Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Laboratory Wares
2.2. Animals, Treatment, and Sample Preparation
2.3. Micro-Computed Tomography (µCT) Analysis
2.4. H&E Staining of Periodontal and Pancreatic Tissue Samples
2.5. Measurement of Osteoclasts Formed in the Periodontium
2.6. Immunohistochemistry (IHC)
2.7. Measurements of Serum RANKL Level and Antioxidant Enzyme Activity
2.8. Isolation of BM Cells from Mice Groups and Osteoclastogenic Assay
2.9. Measurements of Circulating Lymphocytes and Red Blood Cells (RBC)
2.10. In Vitro Assays Using hPDLCs
2.10.1. The hPDLC Culture and Treatment
2.10.2. DCFH-DA Staining and Flow Cytometric Assay
2.10.3. Immunofluorescence Assay
2.10.4. Immunoblot Assay
2.10.5. Proliferation Assay
2.10.6. Mineralization Assay
2.11. Statistical Analyses
3. Results
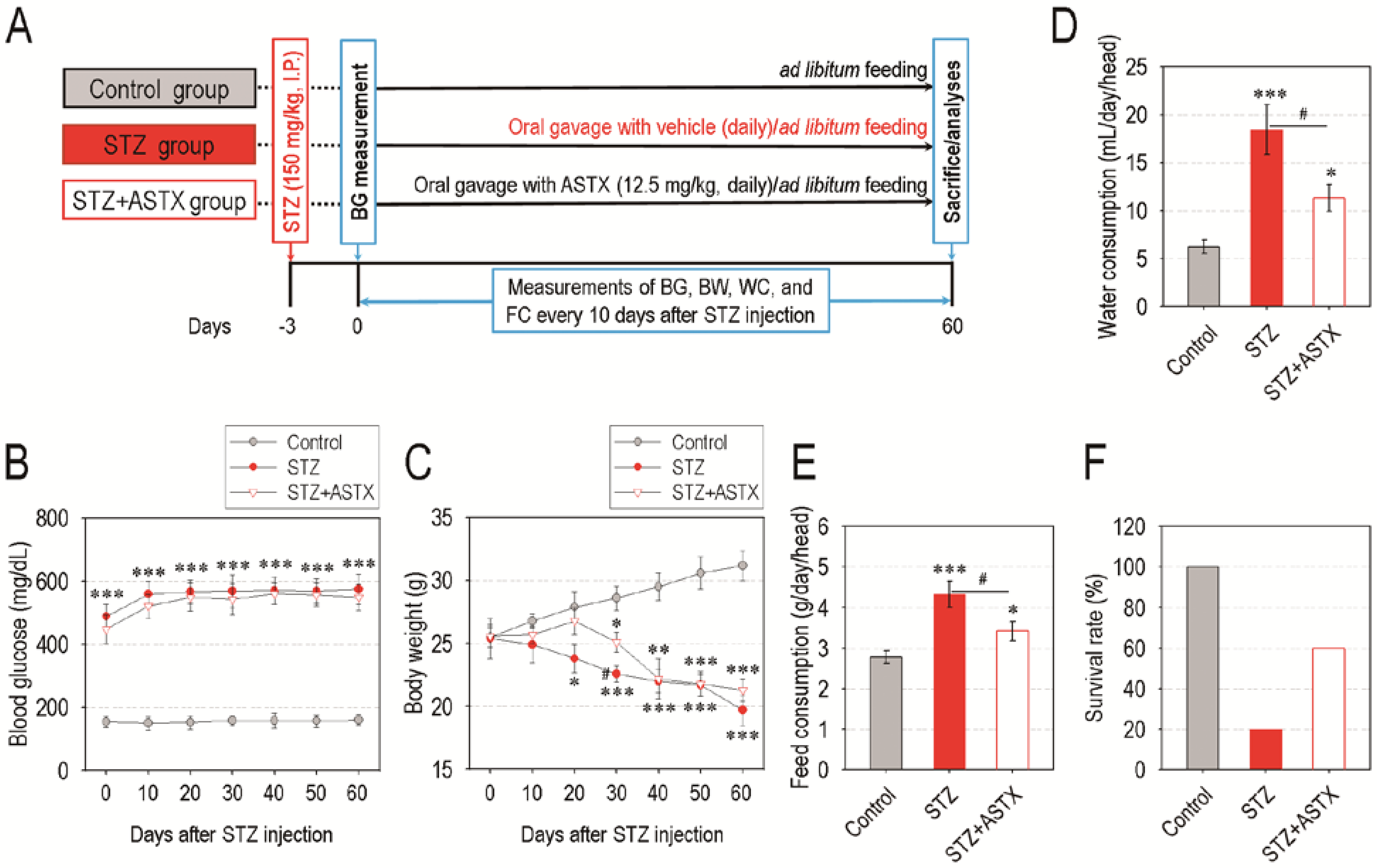
