Changes in Plasma Choline and the Betaine-to-Choline Ratio in Response to 6-Month Lifestyle Intervention Are Associated with the Changes of Lipid Profiles and Intestinal Microbiota: The ICAAN Study
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Measurements of BMI z-Score
2.3. Dietary Assessments
2.4. Measurements of TMAO and Its Precursors
2.5. Microbiota Profiling through 16S rRNA Sequencing
2.6. Analysis of Microbiota Sequencing Data
2.7. Statistical Analysis
3. Results
3.1. The Characteristics of Study Participants
3.2. Changes in TMAO and Its Precursors by the Responses to 6-Month Lifestyle Intervention
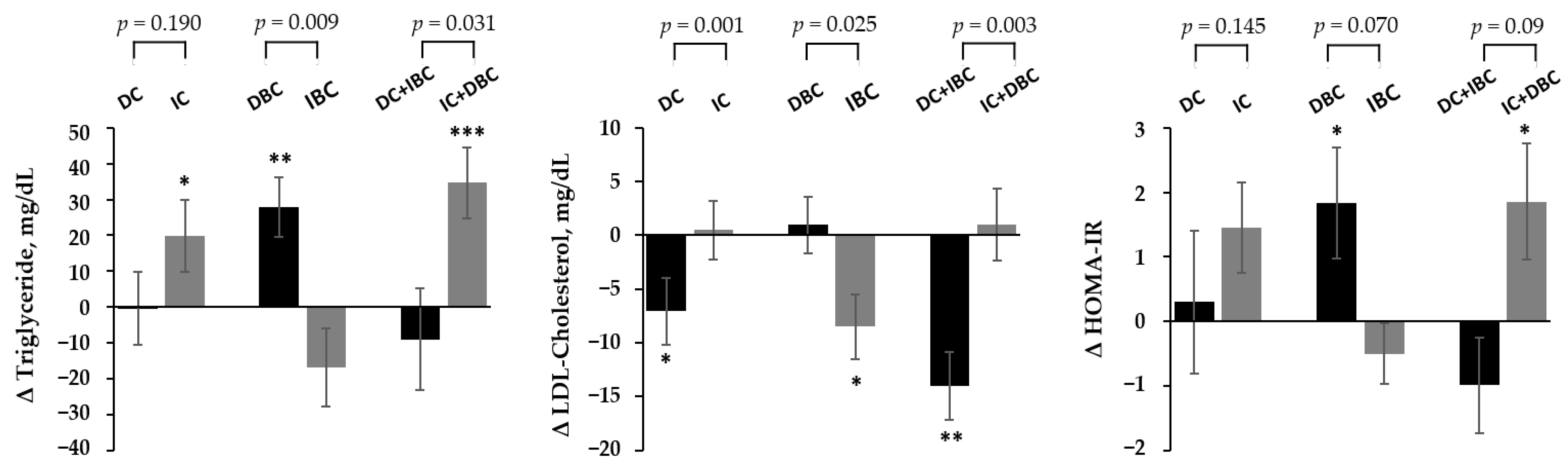
3.3. Association of Changes in Choline and B/C by Lifestyle Intervention with the Improvement in Metabolic Parameters
3.4. Association of Changes in Choline and B/C by Lifestyle Intervention with Gut Microbiota
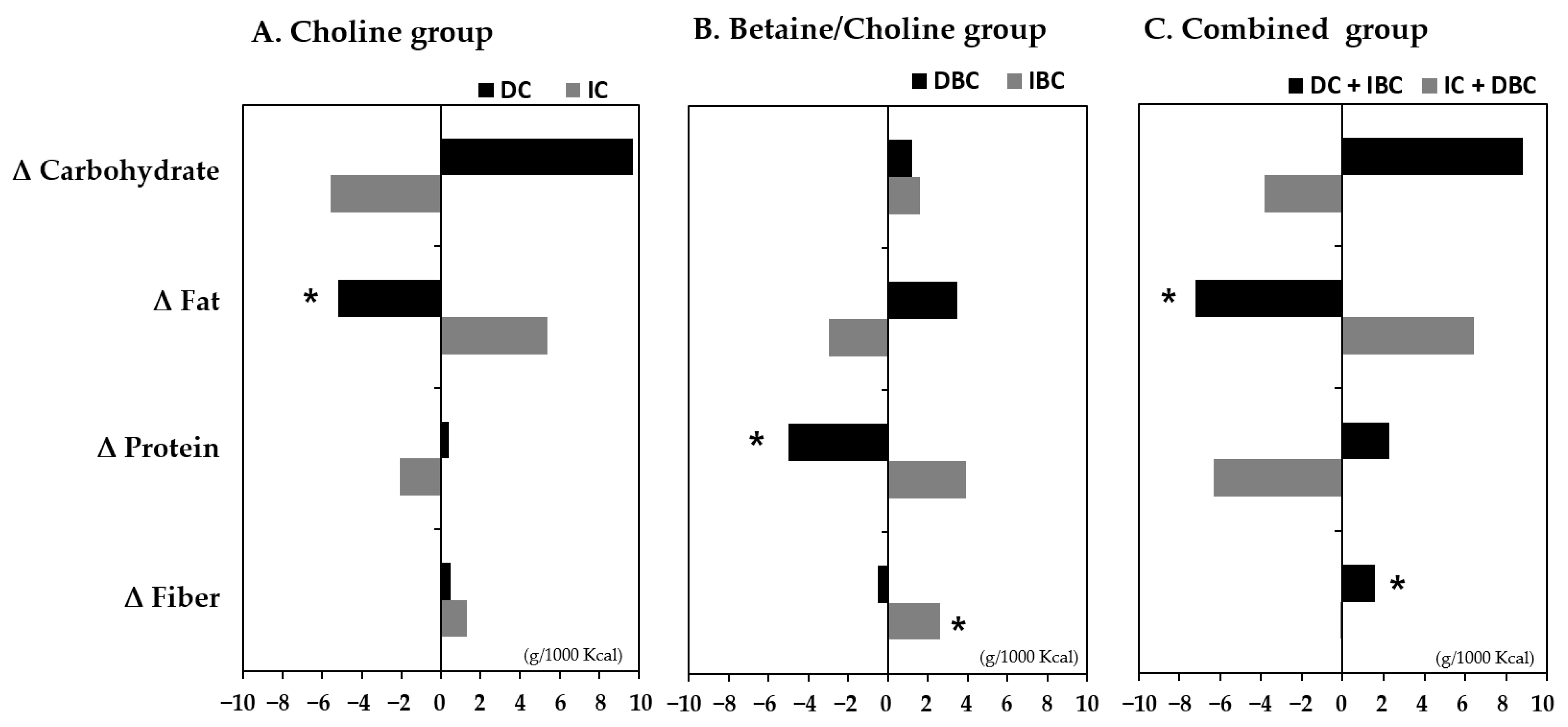
3.5. Association of Changes in Choline and B/C by Lifestyle Intervention with the Intake of Nutrients
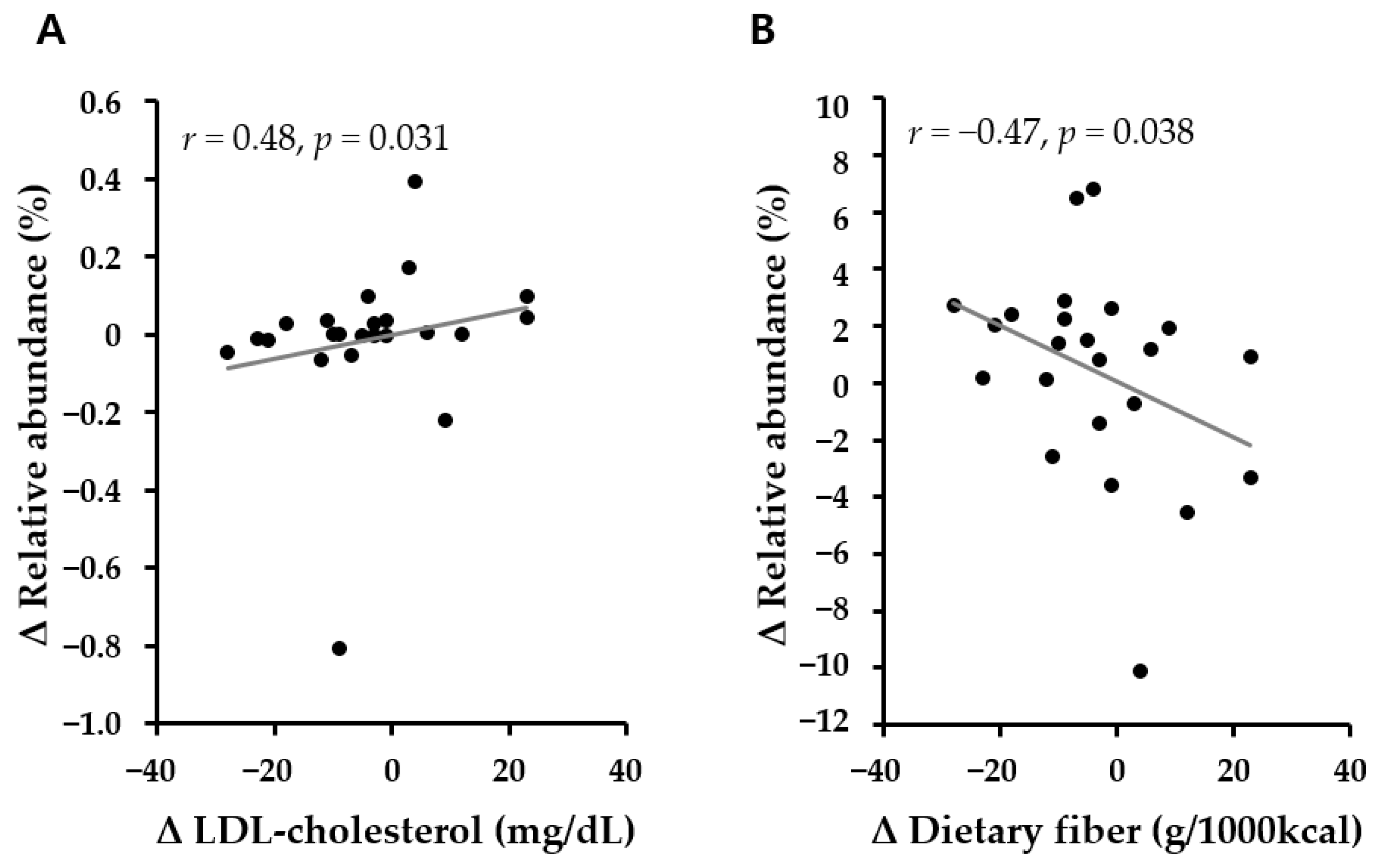
3.6. Association of Microbiota Abundance with Metabolic Parameters and the Intake of Nutrients According to Lifestyle Intervention for 6 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar]
- Singh, A.S.; Mulder, C.; Twisk, J.W.; van Mechelen, W.; Chinapaw, M.J. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, L.G.; Jensen, B.W.; Ängquist, L.; Osler, M.; Sørensen, T.I.A.; Baker, J.L. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N. Engl. J. Med. 2018, 378, 1302–1312. [Google Scholar] [CrossRef]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinvasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejtahed, H.S.; Hasani-Ranjbar, S.; Larijani, B. Human microbiome as an approach to personalized medicine. Altern. Ther. Health Med. 2017, 23, 8–9. [Google Scholar]
