Effects of Plant-Based Diets on Weight Status in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
2.3. Selection Process and Quality Assessment
2.4. Data Extraction
2.5. Quantitative Data Synthesis
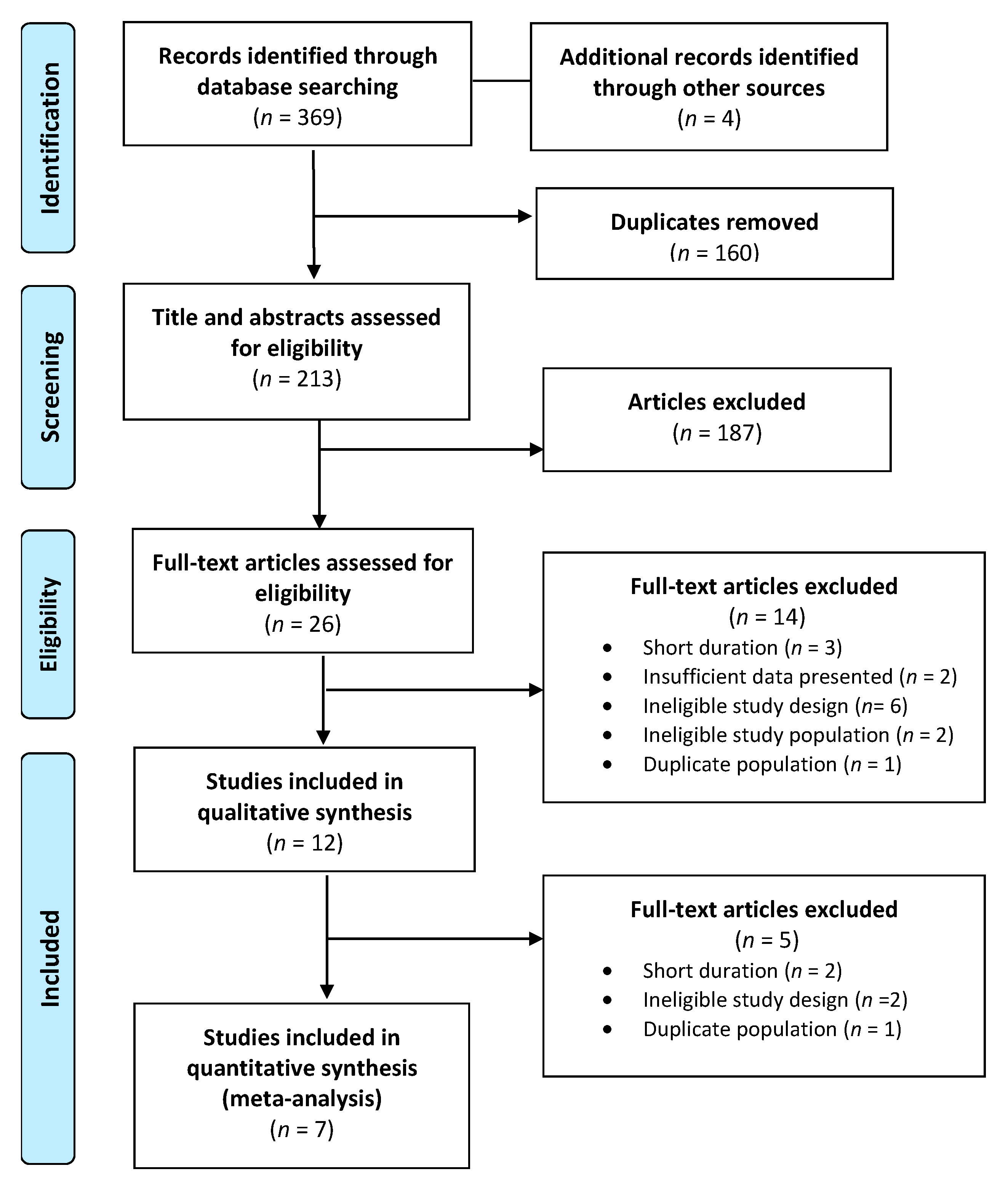
3. Results
3.1. Overview of Publications
3.2. Characteristics of Publications
3.3. Study Quality
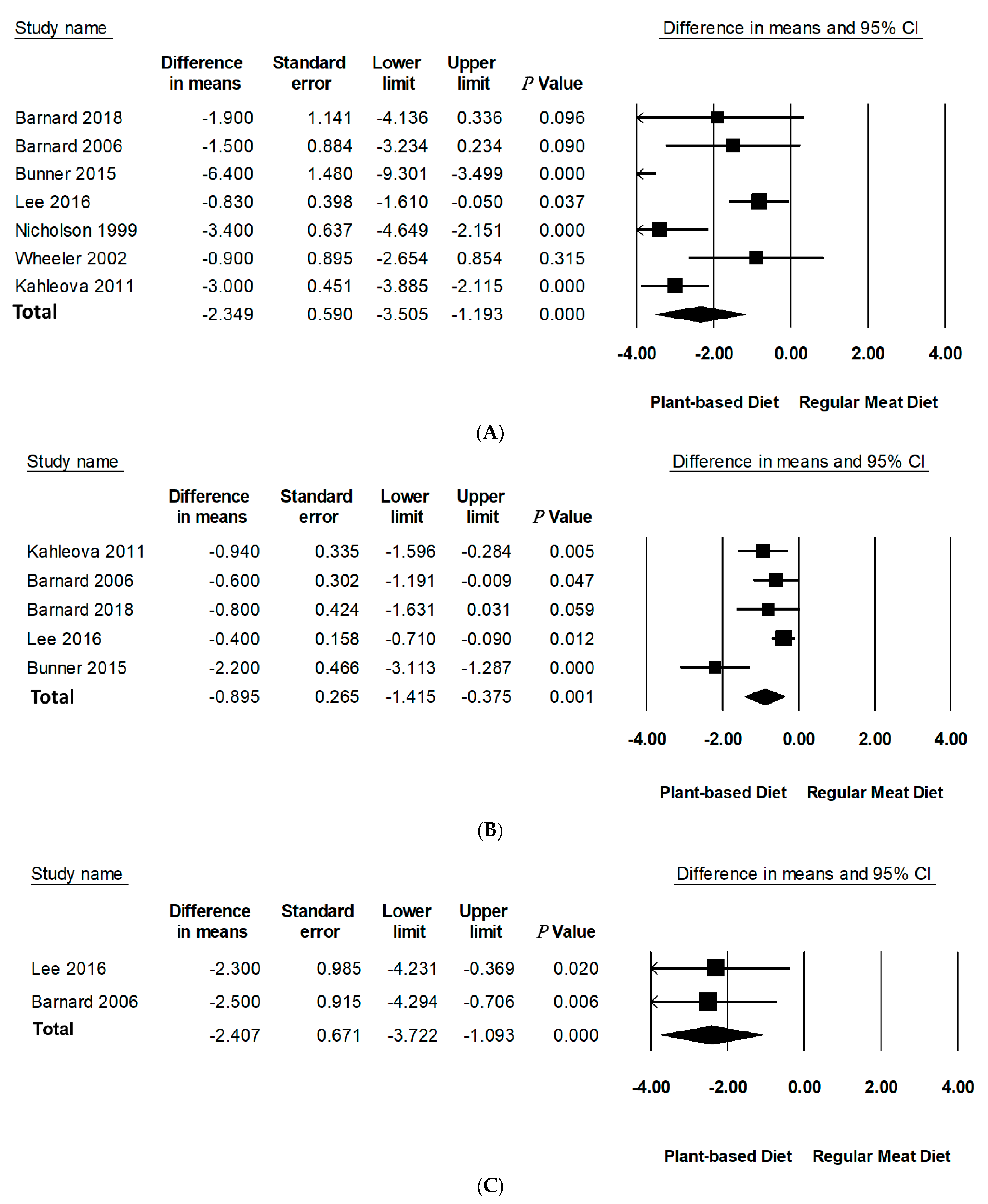
3.4. Effect of PBDs on Body Weight, BMI, and WC in Individuals with T2D
