Frailty Is Associated with Oxidative Stress in Older Patients with Type 2 Diabetes
Abstract
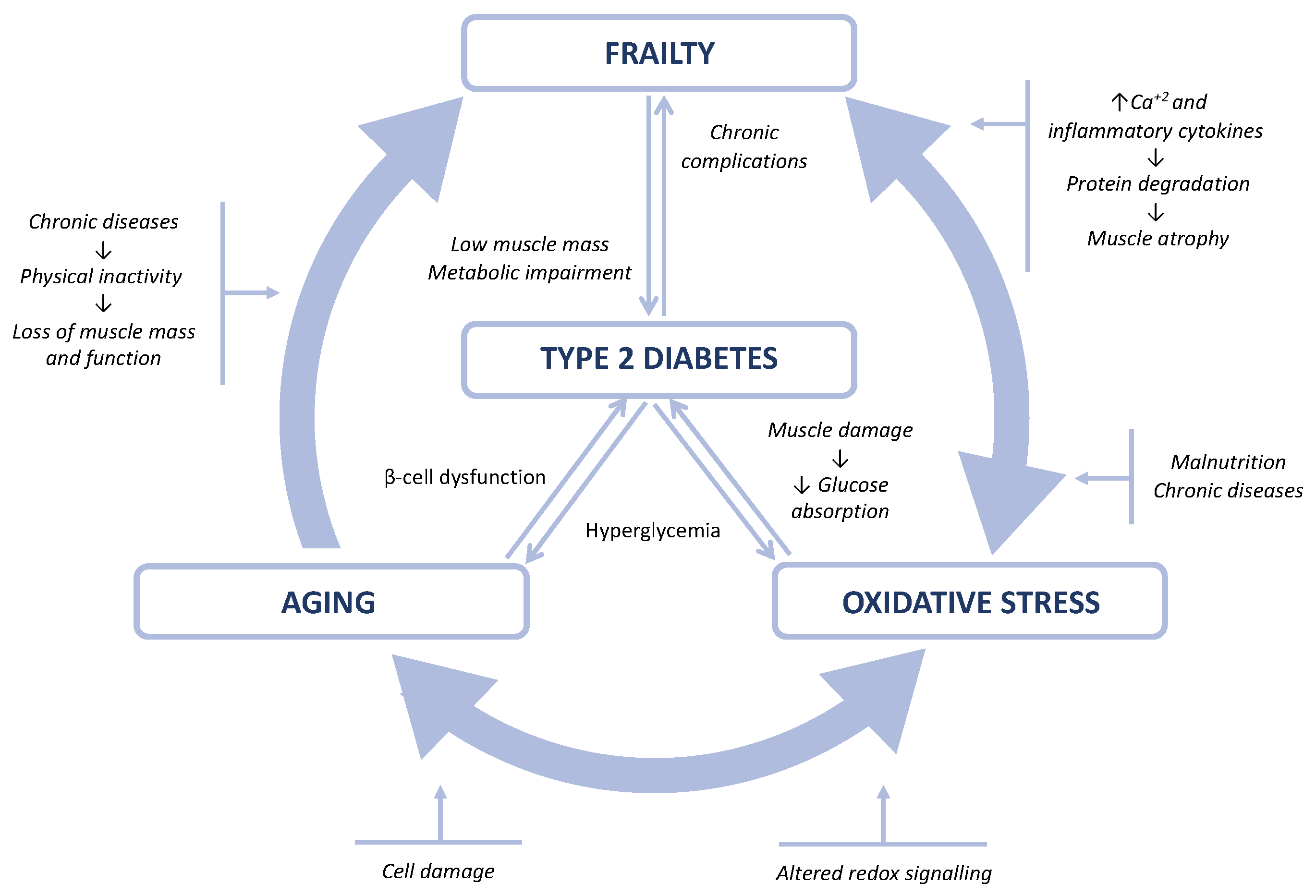
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Anthropometric Parameters
2.3. Body Composition and Functionality Parameters
2.4. Frailty Diagnosis
2.5. Biochemical Parameters
2.6. Oxidative Stress
2.7. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| ASHT | American Society of Hand Therapists |
| ASMI | Appendicular skeletal muscle mass index |
| BMI | Body mass index |
| CRP | C-reactive protein |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ES | Effect size |
| FFMI | Fat-free mass index |
| FMI | Fat mass index |
| HbA1c | Glycated hemoglobin |
| HDLc | High-density lipoprotein cholesterol |
| HPLC | High-performance liquid chromatography |
| LDLc | Low-density lipoprotein cholesterol |
| LM | Lean mass |
| MDA | Malondialdehyde |
| METS | Metabolic equivalent of tasks |
| MNA | Mini Nutritional Assessment |
| OS | Oxidative stress |
| PA | Phase angle |
| SMI | Skeletal muscle mass index |
| T2D | Type 2 diabetes mellitus |
References
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Yanase, T.; Yanagita, I.; Muta, K.; Nawata, H. Frailty in Elderly Diabetes Patients. Endocr. J. 2018, 65, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Mañas, L.; Sinclair, A.J. Frailty, Sarcopenia and Diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar] [CrossRef]
- Peña-Longobardo, L.M.; Oliva-Moreno, J.; Zozaya, N.; Aranda-Reneo, I.; Trapero-Bertran, M.; Laosa, O.; Sinclair, A.; Rodríguez-Mañas, L. Economic Evaluation of a Multimodal Intervention in Pre-Frail and Frail Older People with Diabetes Mellitus: The MID-FRAIL Project. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 111–118. [Google Scholar] [CrossRef]
- Jungert, A.; Eichner, G.; Neuhäuser-Berthold, M. Trajectories of Body Composition during Advanced Aging in Consideration of Diet and Physical Activity: A 20-Year Longitudinal Study. Nutrients 2020, 12, 3626. [Google Scholar] [CrossRef]
- Jafari Nasabian, P.; Inglis, J.E.; Reilly, W.; Kelly, O.J.; Ilich, J.Z. Aging Human Body: Changes in Bone, Muscle and Body Fat with Consequent Changes in Nutrient Intake. J. Endocrinol. 2017, 234, R37–R51. [Google Scholar] [CrossRef] [Green Version]