- Ejtahed, H.S.; Angoorani, P.; Hasani-Ranjbar, S.; Siadat, S.D.; Ghasemi, N.; Larijani, B.; Soroush, A. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: A systematic review. Microb. Pathog. 2018, 116, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum trimethylamin N-oxide (TMAO) levels with early atherosclerosis in Humans. Sci. Rep. 2016, 27, 26745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tang, W.H.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Heianza, Y.; Sun, D.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes in gut microbiota-related metabolites and long-term successful weight loss in response to weight-loss diets: The POUNDS Lost Trial. Diabetes Care 2018, 41, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heianza, Y.; Sun, D.; Li, X.; DiDonato, J.A.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: The POUNDS Lost trial. Gut 2019, 68, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.G.; Lim, H.; Kim, Y.M.; Ju, Y.S.; Choi, Y.; Lee, H.J.; Jang, H.B.; Park, S.I.; Park, K.H. Effects of circuit training or a nutritional intervention on body mass index and other cardiometabolic outcomes in children and adolescents with overweight or obesity. PLoS ONE 2021, 16, e0245875. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.S.; Lee, S.H.; Kim, S. Characterization and analysis of the skin microbiota in acne: Impact of systemic antibiotics. J. Clin. Med. 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinova, S.V.; Tell, G.S.; Vollset, S.E.; Nygard, O.; Bleie, Ø.; Unland, P.M. Divergent association of plasma choline and betaine with component of metabolic syndrome I middle age and elderly men and women. J. Nutr. 2008, 138, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Randell, E.; Tianm, Y.; Zhou, H.; Sun, G. Low serum choline and high serum betaine levels are associated with favorable components of metabolic syndrome in Newfoundland population. J. Diabetic. Complicat. 