3.5. Publication Bias
3.6. Subgroup Analyses
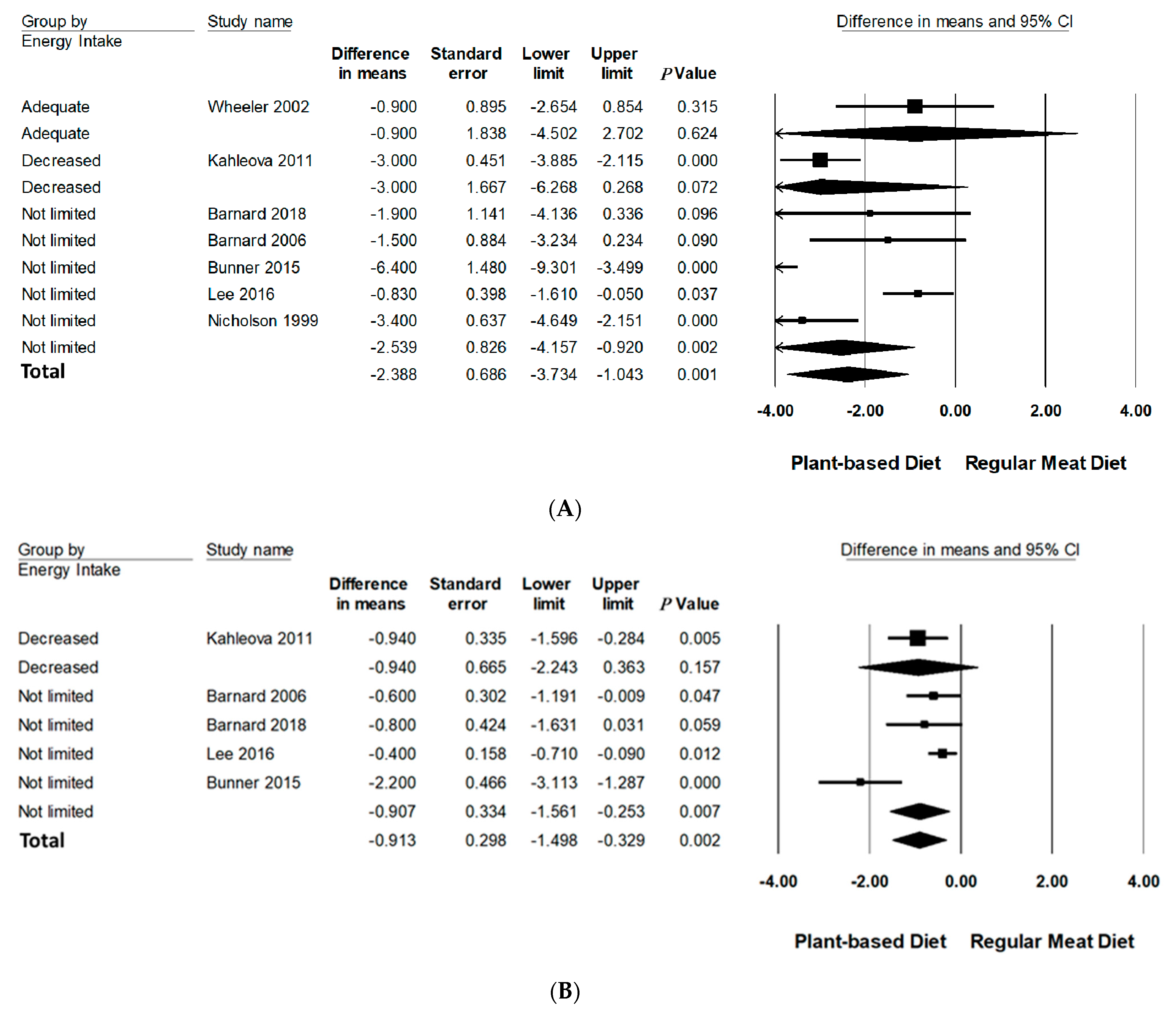
3.6.1. Type of PBD
3.6.2. Energy Intake
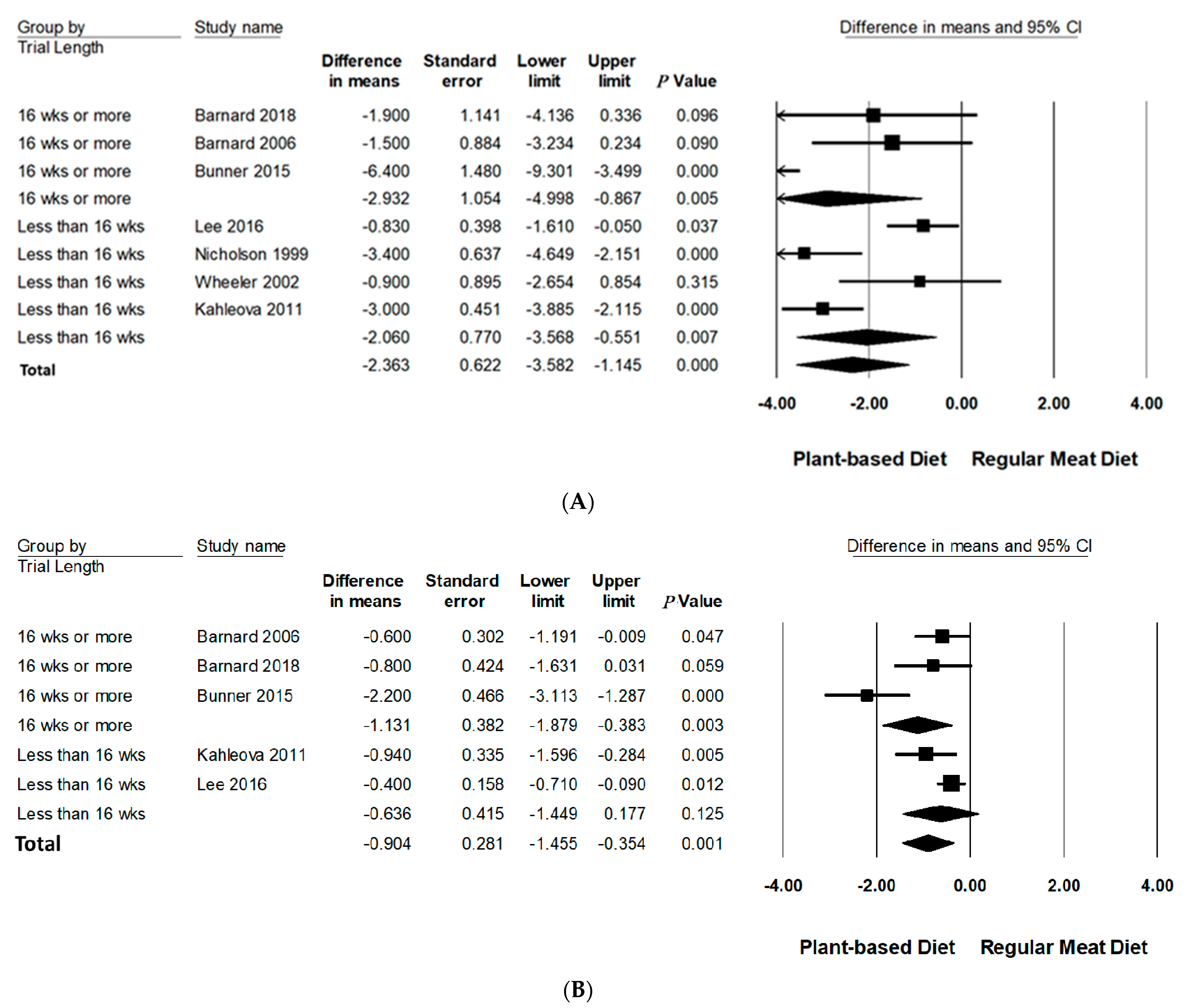
3.6.3. Trial Duration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Australia, D. Diabeties Globally: The National Diabeties Service Scheme. 2021. Available online: https://www.diabetesaustralia.com.au/about-diabetes/diabetes-globally/#:~:text=The%20number%20of%20people%20with,on%20November%2014%20each%20year (accessed on 14 February 2021).
- International Diabetes Federation. IDF Diabetes Atlas 9th edition Diabetes Atlas. 2019. Available online: http://www.diabetesatlas.org/ (accessed on 20 October 2019).
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Ley, S.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2017, 14, 88–98. [Google Scholar] [CrossRef]
- Obesity and Overweight—Facts: WHO. 2003. Available online: https://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf (accessed on 20 May 2021).
- The Royal Australian College of General Practitioners. Management of of Type 2 Diabetes. Available online: https://www.racgp.org.au/getattachment/41fee8dc-7f97-4f87-9d90-b7af337af778/Management-of-type-2-diabetes-A-handbook-for-general-practice.aspx (accessed on 9 June 2021).