- Viña, J.; Borras, C.; Gomez-Cabrera, M.C. A Free Radical Theory of Frailty. Free Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Ganji, S.S.; Kalyani, R.R. Body Composition Changes in Diabetes and Aging. J. Diabetes Complicat. 2019, 33, 451–459. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased Muscle Strength and Quality in Older Adults with Type 2 Diabetes: The Health, Aging, and Body Composition Study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative Stress and Frailty: A Systematic Review and Synthesis of the Best Evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglés, M.; Gambini, J.; Carnicero, J.A.; García-García, F.J.; Rodríguez-Mañas, L.; Olaso-González, G.; Dromant, M.; Borrás, C.; Viña, J. Oxidative Stress Is Related to Frailty, Not to Age or Sex, in a Geriatric Population: Lipid and Protein Oxidation as Biomarkers of Frailty. J. Am. Geriatr. Soc. 2014, 62, 1324–1328. [Google Scholar] [CrossRef]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative Protein Damage Is Associated with Poor Grip Strength among Older Women Living in the Community. J. Appl. Physiol. 2007, 103, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldi, J.C.; Snowling, N. Resistance Training Improves Glycaemic Control in Obese Type 2 Diabetic Men. Int. J. Sports Med. 2003, 24, 419–423. [Google Scholar] [CrossRef]
- Wells, J.C.K. Body Composition and Susceptibility to Type 2 Diabetes: An Evolutionary Perspective. Eur. J. Clin. Nutr. 2017, 71, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, P.; Fauré, I.; Corcoran, N.; Butterly, E.; Lewsey, J.; McAllister, D.; Mair, F.S. Frailty Measurement, Prevalence, Incidence, and Clinical Implications in People with Diabetes: A Systematic Review and Study-Level Meta-Analysis. Lancet Healthy Longev. 2020, 1, e106–e116. [Google Scholar] [CrossRef]
- Kong, L.-N.; Lyu, Q.; Yao, H.-Y.; Yang, L.; Chen, S.-Z. The Prevalence of Frailty among Community-Dwelling Older Adults with Diabetes: A Meta-Analysis. Int. J. Nurs. Stud. 2021, 119, 103952. [Google Scholar] [CrossRef]
- Casals, C.; Casals Sánchez, J.L.; Suárez Cadenas, E.; Aguilar-Trujillo, M.P.; Estébanez Carvajal, F.M.; Vázquez-Sánchez, M.Á. Frailty in older adults with type 2 diabetes mellitus and its relation with glucemic control, lipid profile, blood pressure, balance, disability grade and nutritional status. Nutr. Hosp. 2018, 35, 820–826. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and Its Use in Grading the Nutritional State of Elderly Patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. Edinb. Scotl. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis-Part II: Utilization in Clinical Practice. Clin. Nutr. Edinb. Scotl. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Fess, E.; Moran, C. Clinical Assessment Recommendations. Am. Soc. Hand Ther. 1981, 6–8. Available online: https://www.asht.org/practice/clinical-assessment-recommendations (accessed on 6 November 2021).