3.1. Oral Supplementation with ASTX Does Not Attenuate Blood Glucose Level, but Improves Water and Feed Consumption and Survival Rate in STZ-Induced Diabetic Mice
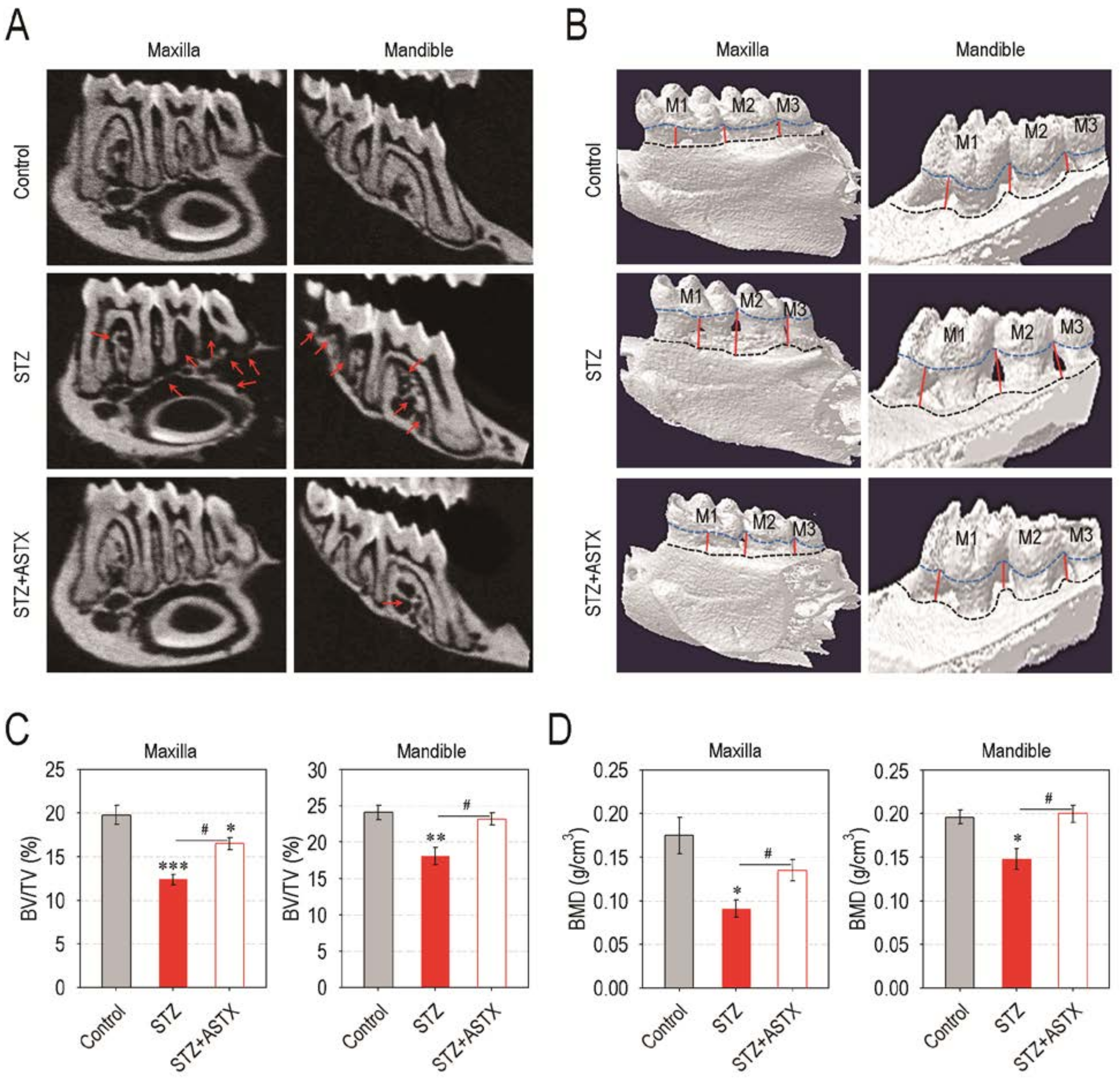
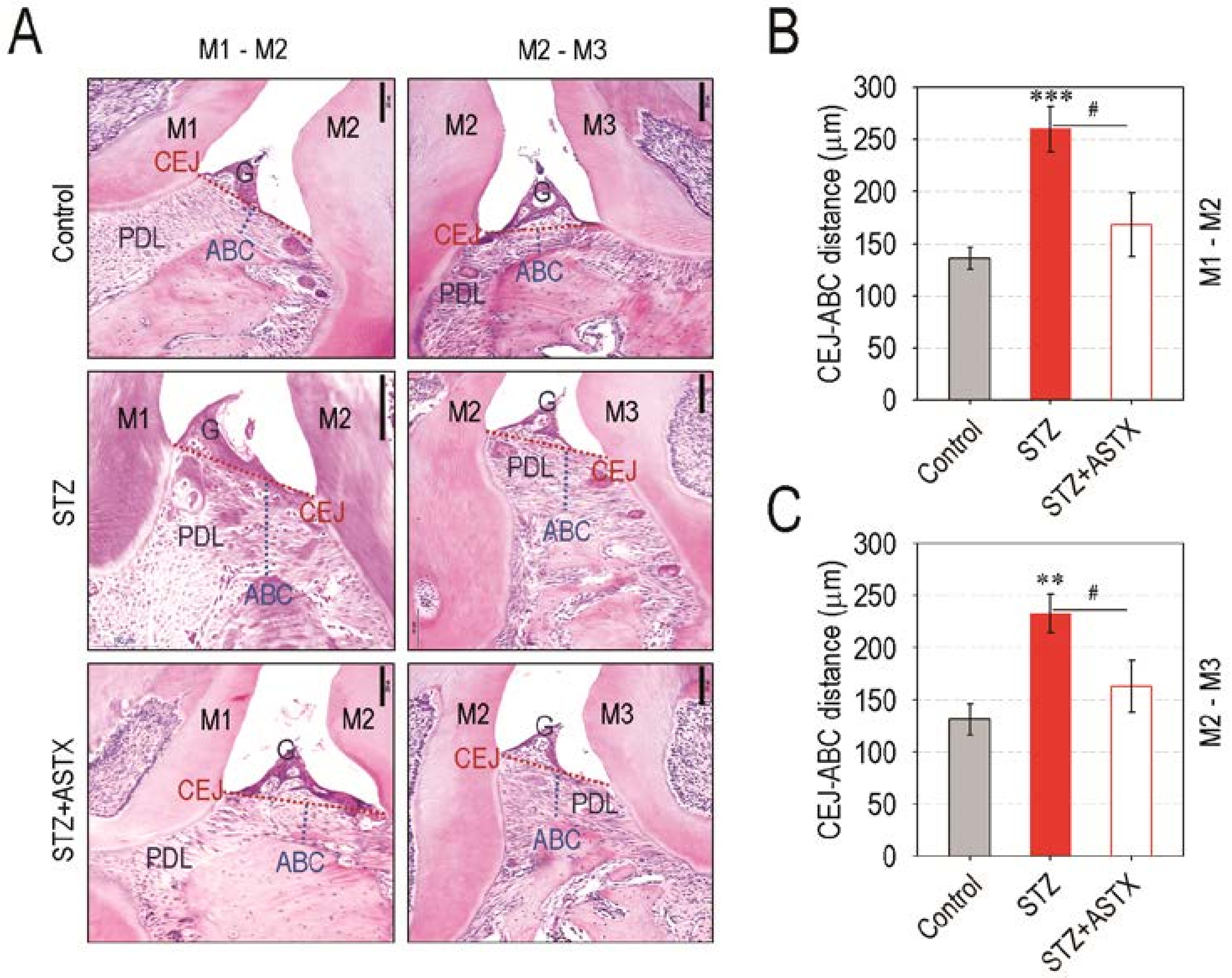
3.2. Supplemental ASTX Protects Mice from Diabetes-Triggered Periodontal Destruction in STZ-Injected Mice
