Interactions between Polygenic Risk Scores, Dietary Pattern, and Menarche Age with the Obesity Risk in a Large Hospital-Based Cohort
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Demographic, Anthropometric, and Biochemical Information
2.3. Definition of Obesity and MetS
2.4. Food and Nutrient Intake and Dietary Patterns
2.5. Dietary Inflammatory Index (DII)
2.6. Genotyping Using a Korean Chip and Quality Control
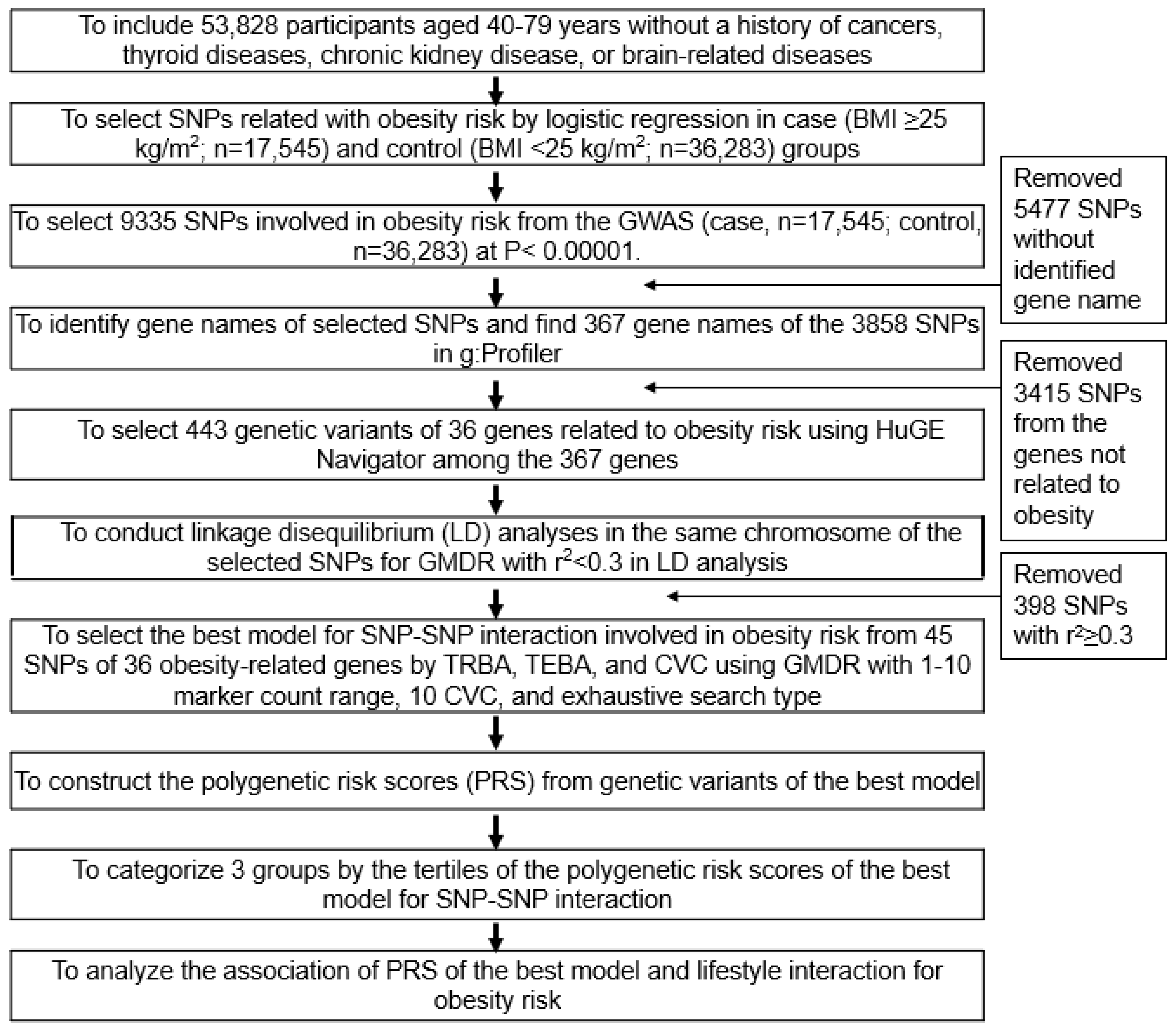
2.7. Genetic Variants Influencing the Obesity Risk and the Best Model with SNP−SNP Interactions
2.8. Statistical Analyses
3. Results
3.1. General and Demographic Characteristics of the Participants According to Gender and Obesity
3.2. Lifestyle Characteristics of the Participants According to Genders and Obesity
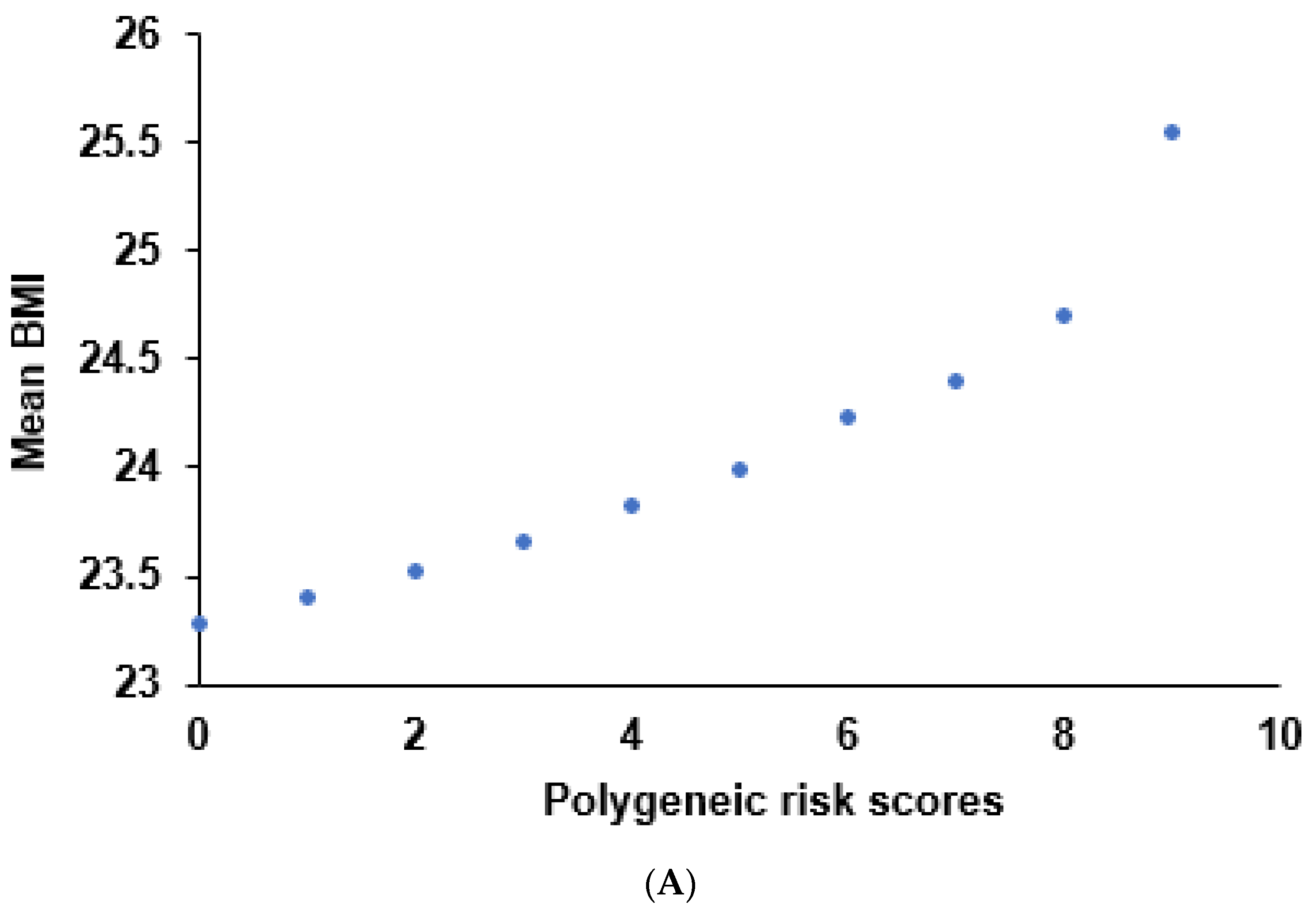
3.3. Genetic Variants Related to the Obesity Risk and the Best Model with SNP-SNP Interaction
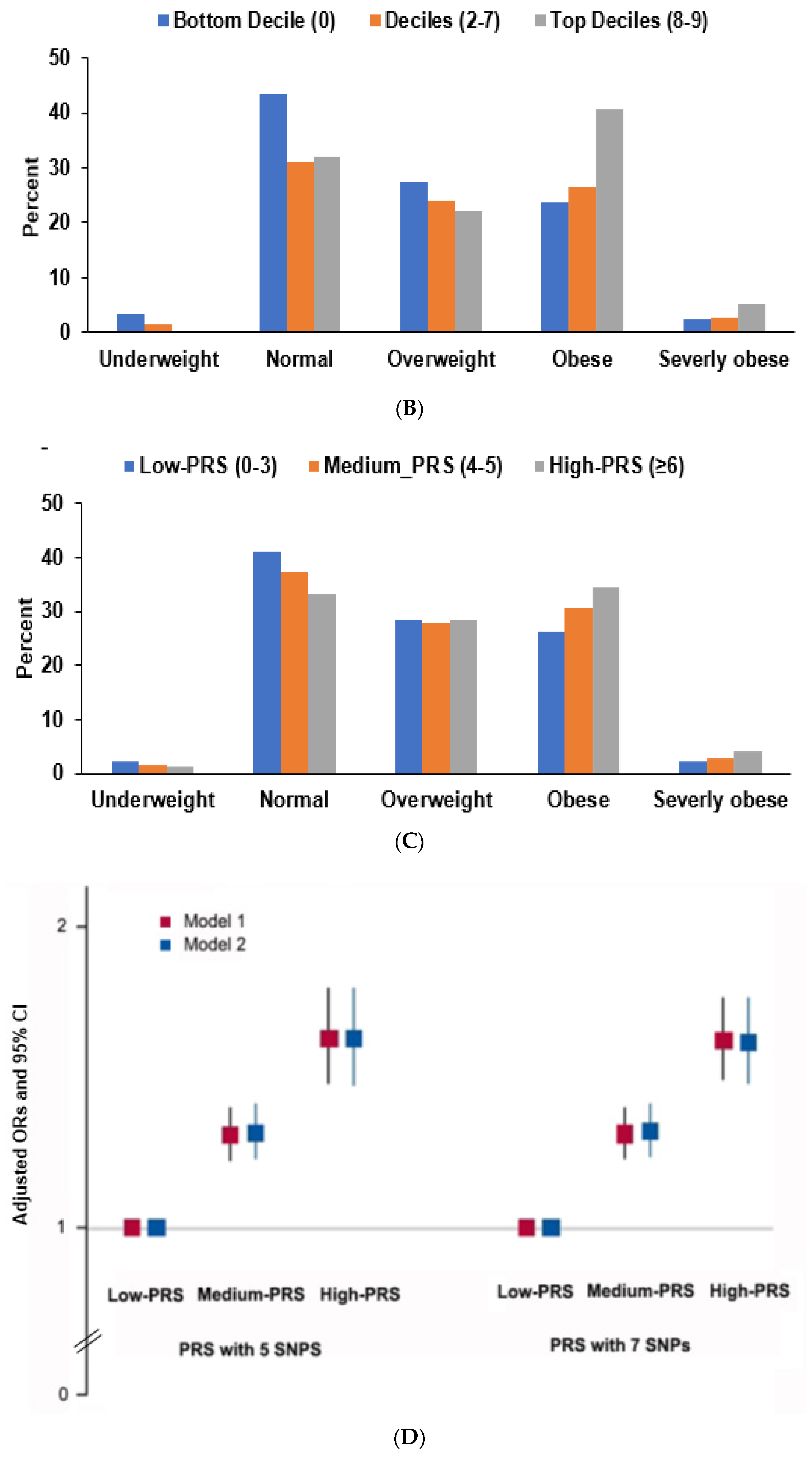
3.4. Interaction of PRS and Nutrient Intake in Obesity Risk
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Haldar, S.; Chia, S.C.; Henry, C.J. Body Composition in Asians and Caucasians: Comparative Analyses and Influences on Cardiometabolic Outcomes. Adv. Food Nutr. Res. 2015, 75, 97–154. [Google Scholar]
- Misra, A. Ethnic-Specific Criteria for Classification of Body Mass Index: A Perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Techol. Ther. 2015, 17, 667–671. [Google Scholar] [CrossRef]
- Golden, A.; Kessler, C. Obesity and genetics. J. Am. Assoc. Nurse Pract. 2020, 32, 493–496. [Google Scholar] [CrossRef]
- Cheng, M.; Mei, B.; Zhou, Q.; Zhang, M.; Huang, H.; Han, L.; Huang, Q. Computational analyses of obesity-associated loci generated by genome-wide association studies. PLoS ONE 2018, 13, e0199987. [Google Scholar] [CrossRef] [PubMed]
- Westerman, K.E.; Miao, J.; Chasman, D.I.; Florez, J.C.; Chen, H.; Manning, A.K.; Cole, J.B. Genome-wide gene-diet interaction analysis in the UK Biobank identifies novel effects on Hemoglobin A1c. Hum. Mol. Genet. 2021, 30, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Song, S.S.; Huang, S.; Park, S. Association of Polygenetic Risk Scores Related to Cell Differentiation and Inflammation with Thyroid Cancer Risk and Genetic Interaction with Dietary Intake. Cancers 2021, 13, 1510. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A.; et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell 2019, 177, 587–596.e589. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Santos, J.L.; Gratacos, M.; Moreno-Aliaga, M.J.; Maiz, A.; Martinez, J.A.; Estivill, X. Association between leptin receptor (LEPR) and brain-derived neurotrophic factor (BDNF) gene variants and obesity: A case-control study. Nutr. Neurosci. 2009, 12, 183–188. [Google Scholar] [CrossRef]
- López-Rodríguez, G.; Estrada-Neria, A.; Suárez-Diéguez, T.; Tejero, M.E.; Fernández, J.C.; Galván, M. Common polymorphisms in MC4R and FTO genes are associated with BMI and metabolic indicators in Mexican children: Differences by sex and genetic ancestry. Gene 2020, 754, 144840. [Google Scholar] [CrossRef] [PubMed]