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Lea, E.; Worsley, A. Benefits and barriers to the consumption of a vegetarian diet in Australia. Public Health Nutr. 2003, 6, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Corrin, T.; Papadopoulos, A. Understanding the attitudes and perceptions of vegetarian and plant-based diets to shape future health promotion programs. Appetite 2017, 109, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.L.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diabetes Rep. 2018, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; I Katcher, H.; Jenkins, D.J.; Cohen, J.; Turner-McGrievy, G. Vegetarian and vegan diets in type 2 diabetes management. Nutr. Rev. 2009, 67, 255–263. [Google Scholar] [CrossRef]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A Systematic Review and Meta-Analysis of Changes in Body Weight in Clinical Trials of Vegetarian Diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Toumpanakis, A.; Turnbull, T.; Alba-Barba, I. Effectiveness of plant-based diets in promoting well-being in the management of type 2 diabetes: A systematic review. BMJ Open Diabetes Res. Care 2018, 6, e000534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern Med. 2014, 174, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern. Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, G.; Azevedo, K.P.M.; Mesquita, G.X.B.; Lima, S.; Silva, D.F.O.; Pimenta, I.; Gonccalves, A.; Lyra, C.d.O.; Piuvezam, G. Red meat consumption, risk of incidence of cardiovascular disease and cardiovascular mortality, and the dose-response effect: Protocol for a systematic review and meta-analysis of longitudinal cohort studies. Medicine 2019, 98, e17271. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-Y.; Huang, C.-C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.W.; Kendall, C.W.; Jenkins, D.J. Importance of Weight Management in Type 2 Diabetes: Review with Meta-analysis of Clinical Studies. J. Am. Coll. Nutr. 2003, 22, 331–339. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2. 2021. Available online: https://training.cochrane.org/handbook/current (accessed on 10 August 2021).
- Evidence Analysis Manual: Steps in the Academy Analysis Process Academy of Nutrition and Dietetics. 2016. Available online: https://www.andeal.org/evidenceanalysis (accessed on 10 August 2021).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.; Identifying and Measuring Heterogeneity. Cochrane Handbook for Systematic Reviews of Interventions Version 6 Cochrane. 2009. Available online: www.training.cochrane.org/handbook (accessed on 12 August 2021).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Levin, S.M.; Gloede, L.; Flores, R. Turning the Waiting Room into a Classroom: Weekly Classes Using a Vegan or a Portion-Controlled Eating Plan Improve Diabetes Control in a Randomized Translational Study. J. Acad. Nutr. Diet. 2018, 118, 1072–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, A.S.; Sklar, M.; Barnard, N.D.; Gore, S.; Sullivan, R.; Browning, S. Toward Improved Management of NIDDM: A Randomized, Controlled, Pilot Intervention Using a Lowfat, Vegetarian Diet. Prev. Med. 1999, 29, 87–91. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, S.-A.; Lee, I.-K.; Kim, J.-G.; Park, K.-G.; Jeong, J.-Y.; Jeon, J.-H.; Shin, J.-Y.; Lee, D.-H. Effect of a Brown Rice Based Vegan Diet and Conventional Diabetic Diet on Glycemic Control of Patients with Type 2 Diabetes: A 12-Week Randomized Clinical Trial. PLoS ONE 2016, 11, e0155918. [Google Scholar] [CrossRef] [Green Version]
