Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. PBMC Isolation and Stimulation
2.3. RNA-Seq Analysis
3. Results
3.1. Impact of Physiological Concentrations of 25(OH)D3 on the Transcriptome of PBMCs
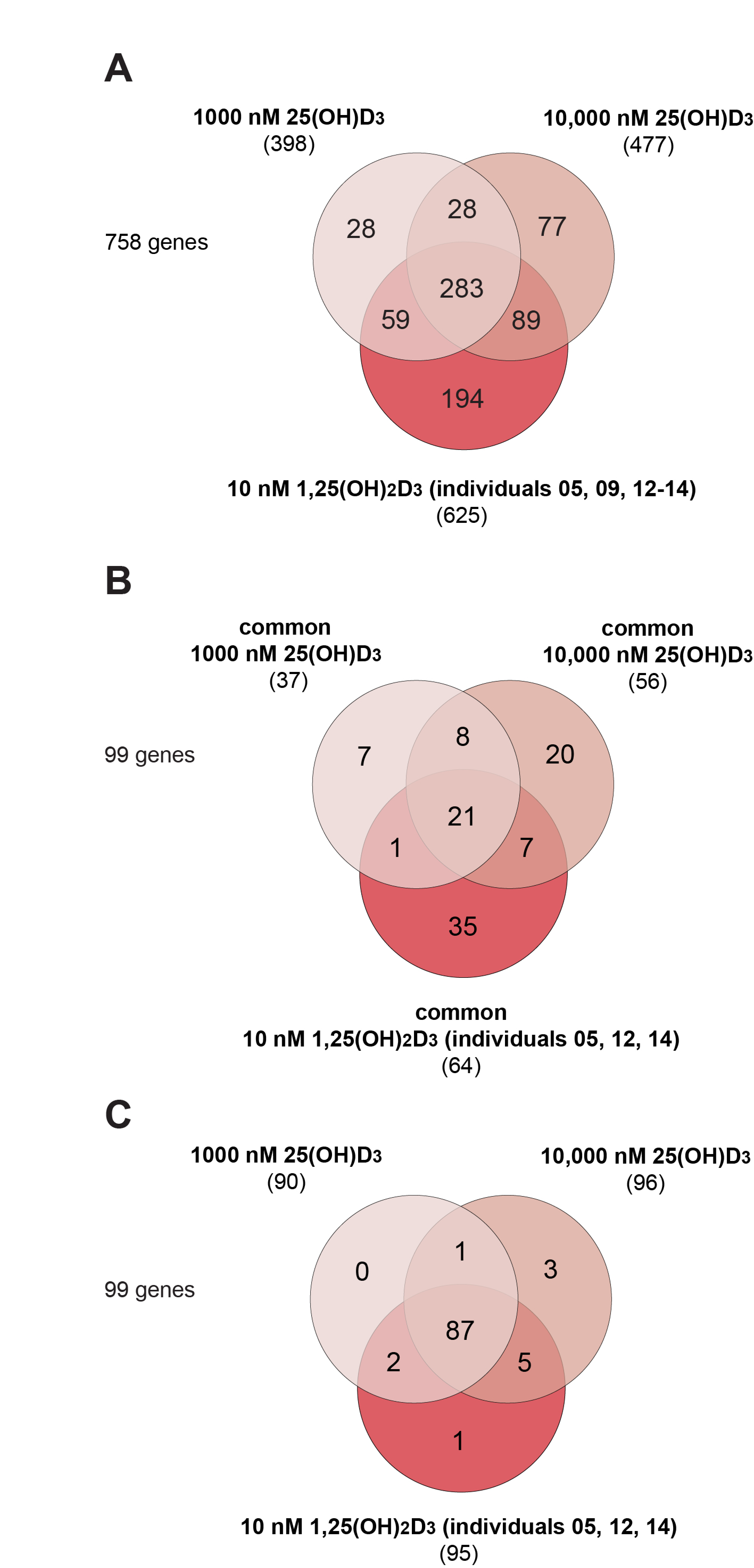
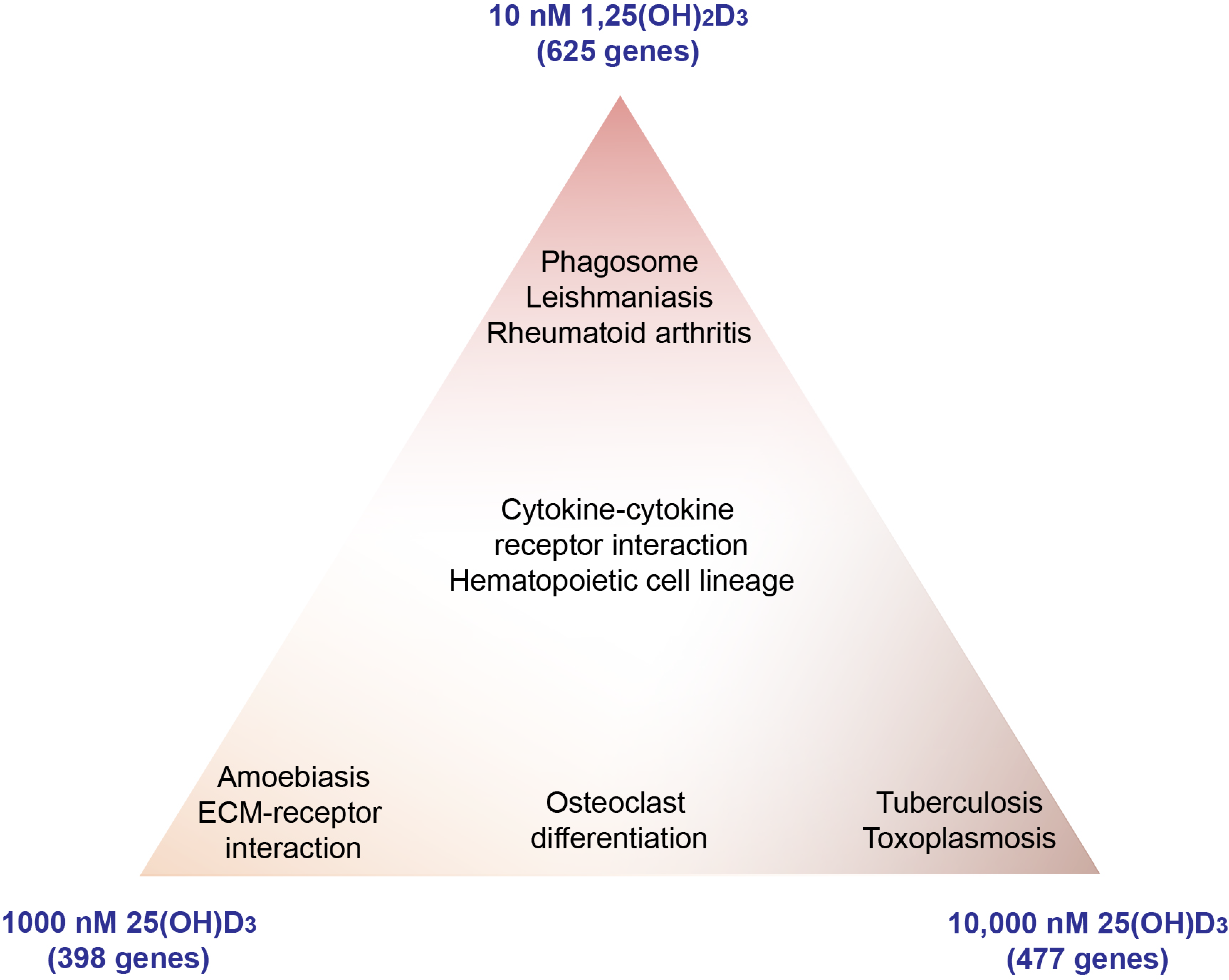
3.2. Effects of High Concentrations of 25(OH)D3 on the PBMC Transcriptome
3.3. Target Genes Specific to 25(OH)D3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogh, M.K.; Schmedes, A.V.; Philipsen, P.A.; Thieden, E.; Wulf, H.C. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J. Investig. Dermatol. 2010, 130, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat. Rev. Rheumatol. 2016, 12, 201–210. [Google Scholar] [CrossRef]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The role of vitamin D in Inflammatory bowel disease: Mechanism to management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.J.; Wang, X.H.; Liu, Z.D.; Cao, W.L.; Han, Y.; Ma, A.G.; Xu, S.F. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug. Des. Dev. Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its potential benefit for the COVID-19 pandemic. Endocr. Pract. 2021, 27, 484–493. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.C.; Slater, S.; Jurutka, P.W. Vitamin D receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008, 66, S98–S112. [Google Scholar] [CrossRef]
- Evans, R.M. The nuclear receptor superfamily: A rosetta stone for physiology. Mol. Endocrinol. 2005, 19, 1429–1438. [Google Scholar] [CrossRef]
- Campbell, M.J. Vitamin D and the RNA transcriptome: More than mRNA regulation. Front. Physiol. 2014, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C. Vitamin D genomics: From in vitro to in vivo. Front. Endocrinol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D signaling in the context of innate immunity: Focus on human monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef] [Green Version]
- Neme, A.; Seuter, S.; Malinen, M.; Nurmi, T.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019, 188, 71–76. [Google Scholar] [CrossRef]
- Hanel, A.; Neme, A.; Malinen, M.; Hamalainen, E.; Malmberg, H.R.; Etheve, S.; Tuomainen, T.P.; Virtanen, J.K.; Bendik, I.; Carlberg, C. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 2020, 10, 21051. [Google Scholar] [CrossRef]