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Ter Welle, H.F.; Baartscheer, T.; Fiolet, J.W. Influence of Free Glycerol on Enzymic Evaluation of Triglycerides. Clin. Chem. 1984, 30, 1102–1103. [Google Scholar] [CrossRef]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid Method for the Isolation of Lipoproteins from Human Serum by Precipitation with Polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Trinder, P. Enzymatic Determination of Glucose in Blood Serum. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Little, R.R. Glycated Hemoglobin Standardization—National Glycohemoglobin Standardization Program (NGSP) Perspective. Clin. Chem. Lab. Med. 2003, 20, 1191–1198. [Google Scholar] [CrossRef]
- Doumas, B.; Peters, T. Origins of Dye-Binding Methods for Measuring Serum Albumin. Clin. Chem. 2009, 55, 583–584. [Google Scholar] [CrossRef]
- Inglés, M.; Serra-Añó, P.; Gambini, J.; Abu-Sharif, F.; Dromant, M.; Garcia-Valles, R.; Pareja-Galeano, H.; Garcia-Lucerga, C.; Gomez-Cabrera, M.C. Active Paraplegics Are Protected against Exercise-Induced Oxidative Damage through the Induction of Antioxidant Enzymes. Spinal Cord 2016, 54, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Knickman, J.R.; Snell, E.K. The 2030 Problem: Caring for Aging Baby Boomers. Health Serv. Res. 2002, 37, 849–884. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Manfredi, G.; Midão, L.; Paúl, C.; Cena, C.; Duarte, M.; Costa, E. Prevalence of Frailty Status among the European Elderly Population: Findings from the Survey of Health, Aging and Retirement in Europe. Geriatr. Gerontol. Int. 2019, 19, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Abizanda, P.; Romero, L.; Sánchez-Jurado, P.M.; Martínez-Reig, M.; Gómez-Arnedo, L.; Alfonso, S.A. Frailty and Mortality, Disability and Mobility Loss in a Spanish Cohort of Older Adults: The FRADEA Study. Maturitas 2013, 74, 54–60. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Murata, K. Relationship between Frailty and Mortality, Hospitalization, and Cardiovascular Diseases in Diabetes: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2019, 18, 81. [Google Scholar] [CrossRef]
- Assar, M.E.; Laosa, O.; Rodríguez Mañas, L. Diabetes and Frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.W.; Auyeung, T.W.; Leung, J.; Kwok, T.; Leung, P.C.; Woo, J. The Effect of Diabetes Mellitus on Age-Associated Lean Mass Loss in 3153 Older Adults. Diabet. Med. J. Br. Diabet. Assoc. 2010, 27, 1366–1371. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, R.R.; Tra, Y.; Egan, J.M.; Ferrucci, L.; Brancati, F. Hyperglycemia Is Associated with Relatively Lower Lean Body Mass in Older Adults. J. Nutr. Health Aging 2014, 18, 737–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kołodziej, M.; Sebastjan, A.; Ignasiak, Z. Appendicular Skeletal Muscle Mass and Quality Estimated by Bioelectrical Impedance Analysis in the Assessment of Frailty Syndrome Risk in Older Individuals. Aging Clin. Exp. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Visser, M.; Schaap, L. Body Weight and Body Composition in Old Age and Their Relationship with Frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Brunani, A.; Perna, S.; Soranna, D.; Rondanelli, M.; Zambon, A.; Bertoli, S.; Vinci, C.; Capodaglio, P.; Lukaski, H.; Cancello, R. Body Composition Assessment Using Bioelectrical Impedance Analysis (BIA) in a Wide Cohort of Patients Affected with Mild to Severe Obesity. Clin. Nutr. Edinb. Scotl. 2021, 40, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Jeong, H.K.; Lee, S.S.; Kim, S.R.; Cha, B.-Y.; Son, H.-Y.; Yoo, S.J. The Effect of Early Intensive Insulin Therapy on Body Fat Distribution and β-Cell Function in Newly Diagnosed Type 2 Diabetes. Endocr. Res. 2013, 38, 160–167. [Google Scholar] [CrossRef]
- Juurinen, L.; Tiikkainen, M.; Häkkinen, A.-M.; Hakkarainen, A.; Yki-Järvinen, H. Effects of Insulin Therapy on Liver Fat Content and Hepatic Insulin Sensitivity in Patients with Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E829–E835. [Google Scholar] [CrossRef] [Green Version]
- Ottenbacher, K.J.; Graham, J.E.; Al Snih, S.; Raji, M.; Samper-Ternent, R.; Ostir, G.V.; Markides, K.S. Mexican Americans and Frailty: Findings from the Hispanic Established Populations Epidemiologic Studies of the Elderly. Am. J. Public Health 2009, 99, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar] [CrossRef]
- Dittmar, M. Reliability and Variability of Bioimpedance Measures in Normal Adults: Effects of Age, Gender, and Body Mass. Am. J. Phys. Anthropol. 2003, 122, 361–370. [Google Scholar] [CrossRef]
- Boittin, F.-X.; Petermann, O.; Hirn, C.; Mittaud, P.; Dorchies, O.M.; Roulet, E.; Ruegg, U.T. Ca2+-Independent Phospholipase A2 Enhances Store-Operated Ca2+ Entry in Dystrophic Skeletal Muscle Fibers. J. Cell Sci. 2006, 119, 3733–3742. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Manwani, B.; Leng, S.X. Frailty, Inflammation, and Immunity. Aging Dis. 2011, 2, 466–473. [Google Scholar] [PubMed]
- Lang, P.-O.; Michel, J.-P.; Zekry, D. Frailty Syndrome: A Transitional State in a Dynamic Process. Gerontology 2009, 55, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J.; et al. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkisas, S.; Vandewoude, M. Where Frailty Meets Diabetes. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 261–267. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-T.; Wang, J.; Chien, K.-L.; COhort of GEriatric Nephrology in NTUH (COGENT) study group. Both Pre-Frailty and Frailty Increase Healthcare Utilization and Adverse Health Outcomes in Patients with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2018, 17, 130. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Rodriguez-Mañas, L. Diabetes and Frailty: Two Converging Conditions? Can. J. Diabetes 2016, 40, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, M.; Laosa, O.; Cadore, E.L.; Abizanda, P.; Garcia-Garcia, F.J.; Hornillos, M.; López-Pavón, I.; Sinclair, A.J.; Rodríguez-Mañas, L. Two-Year Follow-up of a Multimodal Intervention on Functional Capacity and Muscle Power in Frail Patients With Type 2 Diabetes. J. Am. Med. Dir. Assoc. 2021, 22, 1906–1911. [Google Scholar] [CrossRef]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Jivraj, S.; Walters, K. Transitions between Frailty States among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2019, 50, 81–88. [Google Scholar] [CrossRef]
- Gill, T.M.; Gahbauer, E.A.; Allore, H.G.; Han, L. Transitions between Frailty States among Community-Living Older Persons. Arch. Intern. Med. 2006, 166, 418–423. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar]
| Total (n = 100) | Women (n = 52) | Men (n = 48) | Effect Size | |
|---|---|---|---|---|
| Age (years) | 70.3 ± 3.8 | 70.6 ± 3.6 | 70.0 ± 4.0 | |
| Time of T2D evolution (years) | 17.8 ± 10.7 | 20.2 ± 11.2 | 15.3 ± 9.7 * | 0.214 |
| Systolic blood pressure (mmHg) | 131.6 ± 13.6 | 129.6 ± 13.9 | 133.9 ± 13.0 | |
| Diastolic blood pressure (mmHg) | 72.9 ± 11.7 | 71.5 ± 12.9 | 74.4 ± 10.1 | |
| Body mass index (kg/m2) | 30.8 ± 4.2 | 31.0 ± 4.4 | 30.5 ± 4.2 | |
| Mid upper-arm circumference (cm) | 31.5 ± 3.4 | 32.1 ± 3.7 | 30.9 ± 3.0 | |
| Calf circumference (cm) | 37.2 ± 3.1 | 36.7 ± 3.1 | 37.9 ± 3.1 * | 0.387 |
| Waist circumference (cm) | 106.2 ± 14.3 | 104.6 ± 13.4 | 107.9 ± 15.3 | |
| Tricipital skinfold (mm) | 20.5 ± 8.0 | 25.0 ± 6.4 | 15.6 ± 6.6 * | 0.609 |
| Resistance (Ω) | 440.1 ± 67.0 | 474.5 ± 60.8 | 403.5 ± 52.7 * | 1.248 |
| Reactance (Ω) | 42.1 ± 6.2 | 43.1 ± 6.0 | 4.1 ± 6.4 | |
| Phase angle (°) | 5.5 ± 0.7 | 5.2 ± 0.7 | 5.8 ± 0.7 * | 0.327 |
| Fat mass index (kg/m2) | 8.9 ± 3.8 | 10.7 ± 3.8 | 7.0 ± 2.7 * | 1.123 |
| Fat-free mass index (kg/m2) | 21.7 ± 2.6 | 20.1 ± 1.8 | 23.3 ± 2.3 * | 1.550 |
| Skeletal muscle mass index (kg/m2) | 10.1 ± 1.9 | 8.6 ± 1.0 | 11.6 ± 1.4 * | 2.466 |
| Appendicular skeletal muscle mass index (kg/m2) | 7.9 ± 1.0 | 7.3 ± 0.8 | 8.6 ± 0.9 * | 1.527 |
| Cell mass index (kg/m2) | 11.1 ± 2.0 | 10.0 ± 1.5 | 12.3 ± 1.8 * | 1.388 |
| Maximum muscle strength (kg) | 28.6 ± 10.0 | 21.0 ± 4.4 | 36.9 ± 7.6 * | 2.561 |
| Gait speed (m/s) | 0.8 ± 0.2 | 0.86 ± 0.2 | 0.71 ± 0.2 * | 0.441 |
| Physical activity (METS-min/14 days) | 2996.7 ± 1905.0 | 2840.3 ± 1826.1 | 3166.1 ± 1992.3 | |
| Weekly protein rations | 10.4 ± 2.8 | 9.8 ± 2.5 | 11.1 ± 3.0 * | 0.234 |
| MNA score | 26.5 ± 2.1 | 25.8 ± 2.2 | 27.3 ± 1.7 * | 0.352 |
| Glucose (mg/dL) | 148.2 ± 45.7 | 147.1 ± 47.7 | 149.3 ± 43.7 | |
| HbA1c (%) | 7.4 ± 1.1 | 7.3 ± 1.0 | 7.4 ± 1.1 | |
| Total cholesterol (mg/dL) | 156.1 ± 30.2 | 165.7 ± 29.9 | 145.4 ± 27.1 * | 0.711 |
| LDL cholesterol (mg/dL) | 93.0 ± 22.6 | 96.4 ± 23.6 | 89.1 ± 21.1 | |
| HDL cholesterol (mg/dL) | 47.7 ± 12.8 | 51.6 ± 14.4 | 43.4 ± 9.2 * | |
