endoscopic bariatric therapies. Gastrointest. Endosc. 2015, 81, 1073–1086. [Google Scholar] [CrossRef]
- Herve, J.; Wahlen, C.H.; Schaeken, A.; Dallemagne, B.; Dewandre, J.M.; Markiewicz, S.; Monami, B.; Weerts, J.; Jehaes, C. What becomes of patients one year after the intragastric balloon has been removed? Obes. Surg. 2005, 15, 864–870. [Google Scholar] [CrossRef]
- Marinos, G.; Eliades, C.; Muthusamy, V.R.; Greenway, F. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: Clinical results from a 3-and 6-month study. Surg. Obes. Relat. Dis. 2014, 10, 929–934. [Google Scholar] [CrossRef]
- Sauer, N.; Rosch, T.; Pezold, J.; Reining, F.; Anders, M.; Groth, S.; Schachschal, G.; Mann, O.; Aberle, J. A new endoscopically implantable device (SatiSphere) for treatment of obesity—efficacy, safety, and metabolic effects on glucose, insulin, and GLP-1 levels. Obes. Surg. 2013, 23, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Barrichello, S.; Hourneaux de Moura, D.T.; Hourneaux de Moura, E.G.; Jirapinyo, P.; Hoff, A.C.; Fittipaldi-Fernandez, R.J.; Baretta, G.; Felicio Lima, J.H.; Usuy, E.N.; de Almeida, L.S.; et al. Endoscopic sleeve gastroplasty in the management of overweight and obesity: An international multicenter study. Gastrointest. Endosc. 2019, 90, 770–780. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Galvao, M.P.; Bautista-Castano, I.; Fernandez-Corbelle, J.P.; Trell, M.; Lopez, N. Endoscopic Sleeve Gastroplasty for Obesity Treatment: Two Years of Experience. Arq. Bras. Cir. Dig. 2017, 30, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Huberty, V.; Boskoski, I.; Bove, V.; Van Ouytsel, P.; Costamagna, G.; Barthet, M.A.; Deviere, J. Endoscopic sutured gastroplasty in addition to lifestyle modification: Short-term efficacy in a controlled randomised trial. Gut 2020, 70, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Bautista-Castano, I.; Jimenez, A.; de Grado, T.; Fernandez-Corbelle, J.P. The Primary Obesity Surgery Endolumenal (POSE) procedure: One-year patient weight loss and safety outcomes. Surg. Obes. Relat. Dis. 2015, 11, 861–865. [Google Scholar] [CrossRef]
- Sullivan, S.; Swain, J.M.; Woodman, G.; Antonetti, M.; De La Cruz-Muñoz, N.; Jonnalagadda, S.S.; Ujiki, M.; Ikramuddin, S.; Ponce, J.; Ryou, M.; et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: The ESSENTIAL trial. J. Obes. 2017, 25, 294–301. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Asokkumar, R.; Turró Arau, R.; Neto, M.G.; Dayyeh, B.A. Modified primary obesity surgery endoluminal (POSE-2) procedure for the treatment of obesity. VideoGIE 2020, 5, 91–93. [Google Scholar] [CrossRef]
- Kumar, N. Endoscopic therapy for weight loss: Gastroplasty, duodenal sleeves, intragastric balloons, and aspiration. World J. Gastrointest. Endosc. 2015, 7, 847–859. [Google Scholar] [CrossRef]
- Deviere, J.; Ojeda Valdes, G.; Cuevas Herrera, L.; Closset, J.; Le Moine, O.; Eisendrath, P.; Moreno, C.; Dugardeyn, S.; Barea, M.; de la Torre, R.; et al. Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg. Endosc. 2008, 22, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Closset, J.; Dugardeyn, S.; Barea, M.; Mehdi, A.; Collignon, L.; Zalcman, M.; Baurain, M.; Le Moine, O.; Deviere, J. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: Results of the second human pilot study. Endoscopy 2008, 40, 406–413. [Google Scholar] [CrossRef]
- Koehestanie, P.; de Jonge, C.; Berends, F.J.; Janssen, I.M.; Bouvy, N.D.; Greve, J.W. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann. Surg. 2014, 260, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Forner, P.M.; Ramacciotti, T.; Farey, J.E.; Lord, R.V. Safety and Effectiveness of an Endoscopically Placed Duodenal-Jejunal Bypass Device (EndoBarrierA (R)): Outcomes in 114 Patients. Obes. Surg. 2017, 27, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Kaválková, P.; Mráz, M.; Trachta, P.; Kloučková, J.; Cinkajzlová, A.; Lacinová, Z.; Haluzíková, D.; Beneš, M.; Vlasáková, Z.; Burda, V.; et al. Endocrine effects of duodenal-jejunal exclusion in obese patients with type 2 diabetes mellitus. J. Endocrinol. 2016, 231, 11–22. [Google Scholar] [CrossRef]
- Sandler, B.J.; Rumbaut, R.; Swain, C.P.; Torres, G.; Morales, L.; Gonzales, L.; Schultz, S.; Talamini, M.A.; Jacobsen, G.R.; Horgan, S. One-year human experience with a novel endoluminal, endoscopic gastric bypass sleeve for morbid obesity. Surg. Endosc. 2015, 29, 3298–3303. [Google Scholar] [CrossRef]