- Carlberg, C.; Seuter, S.; de Mello, V.D.; Schwab, U.; Voutilainen, S.; Pulkki, K.; Nurmi, T.; Virtanen, J.; Tuomainen, T.P.; Uusitupa, M. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS ONE 2013, 8, e71042. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Wilhelm, F.; Mayer, E.; Norman, A.W. Biological activity assessment of the 26,23-lactones of 1,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 and their binding properties to chick intestinal receptor and plasma vitamin D binding protein. Arch. Biochem. Biophys. 1984, 233, 322–329. [Google Scholar] [CrossRef]
- Kutner, A.; Link, R.P.; Schnoes, H.K.; DeLuca, H.F. Photoactivable analogs for labeling 25-hydroxyvitamin D3 serum binding protein and for 1,25-dihydroxyvitamin D3 intestinal receptor protein. Bioorg. Chem. 1986, 14, 134–147. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Lou, Y.R.; Molnár, F.; Peräkylä, M.; Qiao, S.; Kalueff, A.V.; St-Arnaud, R.; Carlberg, C.; Tuohimaa, P. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J. Steroid Biochem. Mol. Biol. 2010, 118, 162–170. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lun, A.T.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Res 2016, 5, 1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Tarca, A.L.; Draghici, S.; Khatri, P.; Hassan, S.S.; Mittal, P.; Kim, J.S.; Kim, C.J.; Kusanovic, J.P.; Romero, R. A novel signaling pathway impact analysis. Bioinformatics 2009, 25, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Vanhaelen, Q.; Aliper, A.M.; Zhavoronkov, A. A comparative review of computational methods for pathway perturbation analysis: Dynamical and topological perspectives. Mol. Biosyst. 2017, 13, 1692–1704. [Google Scholar] [CrossRef]
- Ryynänen, J.; Neme, A.; Tuomainen, T.P.; Virtanen, J.K.; Voutilainen, S.; Nurmi, T.; de Mello, V.D.; Uusitupa, M.; Carlberg, C. Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Mol. Nutr. Food Res. 2014, 58, 2036–2045. [Google Scholar] [CrossRef]
- Heikkinen, S.; Väisänen, S.; Pehkonen, P.; Seuter, S.; Benes, V.; Carlberg, C. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011, 39, 9181–9193. [Google Scholar] [CrossRef] [Green Version]
- Vukic, M.; Neme, A.; Seuter, S.; Saksa, N.; de Mello, V.D.; Nurmi, T.; Uusitupa, M.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE 2015, 10, e0124339. [Google Scholar] [CrossRef] [Green Version]
- Kreienkamp, R.; Croke, M.; Neumann, M.A.; Bedia-Diaz, G.; Graziano, S.; Dusso, A.; Dorsett, D.; Carlberg, C.; Gonzalo, S. Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes. Oncotarget 2016, 7, 30018–30031. [Google Scholar] [CrossRef] [Green Version]