- Chalazan, B.; Palm, D.; Sridhar, A.; Lee, C.; Argos, M.; Daviglus, M.; Rehman, J.; Konda, S.; Darbar, D. Common genetic variants associated with obesity in an African-American and Hispanic/Latino population. PLoS ONE 2021, 16, e0250697. [Google Scholar] [CrossRef]
- Sun, C.; Kovacs, P.; Guiu-Jurado, E. Genetics of Obesity in East Asians. Front. Genet. 2020, 11, 575049. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, H.J.; Liu, M.; Kim, M.J.; Park, S. Subcutaneous fat mass is associated with genetic risk scores related to proinflammatory cytokine signaling and interact with physical activity in middle-aged obese adults. Nutr. Metab. 2019, 16, 75. [Google Scholar] [CrossRef]
- Daily, J.W.; Liu, M.; Park, S. High genetic risk scores of SLIT3, PLEKHA5 and PPP2R2C variants increased insulin resistance and interacted with coffee and caffeine consumption in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S. A Western-style diet interacts with genetic variants of the LDL receptor to hyper-LDL cholesterolemia in Korean adults. Public Health Nutr. 2021, 24, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef] [PubMed]
- Parastouei, K.; Rostami, H.; Ramezani, A.A.; Tavakoli, H.; Alipour, M. Gene-diet interaction of FTO-rs9939609 gene variant and hypocaloric diet on glycemic control in overweight and obese adults: A systematic review and meta-analysis of clinical trials. Chin. Med. J. 2020, 133, 310–317. [Google Scholar] [CrossRef]
- Isgin-Atici, K.; Alsulami, S.; Turan-Demirci, B.; Surendran, S.; Sendur, S.N.; Lay, I.; Karabulut, E.; Ellahi, B.; Lovegrove, J.A.; Alikasifoglu, M.; et al. FTO gene-lifestyle interactions on serum adiponectin concentrations and central obesity in a Turkish population. Int. J. Food Sci. Nutr. 2021, 72, 375–385. [Google Scholar] [CrossRef]
- Hiraike, Y.; Yang, C.-T.; Liu, W.-J.; Yamada, T.; Lee, C.-L. FTO obesity variant-exercise interaction on changes in body weight and BMI: The Taiwan Biobank study. J. Clin. Endocrin. Metab. 2021, 106, e3673–e3681. [Google Scholar] [CrossRef]
- Park, S.; Daily, J.W.; Song, M.Y.; Kwon, H.K. Gene-gene and gene-lifestyle interactions of AKAP11, KCNMA1, PUM1, SPTBN1, and EPDR1 on osteoporosis risk in middle-aged adults. Nutrition 2020, 79–80, 110859. [Google Scholar] [CrossRef]
- Park, S.; Ahn, J.; Lee, B.K. Self-rated Subjective Health Status Is Strongly Associated with Sociodemographic Factors, Lifestyle, Nutrient Intakes, and Biochemical Indices, but Not Smoking Status: KNHANES 2007-2012. J. Korean Med. Sci. 2015, 30, 1279–1287. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Park, S. An Inverse Relation between Hyperglycemia and Skeletal Muscle Mass Predicted by Using a Machine Learning Approach in Middle-Aged and Older Adults in Large Cohorts. J. Clin. Med. 2021, 10, 2133. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.-Y.; Kim, S.S.; Kang, J.-H.; Kang, J.