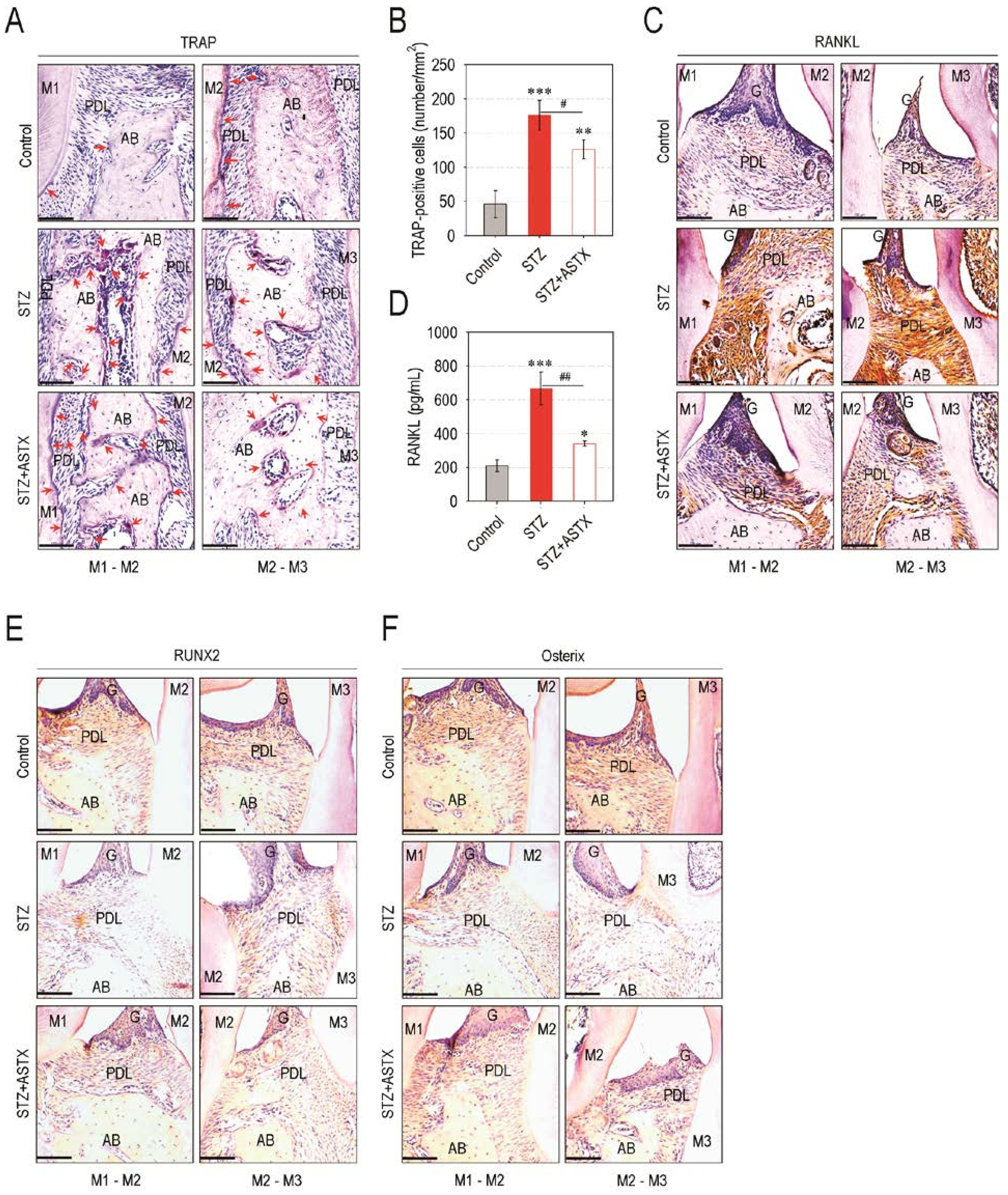
3.3. ASTX Diminishes Osteoclast Formation and the Induction of Osteoclastogenic Factors, but Restores Osteogenic Factor Expression in the Periodontium of STZ-Injected Mice
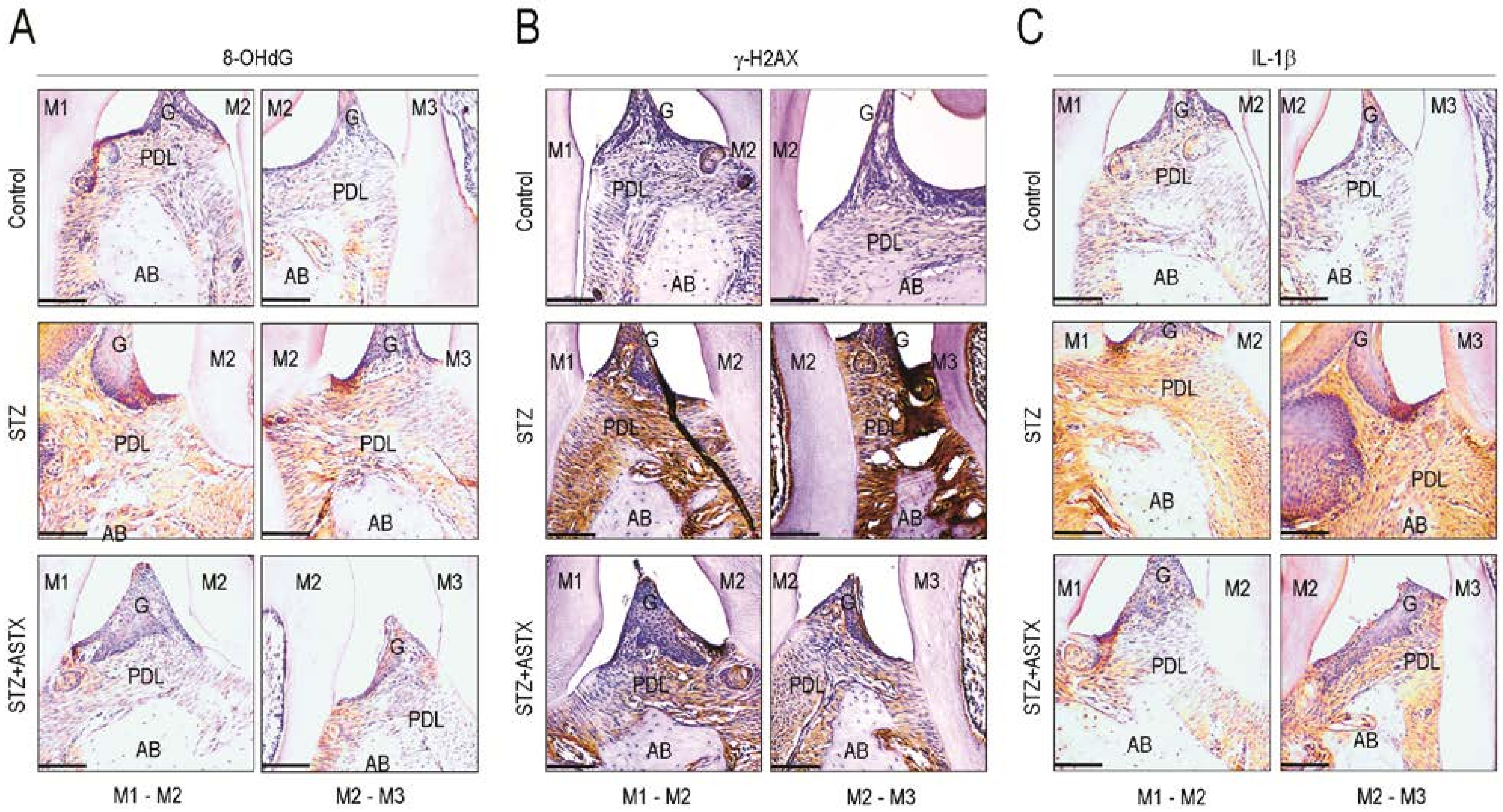