2019, 33, 107398. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Hullar, M.A.J.; Randolph, T.W.; Frnke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Marchand, L.L.; et al. Associations of plama trimethylamine N-oxide, choline, carnitine, and betaine with in flammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiehnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholewa, J.M. Effects of betaine on body composition, performance, and homocysteine thiolactone. J. Int. Soc. Sports Nutr. 2013, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, N.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Xu, Z.; Han, X.; Li, W. Effect of dietary betaine supplementation on lipogenic enzyme activities and fatty acid synthase mRNA Expression in finishing pigs. Anim. Feed Sci. Technol. 2008, 140, 365–375. [Google Scholar] [CrossRef]
- Xing, J.; Kang, L.; Jiang, Y. Effect of dietary betaine supplementation on lipogenesis gene expression and CpG methylation of lipoprotein lipase gene in broilers. Mol. Biol. Rep. 2011, 38, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Chen, Y.; Xia, F.; Abudukerimu, B.; Zhang, W.; Guo, Y.; Wang, N.; Lu, Y. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front. Endocrinol. 2018, 9, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Wang, Y.; Tan, S.; Wang, J. Effects of banana resistant starch on the biochemical indexes and intestinal flora of obses rats induced by a high-fat diet and their correlation analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Singh, R.P.; Halaka, D.A.; Hayouka, A.; Tirosh, O. High-fat diet induced alteration of mice microbiota and the functional ability to utilize fructooligosaccharide for ethanol production. Front. Cell Infect. Microbiol. 2020, 10, 376. [Google Scholar] [CrossRef]