-H.; Kim, K.K.; Kim, B.-Y.; Kim, Y.-H.; Kim, W.-J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- van Woudenbergh, G.J.; Theofylaktopoulou, D.; Kuijsten, A.; Ferreira, I.; van Greevenbroek, M.M.; van der Kallen, C.J.; Schalkwijk, C.G.; Stehouwer, C.D.; Ocké, M.C.; Nijpels, G.; et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: The Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am. J. Clin. Nutr. 2013, 98, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, M.K. Relationship of sodium intake with obesity among Korean children and adolescents: Korea National Health and Nutrition Examination Survey. Br. J. Nutr. 2016, 115, 834–841. [Google Scholar] [CrossRef]
- Rabbee, N.; Speed, T.P. A genotype calling algorithm for affymetrix SNP arrays. Bioinformatics 2006, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Uma Jyothi, K.; Reddy, B.M. Gene-gene and gene-environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene 2015, 5, 9–20. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Song, M.Y.; Park, S. Carbohydrate and sodium intake and physical activity interact with genetic risk scores of four genetic variants mainly related to lipid metabolism to modulate metabolic syndrome risk in Korean middle-aged adults. Br. J. Nutr. 2019, 122, 919–927. [Google Scholar] [CrossRef]
- Theilade, S.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. An overview of obesity mechanisms in humans: Endocrine regulation of food intake, eating behaviour and common determinants of body weight. Diabetes Obes. Metab. 2021, 23 (Suppl. 1), 17–35. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Yeo, G.S.; Keogh, J.M.; Aminian, S.; Jebb, S.A.; Butler, G.; Cheetham, T.; O’Rahilly, S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000, 106, 271–279. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef]
- Fu, J.; Li, G.; Li, L.; Yin, J.; Cheng, H.; Han, L.; Zhang, Q.; Li, N.; Xiao, X.; Grant, S.F.A.; et al. The role of established East Asian obesity-related loci on pediatric leptin levels highlights a neuronal influence on body weight regulation in Chinese children and adolescents: The BCAMS study. Oncotarget 2017, 8, 93593–93607. [Google Scholar] [CrossRef][Green Version]
- Ramírez, D.; Saba, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Melanocortin 4 receptor activates ERK-cFos pathway to increase brain-derived neurotrophic factor expression in rat astrocytes and hypothalamus. Mol. Cell. Endocrinol. 2015, 411, 28–37. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Glick, B.S. Two Mammalian Sec16 Homologues Have Nonredundant Functions in Endoplasmic Reticulum (ER) Export and Transitional ER Organization. Mol. Biol. Cell 2007, 18, 839–849. [Google Scholar] [CrossRef]
- Schmid, P.M.; Heid, I.; Buechler, C.; Steege, A.; Resch, M.; Birner, C.; Endemann, D.H.; Riegger, G.A.; Luchner, A. Expression of fourteen novel obesity-related genes in Zucker diabetic fatty rats. Cardiovasc. Diabetol. 2012, 11, 48. [Google Scholar] [CrossRef]