- Norén, E.; Forssell, H. Aspiration therapy for obesity; a safe and effective treatment. BMC Obes. 2016, 3, 56. [Google Scholar] [CrossRef]
- Thompson, C.C.; Abu Dayyeh, B.K.; Kushnir, V.; Kushner, R.F.; Jirapinyo, P.; Schorr, A.B.; Aronne, L.J.; Amaro, A.; Jaffe, D.L.; Schulman, A.R.; et al. Aspiration therapy for the treatment of obesity: 4-year results of a multicenter randomized controlled trial. Surg. Obes. Relat. Dis. 2019, 15, 1348–1354. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, S.; Fang, L.; Yao, S.; Chen, J.D. Retrograde gastric electrical stimulation suppresses calorie intake in obese subjects. J. Obes. 2014, 22, 1447–1451. [Google Scholar] [CrossRef]
- Bohdjalian, A.; Ludvik, B.; Guerci, B.; Bresler, L.; Renard, E.; Nocca, D.; Karnieli, E.; Assalia, A.; Prager, R.; Prager, G. Improvement in glycemic control by gastric electrical stimulation (TANTALUS) in overweight subjects with type 2 diabetes. Surg. Endosc. 2009, 23, 1955–1960. [Google Scholar] [CrossRef]
- Cha, R.; Marescaux, J.; Diana, M. Updates on gastric electrical stimulation to treat obesity: Systematic review and future perspectives. World J. Gastrointest. Endosc. 2014, 6, 419–431. [Google Scholar] [CrossRef]
- Gniuli, D.; Calcagno, A.; Dalla Libera, L.; Calvani, R.; Leccesi, L.; Caristo, M.E.; Vettor, R.; Castagneto, M.; Ghirlanda, G.; Mingrone, G. High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia 2010, 53, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, H.; Cherrington, A.D.; Thompson, C.C.; Kaplan, L.M.; Rubino, F.; Mingrone, G.; Becerra, P.; Rodriguez, P.; Vignolo, P.; Caplan, J.; et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care 2016, 39, 2254–2261. [Google Scholar] [CrossRef]
- van Baar, A.C.G.; Holleman, F.; Crenier, L.; Haidry, R.; Magee, C.; Hopkins, D.; Rodriguez Grunert, L.; Galvao Neto, M.; Vignolo, P.; Hayee, B.; et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: One year results from the first international, open-label, prospective, multicentre study. Gut 2020, 69, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ye, L.S. Endoscopic applications of magnets for the treatment of gastrointestinal diseases. World J. Gastrointest. Endosc. 2019, 11, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Marrache, M.K.; Itani, M.I.; Farha, J.; Fayad, L.; Sharara, S.L.; Kalloo, A.N.; Khashab, M.A.; Kumbhari, V. Endoscopic gastrointestinal anastomosis: A review of established techniques. Gastrointest. Endosc. 2020, 93, 34–46. [Google Scholar] [CrossRef]
- Machytka, E.; Bužga, M.; Zonca, P.; Lautz, D.B.; Ryou, M.; Simonson, D.C.; Thompson, C.C. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest. Endosc. 2017, 86, 904–912. [Google Scholar] [CrossRef]
- Bang, C.S.; Baik, G.H.; Shin, I.S.; Kim, J.B.; Suk, K.T.; Yoon, J.H.; Kim, Y.S.; Kim, D.J. Effect of intragastric injection of botulinum toxin A for the treatment of obesity: A meta-analysis and meta-regression. Gastrointest. Endosc. 2015, 81, 1141–1149.e17. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Maggard, M.A.; Shugarman, L.R.; Suttorp, M.; Maglione, M.; Sugerman, H.J.; Livingston, E.H.; Nguyen, N.T.; Li, Z.; Mojica, W.A.; Hilton, L.; et al. Meta-analysis: Surgical treatment of obesity. Ann. Intern. Med. 2005, 142, 547–559. [Google Scholar] [CrossRef]
- Coupaye, M.; Puchaux, K.; Bogard, C.; Msika, S.; Jouet, P.; Clerici, C.; Larger, E.; Ledoux, S. Nutritional consequences of adjustable gastric banding and gastric bypass: A 1-year prospective study. Obes. Surg. 2009, 19, 56–65. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.J.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: A meta-analysis. Surg. Obes. Relat. Dis. 2014, 10, 589–597. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Person, B.; Szomstein, S.; Rosenthal, R.J. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part A: Vitamins. Obes. Surg. 2008, 18, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Kaidar-Person, O.; Person, B.; Szomstein, S.; Rosenthal, R.J. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part B: Minerals. Obes. Surg. 2008, 18, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; Boylan, M.; Sriram, K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010, 26, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg. Obes. Relat. Dis. 2013, 9, 159–191. [Google Scholar]