- Nurminen, V.; Seuter, S.; Carlberg, C. Primary vitamin D target genes of human monocytes. Front. Physiol. 2019, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- de Jong, T.V.; Moshkin, Y.M.; Guryev, V. Gene expression variability: The other dimension in transcriptome analysis. Physiol. Genomics 2019, 51, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Hangelbroek, R.W.J.; Vaes, A.M.M.; Boekschoten, M.V.; Verdijk, L.B.; Hooiveld, G.; van Loon, L.J.C.; de Groot, L.; Kersten, S. No effect of 25-hydroxyvitamin D supplementation on the skeletal muscle transcriptome in vitamin D deficient frail older adults. BMC Geriatr. 2019, 19, 151. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Väisänen, S.; Ryhänen, S.; Saarela, J.T.; Peräkylä, M.; Andersin, T.; Mäenpää, P.H. Structurally and functionally important amino acids of the agonistic conformation of the human vitamin D receptor. Mol. Pharmacol. 2002, 62, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. Crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef]
- Gill, H.S.; Londowski, J.M.; Corradino, R.A.; Zinsmeister, A.R.; Kumar, R. Synthesis and biological activity of novel vitamin D analogues: 24,24-difluoro-25-hydroxy-26,27-dimethylvitamin D3 and 24,24-difluoro-1α,25-dihydroxy-26,27-dimethylvitamin D3. J. Med. Chem. 1990, 33, 480–490. [Google Scholar] [CrossRef]
- Tocchini-Valentini, G.; Rochel, N.; Wurtz, J.M.; Mitschler, A.; Moras, D. Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc. Natl. Acad. Sci. USA 2001, 98, 5491–5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehkonen, P.; Welter-Stahl, L.; Diwo, J.; Ryynanen, J.; Wienecke-Baldacchino, A.; Heikkinen, S.; Treuter, E.; Steffensen, K.R.; Carlberg, C. Genome-wide landscape of liver X receptor chromatin binding and gene regulation in human macrophages. BMC Genomics 2012, 13, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddington, K.E.; Robinson, G.A.; Rubio-Cuesta, B.; Chrifi-Alaoui, E.; Andreone, S.; Poon, K.S.; Ivanova, I.; Martin-Gutierrez, L.; Owen, D.M.; Jury, E.C.; et al. LXR directly regulates glycosphingolipid synthesis and affects human CD4+ T cell function. Proc. Natl. Acad. Sci. USA 2021, 118, e2017394118. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sato, R.; Brown, M.S.; Hua, X.; Goldstein, J.L. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell 1994, 77, 53–62. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Sun, L.P.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Direct binding of cholesterol to the purified membrane region of SCAP: Mechanism for a sterol-sensing domain. Mol. Cell 2004, 15, 259–268. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D metabolite, 25-hydroxyvitamin D, regulates lipid metabolism by inducing degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef] [Green Version]