- Magno, F.; Guaraná, H.C.; da Fonseca, A.C.P.; Pedrosa, A.P.; Zembrzuski, V.M.; Cabello, P.H.; Cabello, G.M.K.; Carneiro, J.R.I.; Rosado, E.L. Association of the MC4R rs17782313 polymorphism with plasma ghrelin, leptin, IL6 and TNFα concentrations, food intake and eating behaviors in morbidly obese women. Eat. Weight Disord 2021, 26, 1079–1087. [Google Scholar] [CrossRef]
- Perng, W.; Harte, R.; Ringham, B.M.; Baylin, A.; Bellatorre, A.; Scherzinger, A.; Goran, M.I.; Dabelea, D. A Prudent dietary pattern is inversely associated with liver fat content among multi-ethnic youth. Pediatr. Obes. 2021, 16, e12758. [Google Scholar] [CrossRef]
- Shikany, J.M.; Demmer, R.T.; Johnson, A.J.; Fino, N.F.; Meyer, K.; Ensrud, K.E.; Lane, N.E.; Orwoll, E.S.; Kado, D.M.; Zmuda, J.M.; et al. Association of dietary patterns with the gut microbiota in older, community-dwelling men. Am. J. Clin. Nutr. 2019, 110, 1003–1014. [Google Scholar] [CrossRef]
- Wu, X.; Unno, T.; Kang, S.; Park, S. A Korean-Style Balanced Diet Has a Potential Connection with Ruminococcaceae Enterotype and Reduction of Metabolic Syndrome Incidence in Korean Adults. Nutrients 2021, 13, 495. [Google Scholar] [CrossRef]
- Mills, S.; Lane, J.A.; Smith, G.J.; Grimaldi, K.A.; Ross, R.P.; Stanton, C. Precision Nutrition and the Microbiome Part II: Potential Opportunities and Pathways to Commercialisation. Nutrients 2019, 11, 1468. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Follis, J.L.; Ngwa, J.S.; Smith, C.E.; Ahmad, S.; Tanaka, T.; Wojczynski, M.K.; Voortman, T.; Lemaitre, R.N.; Kristiansson, K.; et al. Gene × dietary pattern interactions in obesity: Analysis of up to 68 317 adults of European ancestry. Hum. Mol. Genet. 2015, 24, 4728–4738. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Robinson, J.G.; Wallace, R.B.; Peterson, L.L.; Bao, W. Association of fried food consumption with all cause, cardiovascular, and cancer mortality: Prospective cohort study. BMJ 2019, 364, k5420. [Google Scholar] [CrossRef]
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djoussé, L. Fried Food Consumption and Cardiovascular Health: A Review of Current Evidence. Nutrients 2015, 7, 8424–8430. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J. Association between fried food consumption and hypertension in Korean adults. Br. J. Nutr. 2016, 115, 87–94. [Google Scholar] [CrossRef]

| Men (n = 19,444) | Women (n = 34,384) | Adjusted ORs and 95% CI | |||
|---|---|---|---|---|---|
| Control (n = 11,690) | Obese (n = 7754) | Control (n = 24,593) | Obese (n = 9791) | ||
| Age (yr) 1 | 56.9 ± 0.08 a | 56.6 ± 0.10 a | 51.4 ± 0.05 d | 52.6 ± 0.08 c***###+++ | 1.106 (1.030–1.188) |
| Height (cm) 2 | 168.6 ± 0.06 a | 168.4 ± 0.07 a | 156.9 ± 0.04 b | 156.2 ± 0.06 c***###+++ | 1.013 (0.934–1.099) |
| BMI (mg/kg2) 3 | 22.7 ± 0.02 b | 27.0 ± 0.02 a | 22.2 ± 0.01 b | 27.2 ± 0.02 a*** | |
| Waist circumference (cm) 4 | 81.8 ± 0.07 c | 91.1 ± 0.08 a | 75.2 ± 0.05 d | 85.7 ± 0.07 b***###+++ | 18.29 (16.46–20.34) |