- Bunner, A.; Wells, C.L.; Gonzales, J.C.; Agarwal, U.; Bayat, E.; Barnard, N.D. A dietary intervention for chronic diabetic neuropathy pain: A randomized controlled pilot study. Nutr. Diabetes 2015, 5, e158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A Low-Fat Vegan Diet Improves Glycemic Control and Cardiovascular Risk Factors in a Randomized Clinical Trial in Individuals With Type 2 Diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.L.; Fineberg, S.E.; Fineberg, N.S.; Gibson, R.G.; Hackward, L.L. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria: Effects on renal, glycemic, and lipid parameters. Diabetes Care 2002, 25, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- A Ferguson, J.J.; Oldmeadow, C.; Mishra, G.D.; Garg, M.L. Plant-based dietary patterns are associated with lower body weight, BMI and waist circumference in older Australian women. Public Health Nutr. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- A Spencer, E.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int. J. Obes. 2003, 27, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Newby, P.K.; Tucker, K.; Wolk, A. Risk of overweight and obesity among semivegetarian, lactovegetarian, and vegan women. Am. J. Clin. Nutr. 2005, 81, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Moore, S.C.; Jacobs, E.J.; Kitahara, C.M.; Rosenberg, P.S.; Adami, H.O.; Ebbert, J.O.; English, D.R.; Gapstur, S.M.; Giles, G.G.; et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014, 89, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benatar, J.R.; Stewart, R.A.H. Cardiometabolic risk factors in vegans; A meta-analysis of observational studies. PLoS ONE. 2018, 13, e0209086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, S.A. A Vegetarian-Style Dietary Pattern Is Associated with Lower Energy, Saturated Fat, and Sodium Intakes; and Higher Whole Grains, Legumes, Nuts, and Soy Intakes by Adults: National Health and Nutrition Examination Surveys 2013–2016. Nutrients 2020, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Koh-Banerjee, P.; Rimm, E.B. Whole grain consumption and weight gain: A review of the epidemiological evidence, potential mechanisms and opportunities for future research. Proc. Nutr. Soc. 2003, 62, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, D. Dietary Glycemic Index and Obesity. J. Nutr. 2000, 130, 280S–283S. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Kendall, C.W.; Kahleová, H.; Rahelić, D.; Salas-Salvadó, J.; Choo, V.L.; Mejia, S.B.; Stewart, S.E.; Leiter, L.A.; Jenkins, D.J.; et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1133–1145. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Davidson, C.; Wingard, E.E.; Wilcox, S.; Frongillo, E.A. Comparative effectiveness of plant-based diets for weight loss: A randomized controlled trial of five different diets. Nutrients 2015, 31, 350–358. [Google Scholar] [CrossRef]
- Mihrshahi, S.; Ding, D.; Gale, J.; Allman-Farinelli, M.; Banks, E.; Bauman, A.E. Vegetarian diet and all-cause mortality: Evidence from a large population-based Australian cohort—The 45 and Up Study. Prev. Med. 2017, 97, 1–7. [Google Scholar] [CrossRef]
| Parameter | Study Selection Criteria |
|---|---|
| Population | Adults with T2D (≥18 years of age) |
| Intervention | PBD interventions (‘semi-vegetarian’, ‘pesco-vegetarian’, ‘lacto-ovo vegetarian’, and ‘vegan’) 1 |
| Comparator | Dietary patterns including meat (‘regular meat eaters’) 2 |
| Outcomes | Weight status (body weight (kg), BMI, WC) |
| Study design | Randomised controlled trials and prospective cohort studies published in English for ≥6 weeks. |
| Participant Characteristics | Treatment Characteristics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference (Quality Score a) | Location, Study Design | Sample Size (% Male) | Mean Age (y) | Baseline wt b | Dietary Intervention Type c | Intervention Delivery d | CHO:PRO:FAT e | Energy Intake f | Total Energy (kcal)(SD) j | Energy Diff (kcal)(SD) k | Length (wks) | Outcomes |
| Barnard et al. (2018) [30] (+) Intervention Control | United States, parallel | 21 (38) 24 (54) | 61.0 61.0 | 34.9 33.0 | VeganPortion-controlled | Dietary advice | 71:14:1850:21:30 | Not limited Decreased | 1491 (129) 1332 (85) | −204 (95) * −305 (100) * | 20 | Wt, BMI |
| Barnard et al. (2006) [34] (+) Intervention Control | United States, parallel | 49 (45) 50 (34) | 56.7 54.6 | 33.9 35.9 | Vegan ADA guidelines | Dietary advice | 75:15:10 70:20:10 | Not limited Decreased g | 1425 (427)1391 (382) | −334 (41) * −455 (215) * | 22 | Wt, BMI, WC |