| Triglycerides (mg/dL) | 134.5 ± 62.3 | 141.4 ± 57.4 | 126.8 ± 67.0 | |
| Albumin (g/dL) | 4.2 ± 0.2 | 4.2 ± 0.2 | 4.2 ± 0.3 | |
| C-reactive protein | 4.5 ± 8.9 | 4.2 ± 6.7 | 4.8 ± 10.9 | |
| Malondialdehyde (µM) | 6.0 ± 5.8 | 6.1 ± 5.6 | 5.8 ± 6.0 | |
| Protein carbonyls (U.A.) | 81.3 ± 20.9 | 84.5 ± 26.3 | 78.0 ± 12.9 |
| Total | Women | Men | |
|---|---|---|---|
| Robust | 28 (20.0) | 7 (13.5) | 21 (43.8) |
| Prefrail | 57 (57.0) | 35 (67.3) | 22 (45.8) |
| Frail | 15 (15.0) | 10 (19.2) | 5 (10.4) |
| Robust (n = 28) | Prefrail (n = 57) | Frail (n = 15) | Effect Size | |
|---|---|---|---|---|
| Age (years) | 69.0 ± 3.0 | 70.4 ± 4.2 | 72.1 ± 2.3 * | 0.06 |
| Time of T2D evolution (years) | 15.6 ± 7.1 | 18.8 ± 11.2 | 17.6 ± 13.7 | |
| Systolic blood pressure (mmHg) | 130.2 ± 11.2 | 132.2 ± 14.4 | 132.1 ± 13.6 | |
| Diastolic blood pressure (mmHg) | 75.4 ± 8.4 | 70.5 ± 13.0 | 77.9 ± 8.7 | |
| Body mass index (kg/m2) | 28.6 ± 3.6 | 31.1 ± 4.1 | 32.6 ± 4.6 * | 0.08 |
| Mid-upper arm circumference (cm) | 30.1 ± 1.9 | 31.8 ± 3.5 * | 32.9 ± 4.6 | |
| Calf circumference (cm) | 37.0 ± 3.1 | 37.2 ± 3.2 | 37.5 ± 3.0 | |
| Waist circumference (cm) | 100.7 ± 16.2 | 106.2 ± 11.7 | 114.0 ± 16.8 * | 0.07 |
| Tricipital skinfold (mm) | 16.6 ± 6.0 | 21.6 ± 8.0 * | 23.1 ± 9.6 * | 0.09 |
| Resistance (Ω) | 422.9 ± 46.1 | 446.9 ± 75.7 | 443.4 ± 58.1 | |
| Reactance (Ω) | 41.5 ± 5.4 | 43.2 ± 6.2 | 39.7 ± 6.4 | |
| Phase angle (°) | 5.6 ± 0.6 | 5.5 ± 0.7 | 5.1 ± 0.7 * | 0.07 |
| Fat mass index (kg/m2) | 6.5 ± 2.3 | 9.7 ± 3.6 * | 10.5 ± 4.7 * | 0.16 |
| Fat-free mass index (kg/m2) | 22.1 ± 2.4 | 21.7 ± 3.0 | 21.3 ± 2.0 | |
| Skeletal muscle mass index (kg/m2) | 10.7 ± 1.5 | 9.9 ± 2.1 | 9.6 ± 1.5 | |
| Appendicular skeletal muscle mass index (kg/m2) | 8.0 ± 0.9 | 7.9 ± 1.2 | 7.8 ± 0.8 | |
| Cell mass index (kg/m2) | 11.5 ± 1.7 | 11.2 ± 2.4 | 10.3 ± 1.7 | |
| Maximum muscle strength (kg) | 35.1 ± 9.1 | 27.6 ± 9.4 * | 20.9 ± 7.6 *,** | 0.21 |
| Gait speed (m/s) | 0.68 ± 0.1 | 0.8 ± 0.2 * | 1.0 ± 0.3 *,** | 0.32 |
| Physical activity (METS-min/14 days) | 3979.2 ± 2179.1 | 2925.2 ± 1559.5 | 1460.4 ± 1679.2 *,** | 0.16 |
| Weekly protein rations | 11.4 ± 2.8 | 10.4 ± 3.3 | 9.6 ± 2.6 | |
| MNA score | 27.9 ± 1.4 | 26.4 ± 1.8 * | 24.5 ± 2.5 *,** | 0.22 |
| Glucose (mg/dL) | 138.7 ± 34.6 | 156.1 ± 49.3 | 131.6 ± 41.9 | |
| HbA1c (%) | 7.1 ± 1.0 | 7.5 ± 1.1 | 7.4 ± 1.1 | |
| Total cholesterol (mg/dL) | 159.3 ± 32.0 | 155.5 ± 28.4 | 152.5 ± 35.9 | |
| LDL cholesterol (mg/dL) | 97.9 ± 22.7 | 92.7 ± 21.4 | 85.2 ± 26.5 | |
| HDL cholesterol (mg/dL) | 49.2 ± 11.7 | 46.7 ± 12.6 | 49.0 ± 15.9 | |
| Triglycerides (mg/dL) | 121.3 ± 68.1 | 144.2 ± 77.7 | 146.1 ± 65.3 | |
| Albumin (g/dL) | 4.2 ± 0.2 | 4.2 ± 0.2 | 4.1 ± 0.3 | |
| C-reactive protein | 4.6 ± 11.6 | 3.7 ± 6.6 | 7.8 ± 11.6 | |
| Malondialdehyde (µM) | 3.7 ± 3.2 | 7.3 ± 6.6 * | 5.9 ± 6.2 | |
| Protein carbonyls (U.A.) | 75.1 ± 13.3 | 79.7 ± 22.3 | 99.1 ± 16.8 *,** | 0.14 |