| SMI (%) 5 | 29.8 ± 0.02 b | 32.9 ± 0.03 a | 23.4 ± 0.02 d | 25.9 ± 0.02 c***###+++ | 3.380 (3.111–3.673) |
| Fat mass (%) 6 | 21.3 ± 0.02 d | 26.2 ± 0.03 c | 29.4 ± 0.02 b | 35.3 ± 0.03 a***###+++ | 20.71 (17.83–24.16) |
| Menarche age 7 | 15.2 ± 0.01 | 15.0 ± 0.02 *** | 0.778 (0.725–0.834) | ||
| Menopausal age 8 | 49.3 ± 0.04 | 49.3 ± 0.06 | 1.410 (0.975–2.038) | ||
| Education ≤Middle school High school ≥College | 954 (13.2) 1431 (19.8) 4843 (67.0) | 592 (12.9) 941 (20.4) 3070 (66.7) | 3255 (17.6) 4004 (21.6) 11,254(60.8) | 2296 (28.0) *** 2084 (25.5) 3809 (46.5) | 1 0.812 (0.756–0.872) 0.627 (0.587–0.671) |
| Income ≤USD 2000 USD 2000–4000 >USD 4000 | 965 (8.68) 4936 (44.4) 5222 (47.0) | 544 (7.38) *** 2900 (39.3) 3929 (53.3) | 2338 (10.1) 9973 (43.0) 10,896(47.0) | 1378 (15.1) *** 4299 (47.1) 3460 (37.9) | 1 0.985 (0.915–1.061) 0.915 (0.854–0.980) |
| MetS (%) 9 | 942 (8.1) | 2514(32.4) *** | 1478 (6.0) | 2725 (27.8) *** | 5.860 (5.307–6.471) |
| Serum glucose (mg/dL) 10 | 96.4 ± 0.23 b | 100.4 ± 0.27 a | 92.3 ± 0.15 c | 97.2 ± 0.23 b***###+ | 1.668 (1.548–1.796) |
| HbA1c (%) 11 | 5.61 ± 0.01 d | 5.80 ± 0.01 b | 5.66 ± 0.01 c | 5.87 ± 0.01 a***### | 1.695 (1.517–1.893) |
| Serum total cholesterol 12 | 188.5 ± 0.41 d | 192.1 ± 0.48 c | 200.4 ± 0.27 b | 204.8 ± 0.41 a***### | 1.475 (1.352–1.610) |
| Serum HDL 13 | 51.0 ± 0.15 c | 46.3 ± 0.17 d | 57.7 ± 0.10 a | 53.0 ± 0.14 b***### | 1.913 (1.756–2.083) |
| Serum LDL 14 | 113.0 ± 0.38 c | 113.8 ± 0.45 c | 120.3 ± 0.25 b | 124.4 ± 0.37 a***###+++ | 1.484 (1.338–1.647) |
| Serum Triglyceride 15 | 122.6 ± 0.97 b | 160.6 ± 1.14 a | 111.8 ± 0.64 d | 137.3 ± 0.96 c***###+++ | 2.157 (2.006–2.320) |
| Serum hs-CRP 16 | 0.17 ± 0.01 ab | 0.19 ± 0.01 a | 0.12 ± 0.02 b | 0.23 ± 0.03 a***+ | 1.266 (1.008–1.589) |
| SBP (mmHg) 17 | 123.7 ± 0.16 c | 128.9 ± 0.19 a | 119.0 ± 0.11 d | 125.3 ± 0.16 b***###+++ | 1.782 (1.657–1.916) |
| DBP (mmHg) 18 | 77.2 ± 0.11 b | 80.5 ± 0.13 a | 73.1 ± 0.07 c | 76.9 ± 0.11 b***###++ | 1.946 (1.750–2.164) |
| eGFR (ml/min) 19 | 84.7 ± 0.18 c | 83.3 ± 0.24 d | 86.9 ± 0.13 a | 88.0 ± 0.21 b***+++ | 1.140 (1.039–1.251) |
| Serum AST (U/L) 20 | 24.3 ± 0.25 b | 26.6 ± 0.31 a | 22.3 ± 0.17 c | 24.4 ± 0.27 b***### | 2.013 (1.837–2.205) |
| Serum ALT(U/L) 21 | 23.8 ± 0.24 b | 30.8 ± 0.29 a | 18.6 ± 0.16 c | 23.9 ± 0.26 b***###++ | 2.724 (2.566–2.892) |
| Serum hs-CRP (mg/L) 22 | 0.17 ± 0.01 ab | 0.19 ± 0.01 a | 0.12 ± 0.02 b | 0.23 ± 0.03 a**+ | 1.645 (1.201–2.254) |
| Men (n = 19,444) | Women (n = 38,384) | Adjusted ORs and 95% CI 1 | |||
|---|---|---|---|---|---|
| Control (n = 11,690) | Obese (n = 7754) | Control (n = 24,593) | Obese (n = 9791) | ||
| Energy (<EER %) 2 | 90.2 ± 0.32 3 | 92.4 ± 0.39 | 92.8 ± 1.07 | 103.5 ± 1.62 ***###+++ | 1.244 (1.153–1.342) |
| CHO (<70 En %) | 71.0 ± 0.07 a | 70.7 ± 0.09 a | 69.6 ± 0.26 b | 70.1 ± 0.39 ab### | 0.946 (0.895–1.000) |
| Protein(<14 En%) | 13.6 ± 0.03 b | 13.5 ± 0.03 b | 14.2 ± 0.10 a | 14.1 ± 0.15 a### | 1.047 (0.998–1.090) |