- Behrns, K.E.; Smith, C.D.; Sarr, M.G. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig. Dis. Sci. 1994, 39, 315–320. [Google Scholar] [CrossRef]
- Aarts, E.O.; Janssen, I.M.; Berends, F.J. The gastric sleeve: Losing weight as fast as micronutrients? Obes. Surg. 2011, 21, 207–211. [Google Scholar] [CrossRef][Green Version]
- Belfiore, A.; Cataldi, M.; Minichini, L.; Aiello, M.L.; Trio, R.; Rossetti, G.; Guida, B. Short-term changes in body composition and response to micronutrient supplementation after laparoscopic sleeve gastrectomy. Obes. Surg. 2015, 25, 2344–2351. [Google Scholar] [CrossRef]
- Punchai, S.; Hanipah, Z.N.; Meister, K.M.; Schauer, P.R.; Brethauer, S.A.; Aminian, A. Neurologic manifestations of vitamin b deficiency after bariatric surgery. Obes. Surg. 2017, 27, 2079–2082. [Google Scholar] [CrossRef]
- Lewis, C.A.; de Jersey, S.; Hopkins, G.; Hickman, I.; Osland, E. Does bariatric surgery cause vitamin a, b1, c or e deficiency? A systematic review. Obes. Surg. 2018, 28, 3640–3657. [Google Scholar] [CrossRef] [PubMed]
- Koffman, B.M.; Greenfield, L.J.; Ali, I.I.; Pirzada, N.A. Neurologic complications after surgery for obesity. Muscle Nerve 2006, 33, 166–176. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Friedrich, A.; Kramer, K.M.; Stingel, K.; Meile, T.; Küper, M.A.; Königsrainer, A.; Bischoff, S.C. Pre- and postoperative nutritional deficiencies in obese patients undergoing laparoscopic sleeve gastrectomy. Obes. Surg. 2012, 22, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Cambi, M.P.C.; Baretta, G.A.P.; Spagnol, M.; Zilio, R.; Rossoni, C. Systematization of nutritional care in endoscopic treatment for obesity. Obes. Surg. 2019, 29, 1074–1080. [Google Scholar] [CrossRef]
- Compher, C.W.; Badellino, K.O.; Boullata, J.I. Vitamin D and the bariatric surgical patient: A review. Obes. Surg. 2008, 18, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic burden of obesity: A systematic literature review. Int. J. Env. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
| Classification | BMI (kg/m2) |
|---|---|
| Underweight | <18.5 |
| Normal weight | 18.5–24.9 |
| Overweight | 25.0–29.9 |
| Obese class I | 30.0–34.9 |
| Obese class II | 35.0–39.9 |
| Obese class III | ≥40 |
| Type of Balloon | Volume | Filling | Material | Duration of Implantation | Form of Implantation |
|---|---|---|---|---|---|
| Orbera™ | 400–700 mL | normal saline with methylene blue | 6 months | Gastroscopic | |
| Heliosphere | 550 mL | air | 6 months | Gastroscopic | |
| Medsil | 400–700 mL | normal saline with methylene blue | 6 months | Gastroscopic | |
| ReShapeDuo™ | 900 mL | normal saline with methylene blue | 6 months | Gastroscopic | |
| Silimed | 250–700 mL | normal saline with methylene blue | 6 months | Gastroscopic | |
| Spatz™ | 700 mL max. | normal saline with methylene blue | 12 months | Gastroscopic | |
| Elipse® | 550 mL | normal saline with methylene blue | 3 months | swallowed balloon | |
| Obalon® | 250–450 mL | Air | 4 months | swallowed balloon | |
| Ullorex® | 300 mL | carbon dioxide | 1 month | swallowed balloon |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Král, J.; Machytka, E.; Horká, V.; Selucká, J.; Doleček, F.; Špičák, J.; Kovářová, V.; Haluzík, M.; Bužga, M. Endoscopic Treatment of Obesity and Nutritional Aspects of Bariatric Endoscopy. Nutrients 2021, 13, 4268. https://doi.org/10.3390/nu13124268
Král J, Machytka E, Horká V, Selucká J, Doleček F, Špičák J, Kovářová V, Haluzík M, Bužga M. Endoscopic Treatment of Obesity and Nutritional Aspects of Bariatric Endoscopy. Nutrients. 2021; 13(12):4268. https://doi.org/10.3390/nu13124268
Chicago/Turabian StyleKrál, Jan, Evžen Machytka, Veronika Horká, Jana Selucká, Filip Doleček, Julius Špičák, Viktorie Kovářová, Martin Haluzík, and Marek Bužga. 2021. "Endoscopic Treatment of Obesity and Nutritional Aspects of Bariatric Endoscopy" Nutrients 13, no. 12: 4268. https://doi.org/10.3390/nu13124268
APA StyleKrál, J., Machytka, E., Horká, V., Selucká, J., Doleček, F., Špičák, J., Kovářová, V., Haluzík, M., & Bužga, M. (2021). Endoscopic Treatment of Obesity and Nutritional Aspects of Bariatric Endoscopy. Nutrients, 13(12), 4268. https://doi.org/10.3390/nu13124268