- Boutagy, N.E.; Nelison, A.P.; Osterverg, K.L.; Andrew, T.S.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Short-term high-fat diet increases postprandial trimethylamin-N-oxide in human. Nutr. Res. 2015, 35, 858–864. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, environments, and gut microbiota. A preliminary investigation in children libing in rural and urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef] [Green Version]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basa-gila, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]

| Responder (N = 27) | Non-Responder (N = 13) | p-Value | |||
|---|---|---|---|---|---|
| Diff ± SD | Paired t-Test | Diff ± SD | Paired t-Test | ||
| Metabolic parameters (Δ Baseline—6 month) | |||||
| BMI z-score | –0.27 ± 0.28 | <0.0001 | 0.22 ± 0.17 | 0.0007 | <0.0001 |
| Triglyceride §, mg/dL | 4.96 ± 37.3 | 0.3482 | 27.2 ± 61.3 | 0.0565 | 0.1074 |
| HDL-C, mg/dL | 0.04 ± 4.97 | 0.9694 | −2.46 ± 7.09 | 0.2345 | 0.1955 |
| LDL-C, mg/dL | −2.52 ± 12.7 | 0.3122 | −2.00 ± 15.2 | 0.6444 | 0.5620 |
| Glucose, mg/dL | 1.22 ± 7.76 | 0.4207 | 5.69 ± 10.6 | 0.0762 | 0.4227 |
| HOMA-IR § | 0.32 ± 3.22 | 0.8039 | 2.45 ± 4.62 | 0.0607 | 0.1038 |
| Nutrients intake, g/1000 kcal/day (Δ Baseline—6 month) | |||||
| Carbohydrate | 1.88 ± 20.1 | 0.7123 | −0.08 ± 30.9 | 0.9953 | 0.1326 |
| Protein | −1.06 ± 8.10 | 0.6095 | −0.78 ± 61.3 | 0.8342 | 0.7425 |
| Fat | −0.09 ± 7.80 | 0.9624 | 2.37 ± 7.09 | 0.6809 | 0.3709 |
| Dietary fiber | 1.07 ± 2.45 | 0.1007 | 0.48 ± 15.2 | 0.7803 | 0.2695 |
| Responder (N = 27) | Non-Responder (N = 13) | p-Value | |||
|---|---|---|---|---|---|
| Diff ± SD | Paired t-Test | Diff ± SD | Paired t-Test | ||
| Choline metabolism related metabolites (Δ Baseline—6 month) | |||||