| Total fat (<15 En%) | 14.5 ± 0.06 b | 14.6 ± 0.07 b | 15.4 ± 0.20 a | 14.9 ± 0.30 ab## | 1.020 (0.977–1.064) |
| Saturated fat (<4.7 En%) | 0.44 ± 0.002 b | 0.46 ± 0.003 a | 0.45 ± 0.002 a | 0.44 ± 0.003 b+++ | 1.032 (0.986–1.080) |
| Monounsaturated fat (<6.0 En%) | 0.56 ± 0.003 b | 0.58 ± 0.004 a | 0.55 ± 0.002 c | 0.54 ± 0.003 d###+++ | 1.001 (0.955–1.049) |
| Polyunsaturated fat (2.5 En%) | 0.32 ± 0.003 ab | 0.33 ± 0.003 a | 0.31 ± 0.002 b | 0.31 ± 0.003 b###++ | 1.038 (0.992–1.087) |
| Cholesterol (<200 mg/d) | 179 ± 1.13 a | 181 ± 1.33 a | 165 ± 0.74 b | 162 ± 1.12 c###++ | 0.985 (0.930–1.043) |
| Fiber (6 g/d) | 5.98 ± 0.02 a | 5.94 ± 0.03 a | 5.51 ± 0.02 b | 5.49 ± 0.02 b### | 0.985 (0.892–1.086) |
| DII (<2374 scores) | 2096 ± 15.9 a | 2088 ± 18.8 a | 1917 ± 10.5 b | 1939 ± 15.9 b### | 0.980 (0.933–1.030) |
| Fried foods (<0.6/week) | 0.53 ± 0.01 b | 0.60 ± 0.01 a | 0.42 ± 0.01 c | 0.50 ± 0.01 b***### | 1.217 (1.117–1.326) |
| Sugar-containing foods | 3.05 ± 0.09 a | 2.98 ± 0.10 a | 2.79 ± 0.06 a | 2.45 ± 0.09 b**## | 0.984 (0.905–1.070) |
| Balanced Korean diet (<70th percentile) | 10,984 (66.9) | 2114 (69.9) ** | 19,746 (64.6) | 2843(67.2) ** | 1.137 (1.089–1.186) |
| Plant-based diet (<70th percentile) | 8721 (53.1) 4 | 1552 (51.3) | 21,961 (72.8) | 2857 (67.5) *** | 0.868 (0.832–0.907) |
| Western-style diet (<70th percentile) | 12,949 (78.9) | 2487 (82.2) *** | 17,898 (59.4) | 2552 (60.3) | 1.142 (1.092–1.195) |
| Rice-based diet (<70th percentile) | 10,949 (66.7) | 1974 (65.2) | 19,580 (64.9) | 2828 (66.8) * | 1.001 (0.960–1.045) |
| Alcohol drinking (<100 g/week) | 199 ± 3.37 b | 241 ± 3.96 a | 57.8 ± 2.22 d | 64.1 ± 3.36 c***###+++ | 1.139 (1.060–1.225) |
| Smoking status (current smokers) | 3423 (29.4) | 2106 (27.2) *** | 469 (1.91) | 212 (2.17) | 0.820 (0.761–0.884) |
| Regular Exercise 5 | 6897 (59.0) | 4575 (59.0) | 12,961 (52.7) | 4523 (46.2) *** | 0.444 (0.203–0.974) |
| Chr 1 | SNP 2 | Position | Mi 3 | Ma 4 | OR and 95% CI for City 5 | p-Value Adjusted (City) 6 | p-Value Adjusted (Urban) 7 | MAF 8 | p-Value for HWE 9 | Gene | Functional Consequence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rs543874 | 177889480 | G | A | 1.13 (1.10–1.17) | 6.65 × 10−16 | 1.52 × 10−3 | 0.249 | 0.581 | SEC16B | exon |
| 2 | rs713586 | 25158008 | C | T | 1.08 (1.05–1.11) | 2.16 × 10−8 | 2.25 × 10−3 | 0.485 | 0.225 | DNAJC27 | exon |
| 6 | rs9356744 | 20685486 | C | T | 0.95 (0.93–0.98) | 4.06 × 10−5 | 8.80 × 10−5 | 0.466 | 0.102 | CDKAL1 | intron |
| 6 | rs2206277 | 50798526 | T | C | 1.07 (1.04–1.11) | 4.35 × 10−7 | 5.06 × 10−2 | 0.310 | 0.969 | TFAP2B | intron |
| 11 | rs6265 | 27679916 | T | C | 0.92 (0.89–0.94) | 2.04 × 10−10 | 2.84 × 10−1 | 0.459 | 0.148 | BDNF | missense |
| 12 | rs3782889 | 111350655 | G | A | 0.94 (0.91–0.97) | 4.84 × 10−4 | 8.11 × 10−3 | 0.171 | 0.449 | MYL2 | intron |
| 13 | rs9568856 | 54064981 | A | G | 1.08 (1.05–1.11) | 1.33 × 10−7 | 3.17 × 10−1 | 0.285 | 0.620 | OLFM4 | intron |