| Individual Number | Treatment | Concentration (nM) | Target Genes Total | Target Genes Up | Target Genes Down | Genes Expressed |
|---|---|---|---|---|---|---|
| 05 | Vitamin D3 | 250 | 0 | 0 | 0 | 13,284 |
| 05 | 25(OH)D3 | 250 | 0 | 0 | 0 | 13,284 |
| 05 | 1,25(OH)2D3 | 10 | 382 | 122 | 260 | 13,284 |
| 09 | Vitamin D3 | 250 | 0 | 0 | 0 | 12,742 |
| 09 | 25(OH)D3 | 250 | 0 | 0 | 0 | 12,742 |
| 09 | 1,25(OH)2D3 | 10 | 235 | 57 | 178 | 12,742 |
| 12 | Vitamin D3 | 250 | 0 | 0 | 0 | 13,337 |
| 12 | 25(OH)D3 | 250 | 0 | 0 | 0 | 13,337 |
| 12 | 1,25(OH)2D3 | 10 | 377 | 131 | 246 | 13,337 |
| 13 | Vitamin D3 | 250 | 0 | 0 | 0 | 12,530 |
| 13 | 25(OH)D3 | 250 | 0 | 0 | 0 | 12,530 |
| 13 | 1,25(OH)2D3 | 10 | 256 | 53 | 203 | 12,530 |
| 14 | Vitamin D3 | 250 | 0 | 0 | 0 | 13,140 |
| 14 | 25(OH)D3 | 250 | 0 | 0 | 0 | 13,140 |
| 14 | 1,25(OH)2D3 | 10 | 83 | 20 | 63 | 13,140 |
| Individual Number | Treatment | Concentration (nM) | Target Genes Total | Target Genes Up | Target Genes Down | Genes Expressed |
|---|---|---|---|---|---|---|
| 05 | 25(OH)D3 | 100 | 0 | 0 | 0 | 13,284 |
| 05 | 25(OH)D3 | 1000 | 332 | 122 | 210 | 13,284 |
| 05 | 25(OH)D3 | 10,000 | 386 | 90 | 296 | 13,284 |
| 12 | 25(OH)D3 | 100 | 0 | 0 | 0 | 13,337 |
| 12 | 25(OH)D3 | 1000 | 265 | 99 | 166 | 13,337 |
| 12 | 25(OH)D3 | 10,000 | 341 | 63 | 278 | 13,337 |
| 14 | 25(OH)D3 | 100 | 0 | 0 | 0 | 13,140 |
| 14 | 25(OH)D3 | 1000 | 47 | 15 | 32 | 13,140 |
| 14 | 25(OH)D3 | 10,000 | 66 | 14 | 52 | 13,140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanel, A.; Bendik, I.; Carlberg, C. Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood. Nutrients 2021, 13, 4100. https://doi.org/10.3390/nu13114100
Hanel A, Bendik I, Carlberg C. Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood. Nutrients. 2021; 13(11):4100. https://doi.org/10.3390/nu13114100
Chicago/Turabian StyleHanel, Andrea, Igor Bendik, and Carsten Carlberg. 2021. "Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood" Nutrients 13, no. 11: 4100. https://doi.org/10.3390/nu13114100
APA StyleHanel, A., Bendik, I., & Carlberg, C. (2021). Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood. Nutrients, 13(11), 4100. https://doi.org/10.3390/nu13114100