| Bunner et al. (2015) [33] (+) Intervention Control | United States, parallel | 17 (35) 17 (53) | 57.0 58.0 | 35.9 36.2 | Vegan Usual diet | Dietary advice | NR NR | Not limited h Not limited | - | - | 20 | Wt, BMI |
| Kahleova et al. (2011) [29] (+) Intervention Control | Czech Republic parallel | 37 (46) 37 (49) | 54.6 57.7 | 35.1 35.0 | Lacto-ovo veg DNSG guidelines | Dietary advice | 60:15:25 50:20:30 | Decreased Decreased | 1736 1795 | −99 (438) * −37 (837) * | 12 | Wt, BMI, WC |
| Lee et al. (2016) [32] (+) Intervention Control | South Korea, parallel | 46 (13) 47 (25) | 57.5 58.3 | 23.9 23.1 | VeganKDA guidelines | Dietary advice | NR 60:20:25 | Not limited Adequate | 1409 (549) 1526 (314) | −71 (281) −67 (301) | 12 | Wt, BMI, WC |
| Nicholson et al. (1999) [31] (ø) Intervention Control | Unites States, parallel | 7 (50) 4 (57) | 51.0 60.0 | 97.7 97.0 | Vegan Low-fat diet | Meal supps | 75:15:10 60:15:25 | Not limited i Not limited | 1409 (549) 1526 (314) | −274 (114) +96 (89) | 12 | Wt |
| Wheeler et al. (2002) [35] (+) Intervention Control | Unites States, cross-over | 17 (82) 17 (82) | 56 56 | 33.1 33.1 | Vegan Animal protein | Meal supps | 53:17:30 53:17:30 | Adequate Adequate | - | - | 6 | Wt |
| Outcome | Subgroup | No. of Intervention Groups | No. Participant (Intervention/Control) | MD (95% CI) a | p | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| I2 | P c | %Change b | ||||||
| Body weight (kg) | Within PBD group change | 7 | 192 | −4.95(−7.34 to −2.55) | <0.001 | 97.04 | <0.001 | 5.1% d |
| Between group differences | 7 | 192/192 | −2.35(−3.51 to −1.19) | <0.001 | 78.63 | <0.001 | 2.4% d | |
| PBD Type | ||||||||
| Lacto-ovo veg | 1 | 37/37 | −3.00(−5.97 to −0.32) | <0.05 | - | - | 2.5% | |
| Vegan | 6 | 155/155 | −2.23(−3.60 to −0.87) | =0.001 | 78.12 | <0.001 | 2.3% | |
| Energy Intake | ||||||||
| Not limited | 5 | 138/138 | −2.54(−4.16 to −0.92) | <0.001 | 87.60 | <0.005 | 2.4% | |
| Adequate | 1 | 17/17 | −0.90 (−4.50 to 2.70) | 0.62 | - | - | - | |
| Decreased | 1 | 37/37 | −3.00 (−6.27 to −0.27) | 0.072 | - | - | - | |
| Trial length | ||||||||
| <16 weeks | 4 | 107/104 | −2.06(−3.57 to −0.55) | <0.01 | 84.91 | <0.001 | 2.1% | |
| ≥16 weeks | 3 | 85/88 | −2.93(−5.00 to −0.87) | =0.005 | 76.46 | <0.05 | 3.0% | |
| BMI (kg/m2) | Within PBD group change | 5 | 168 | −1.87(−2.78 to −0.95) | <0.001 | 94.95 | =0.001 | 5.4% |
| Between group differences | 5 | 168/171 | −0.90(−1.42 to −0.38) | <0.001 | 72.35 | <0.01 | 2.4% | |
| PBD Type | ||||||||
| Lacto-ovo veg | 1 | 37/37 | −0.94(−2.24 to 0.36) | 0.16 | - | - | - | |
| Vegan | 4 | 114/117 | −0.91(−1.56 to −0.25) | <0.01 | 77.95 | <0.005 | 2.4% | |
| Energy Intake | ||||||||
| Not limited | 4 | 131/134 | −0.91(−1.56 to −0.25) | <0.01 | 77.95 | <0.005 | 2.4% | |
| Decreased | 1 | 37/37 | −0.94(−2.24 to 0.36) | 0.16 | - | - | - | |
| Trial length | ||||||||
| <16 weeks | 2 | 83/83 | −0.64(−1.45 to 0.18) | 0.125 | - | - | - | |
| ≥16 weeks | 3 | 85/88 | −1.13(−1.89 to −0.38) | <0.005 | 79.79 | <0.05 | 3.0% | |
| WC (cm) | Within PBD group change | 2 | 95 | −4.23(−6.38 to −2.07) | <0.001 | 81.01 | <0.05 | 4.3% |
| Between group differences | 2 | 95/96 | −2.41(−3.72 to −1.09) | <0.001 | - | - | 2.2% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austin, G.; Ferguson, J.J.A.; Garg, M.L. Effects of Plant-Based Diets on Weight Status in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 4099. https://doi.org/10.3390/nu13114099
Austin G, Ferguson JJA, Garg ML. Effects of Plant-Based Diets on Weight Status in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2021; 13(11):4099. https://doi.org/10.3390/nu13114099
Chicago/Turabian StyleAustin, Grace, Jessica J. A. Ferguson, and Manohar L. Garg. 2021. "Effects of Plant-Based Diets on Weight Status in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Nutrients 13, no. 11: 4099. https://doi.org/10.3390/nu13114099
APA StyleAustin, G., Ferguson, J. J. A., & Garg, M. L. (2021). Effects of Plant-Based Diets on Weight Status in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients, 13(11), 4099. https://doi.org/10.3390/nu13114099