| 16 | rs1421085 | 53800954 | C | T | 1.18 (1.13–1.22) | 1.82 × 10−16 | 1.54 × 10−6 | 0.125 | 0.460 | FTO | intron |
| 18 | rs17782313 | 57829135 | C | T | 1.11 (1.08–1.15) | 3.16 × 10−12 | 2.14 × 10−4 | 0.239 | 0.585 | MC4R | exon |
| 19 | rs1444988703 | 46175046 | A | T | 1.10 (1.07–1.13) | 2.86 × 10−12 | 6.22 × 10−5 | 0.407 | 0.441 | GIPR | intron |
| GMDR | Adjusted for Gender, Age, and Residence Area | Adjusted for Gender, Age, Residence Area, Regular Exercise, and Smoking Status | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | TRBA | TEBA | p-Value | CVC | TRBA | TEBA | p-Value | CVC |
| SEC16B_ rs543874 | 0.5171 | 0.5144 | 10 (0.0010) | 8/10 | 0.5174 | 0.5156 | 10 (0.0010) | 9/10 |
| Model 1 plus BDNF_ rs6265 | 0.5237 | 0.5178 | 10 (0.0010) | 6/10 | 0.5239 | 0.5179 | 10 (0.0010) | 6/10 |
| model 2 plus GIPR_ rs1444988703 | 0.5275 | 0.5180 | 10 (0.0010) | 4/10 | 0.5276 | 0.5183 | 10 (0.0010) | 4/10 |
| Model 3 plus FTO_ rs1421085 | 0.5322 | 0.5228 | 10 (0.0010) | 6/10 | 0.5326 | 0.5222 | 10 (0.0010) | 4/10 |
| Model 3 plus DNAJC27_ rs713586, MC4R_ rs17782313 | 0.5413 | 0.5260 | 10 (0.0010) | 10/10 | 0.5416 | 0.5254 | 10 (0.0010) | 10/10 |
| Model 5 plus OLFM4_ rs9568856 | 0.5536 | 0.5232 | 10 (0.0010) | 9/10 | 0.5539 | 0.5210 | 10 (0.0010) | 6/10 |
| Model 6 plus CDKAL1_ rs9356744 | 0.5774 | 0.5224 | 10 (0.0010) | 10/10 | 0.5778 | 0.5197 | 10 (0.0010) | 10/10 |
| Model 7 plus TFAP2B_ rs2206277 | 0.6129 | 0.5165 | 10 (0.0010) | 10/10 | 0.6139 | 0.5145 | 10 (0.0010) | 10/10 |
| Model 8 plus MYL2_ rs3782889 | 0.6550 | 0.5163 | 10 (0.0010) | 10/10 | 0.6568 | 0.5163 | 10 (0.0010) | 10/10 |
| Model 4 plus DNAJC27, CDKAL1, TFAP2B, MYL2, OLFM4, MC4R | 0.6978 | 0.5129 | 10 (0.0010) | 10/10 | 0.6996 | 0.5122 | 10 (0.0010) | 10/10 |
| Men | Women | ||||
|---|---|---|---|---|---|
| Low PRS 1 | Medium PRS (n = 13,024) | High PRS (n = 1931) | Medium PRS (n = 23,094) | High PRS (n = 3344) | |
| Age (<55 year) | 1 | 0.965 (0.893–1.043) | 1.019 (0.901–1.151) | 0.986 (0.926–1.049) | 0.975 (0.883–1.076) |
| Waist circumference (M: 95; F 85 cm) | 1 | 0.873 (0.772–1.000) | 0.907 (0.764–1.077) | 0.971 (0.881–1.069) | 0.886 (0.764–1.027) |
| BMI (<25 mg/kg2) | 1 | 1.252 (1.161–1.350) | 1.430 (1.272–1.608) | 1.278 (1.199–1.362) | 1.554 (1.412–1.711) |
| BMI (<27 mg/kg2) | 1 | 1.232 (1.109–1.369) | 1.479 (1.267–1.727) | 1.375 (1.255–1.506) | 1.742 (1.531–1.983) |
| Skeletal muscle index 2 (%) | 1 | 0.976 (0.897–1.063) | 0.940 (0.822–1.075) | 0.998 (0.937–1.063) | 0.928 (0.839–1.026) |
| Fat mass (%) | 1 | 1.233 (1.136–1.338) | 1.463 (1.291–1.657) | 0.893 (0.785–1.015) | 0.824 (0.656–1.001) |
| Metabolic syndrome (No) | 1 | 1.062 (0.956–1.181) | 1.106 (0.939–1.304) | 1.006 (0.915–1.106) | 0.892 (0.774–1.028) |
| Serum glucose (<126 mg/dL) | 1 | 1.012 (0.936–1.094) | 0.972 (0.859–1.099) | 0.947 (0.881–1.017) | 1.063 (0.952–1.187) |
| HbA1c (<6.5%) | 1 | 1.021 (0.905–1.151) | 1.058 (0.879–1.274) | 1.009 (0.897–1.136) | 1.182 (1.002–1.401) |
| Serum total cholesterol (<230 mg/dL) | 1 | 0.884 (0.805–0.970) | 0.826 (0.710–0.961) | 1.012 (0.948–1.081) | 1.055 (0.953–1.169) |