| A. Dependent Variable: Frailty | Unstandardized Coefficients | Standardized Coefficients | |||
|---|---|---|---|---|---|
| B | SE | β | t | Significance | |
| (Constant) | −2.148 | 1.261 | −1.704 | 0.092 | |
| Malondialdehyde (µM) | 0.008 | 0.012 | 0.70 | 0.716 | 0.476 |
| Protein carbonyls (U.A.) | 0.009 | 0.003 | 0.284 | 2.842 | 0.006 |
| Age (years old) | 0.041 | 0.017 | 0.236 | 2.404 | 0.018 |
| HbA1c (%) | 0.047 | 0.059 | 0.079 | 0.809 | 0.420 |
| B. Dependent Variable: Malondialdehyde | Unstandardized Coefficients | Standardized Coefficients | |||
| Model | B | SE | β | t | Significance |
| 1 (Constant) | 5.761 | 4.285 | 1.345 | 0.182 | |
| Maximum muscle strength (kg) | −0.034 | 0.067 | −0.061 | −0.510 | 0.612 |
| Gait speed (m/s) | 1.079 | 3.499 | 0.037 | 0.308 | 0.758 |
| Physical activity (METS-min/14 days) | 0.0001 | 0.0003 | 0.037 | 0.350 | 0.727 |
| 2 (Constant) | 2.800 | 6.037 | 0.464 | 0.644 | |
| Fat mass index (kg/m2) | 0.155 | 0.232 | 0.102 | 0.667 | 0.506 |
| Fat-free mass index (kg/m2) | 0.552 | 0.764 | 0.246 | 0.723 | 0.471 |
| Skeletal muscle mass index (kg/m2) | −1.014 | 1.169 | −0.320 | −0.868 | 0.388 |
| C. Dependent Variable: Protein Carbonyls | Unstandardized Coefficients | Standardized Coefficients | |||
| Model | B | SE | β | t | Significance |
| 1 (Constant) | 65.286 | 14.611 | 4.468 | 0.0001 | |
| Maximum muscle strength (kg) | −0.047 | 0.231 | −0.023 | −0.204 | 0.839 |
| Gait speed (m/s) | 26.618 | 11.990 | 0.253 | 2.220 | 0.029 |
| Physical activity (METS-min/14 days) | −0.001 | 0.001 | −0.114 | −1.118 | 0.267 |
| 2 (Constant) | 72.266 | 21.674 | 3.334 | 0.001 | |
| Fat mass index (kg/m2) | 1.445 | 0.800 | 0.276 | 1.806 | 0.074 |
| Fat-free mass index (kg/m2) | −0.873 | 2.690 | −0.108 | −0.324 | 0.746 |
| Skeletal muscle mass index (kg/m2) | 1.609 | 4.074 | 0.143 | 0.395 | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabadi, B.; Civera, M.; De la Rosa, A.; Martinez-Hervas, S.; Gomez-Cabrera, M.C.; Real, J.T. Frailty Is Associated with Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients 2021, 13, 3983. https://doi.org/10.3390/nu13113983
Alabadi B, Civera M, De la Rosa A, Martinez-Hervas S, Gomez-Cabrera MC, Real JT. Frailty Is Associated with Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients. 2021; 13(11):3983. https://doi.org/10.3390/nu13113983
Chicago/Turabian StyleAlabadi, Blanca, Miguel Civera, Adrián De la Rosa, Sergio Martinez-Hervas, Mari Carmen Gomez-Cabrera, and José T. Real. 2021. "Frailty Is Associated with Oxidative Stress in Older Patients with Type 2 Diabetes" Nutrients 13, no. 11: 3983. https://doi.org/10.3390/nu13113983
APA StyleAlabadi, B., Civera, M., De la Rosa, A., Martinez-Hervas, S., Gomez-Cabrera, M. C., & Real, J. T. (2021). Frailty Is Associated with Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients, 13(11), 3983. https://doi.org/10.3390/nu13113983