| Carnitine | −0.0127 ± 0.0488 | 0.189 | −0.0177 ± 0.0293 | 0.051 | 0.493 |
| Choline § | 0.0028 ± 0.0174 | 0.335 | 0.0102 ± 0.0153 | 0.035 | 0.119 |
| TMAO § | −0.0001 ± 0.0146 | 0.840 | 0.0001 ± 0.0055 | 0.850 | 0.233 |
| Betaine | −0.0062 ± 0.0479 | 0.509 | −0.0027 ± 0.0442 | 0.830 | 0.564 |
| Betaine/Choline § | −1.4030 ± 5.6760 | 0.192 | −2.3440 ± 3.1800 | 0.024 | 0.660 |
| TMAO/Choline | 0.0270 ± 1.3670 | 0.919 | 0.0809 ± 0.4270 | 0.508 | 0.283 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Lim, H.; Park, K.-H.; Park, S.; Lee, H.-J. Changes in Plasma Choline and the Betaine-to-Choline Ratio in Response to 6-Month Lifestyle Intervention Are Associated with the Changes of Lipid Profiles and Intestinal Microbiota: The ICAAN Study. Nutrients 2021, 13, 4006. https://doi.org/10.3390/nu13114006
Jang H, Lim H, Park K-H, Park S, Lee H-J. Changes in Plasma Choline and the Betaine-to-Choline Ratio in Response to 6-Month Lifestyle Intervention Are Associated with the Changes of Lipid Profiles and Intestinal Microbiota: The ICAAN Study. Nutrients. 2021; 13(11):4006. https://doi.org/10.3390/nu13114006
Chicago/Turabian StyleJang, HanByul, Hyunjung Lim, Kyung-Hee Park, SangIck Park, and Hye-Ja Lee. 2021. "Changes in Plasma Choline and the Betaine-to-Choline Ratio in Response to 6-Month Lifestyle Intervention Are Associated with the Changes of Lipid Profiles and Intestinal Microbiota: The ICAAN Study" Nutrients 13, no. 11: 4006. https://doi.org/10.3390/nu13114006
APA StyleJang, H., Lim, H., Park, K.-H., Park, S., & Lee, H.-J. (2021). Changes in Plasma Choline and the Betaine-to-Choline Ratio in Response to 6-Month Lifestyle Intervention Are Associated with the Changes of Lipid Profiles and Intestinal Microbiota: The ICAAN Study. Nutrients, 13(11), 4006. https://doi.org/10.3390/nu13114006