| Serum HDL (M: 40 F: 50 mg/dL) | 1 | 1.006 (0.917–1.103) | 0.992 (0.858–1.147) | 1.031 (0.938–1.132) | 1.007 (0.949–1.070) |
| Serum LDL (<140 mg/dL) | 1 | 0.925 (0.827–1.034) | 0.915 (0.767–1.092) | 0.986 (0.915–1.063) | 1.018 (0.906–1.144) |
| Serum triglyceride (<150 mg/dL) | 1 | 0.931 (0.862–1.006) | 0.869 (0.769–0.982) | 0.942 (0.880–1.007) | 1.030 (0.928–1.142) |
| SBP (<130 mmHg) | 1 | 1.029 (0.954–1.110) | 0.975 (0.865–1.100) | 0.997 (0.935–1.063) | 1.011 (0.916–1.117) |
| DBP (<90 mmHg) | 1 | 0.977 (0.875–1.090) | 1.026 (0.865–1.217) | 0.999 (0.894–1.117) | 1.071 (0.905–1.268 |
| eGFR (<70 mL/min) | 1 | 1.070 (0.879–1.304) | 1.206 (0.898–1.619) | 0.971 (0.817–1.154) | 1.127 (0.869–1.462) |
| Serum hs-CRP (<0.5 mg/L) | 1 | 1.606 1.106 2.332 | 1.719 (1.020–2.896) | 0.491 (0.110–2.193) | 0.613 (0.059–6.409) |
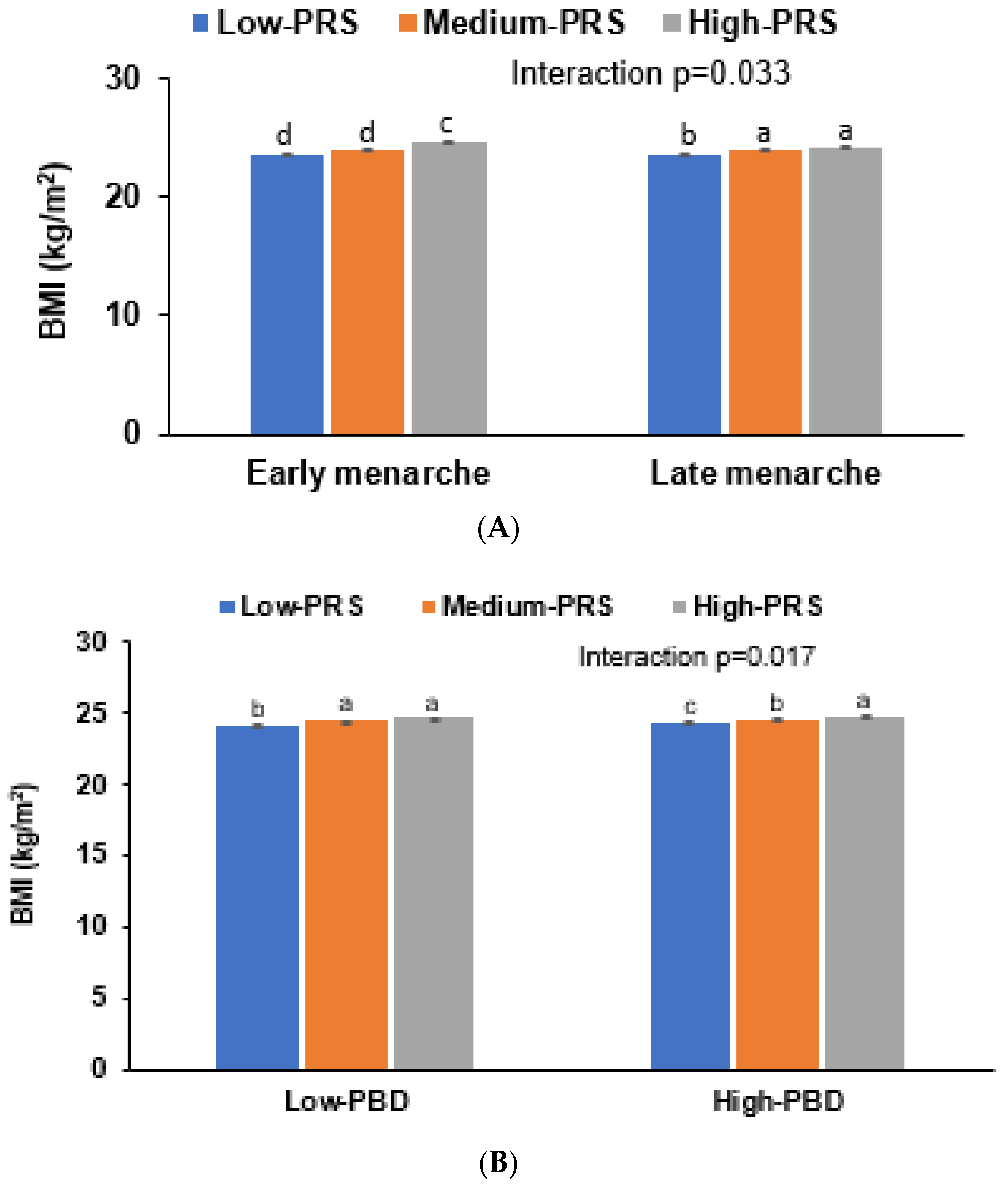
| Menarche age (<14 yr) | 1 | 0.997 (0.926 1.074) | 1.014 (0.903–1.140) | ||
| Menopausal age (<50 yr) | 1 | 1.060 (0.995 1.129) | 1.118 (0.998–1.242) | ||
| Low PRS (n = 7939) | Medium PRS (n = 23,094) | High PRS (n = 3344) | PRS−Lifestyle Interaction p-Value 3 | |
|---|---|---|---|---|
| Early menarche (<14 yr) 2 | 1 | 1.152 (0.990–1.341) 1 | 1.785 (1.427–2.233) | 0.0174 |
| Late menarche | 1 | 1.283 (1.219–1.351) | 1.479 (1.367–1.600) | |
| Low PRS (12,424) | Medium PRS (n = 36,118) | High PRS (n = 5275) | PRS−lifestyle interaction p-value | |
| Low plant-based diet (<70th percentile) | 1 | 1.241 (1.138–1.353) | 1.462 (1.279–1.670) | 0.0273 |
| High plant-based diet | 1 | 1.268 (1.118–1.437) | 1.392 (1.141–1.699) | |
| Low intake of fried food (<1 times/w) | 1 | 1.288 (1.220–1.359) | 1.472 (1.355–1.600) | 0.0364 |
| High intake of fried food | 1 | 1.196 (1.072–1.335) | 1.616 (1.374–1.902) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Yang, H.J.; Kim, M.J.; Hur, H.J.; Kim, S.-H.; Kim, M.-S. Interactions between Polygenic Risk Scores, Dietary Pattern, and Menarche Age with the Obesity Risk in a Large Hospital-Based Cohort. Nutrients 2021, 13, 3772. https://doi.org/10.3390/nu13113772
Park S, Yang HJ, Kim MJ, Hur HJ, Kim S-H, Kim M-S. Interactions between Polygenic Risk Scores, Dietary Pattern, and Menarche Age with the Obesity Risk in a Large Hospital-Based Cohort. Nutrients. 2021; 13(11):3772. https://doi.org/10.3390/nu13113772
Chicago/Turabian StylePark, Sunmin, Hye Jeong Yang, Min Jung Kim, Haeng Jeon Hur, Soon-Hee Kim, and Myung-Sunny Kim. 2021. "Interactions between Polygenic Risk Scores, Dietary Pattern, and Menarche Age with the Obesity Risk in a Large Hospital-Based Cohort" Nutrients 13, no. 11: 3772. https://doi.org/10.3390/nu13113772
APA StylePark, S., Yang, H. J., Kim, M. J., Hur, H. J., Kim, S.-H., & Kim, M.-S. (2021). Interactions between Polygenic Risk Scores, Dietary Pattern, and Menarche Age with the Obesity Risk in a Large Hospital-Based Cohort. Nutrients, 13(11), 3772. https://doi.org/10.3390/nu13113772