3.4. ASTX Attenuates STZ-Induced Oxidative Damage and Inflammatory Response in the Periodontium of Diabetic Mice
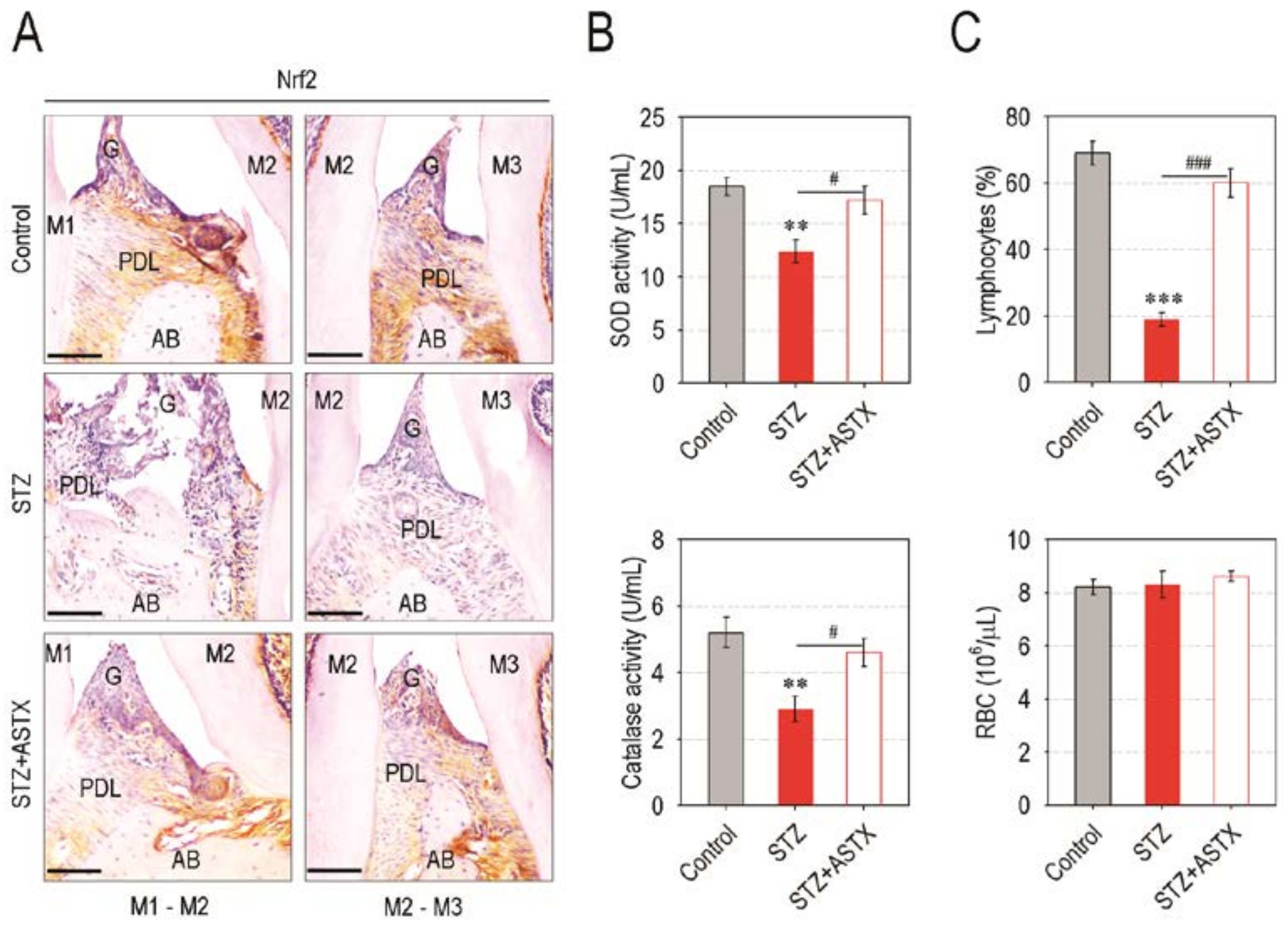
3.5. Supplemental ASTX Restores Levels of Periodontal Nrf2, Serum SOD and Catalase, and Circulating Lymphocytes in STZ-Injected Mice up to Those of Control Mice
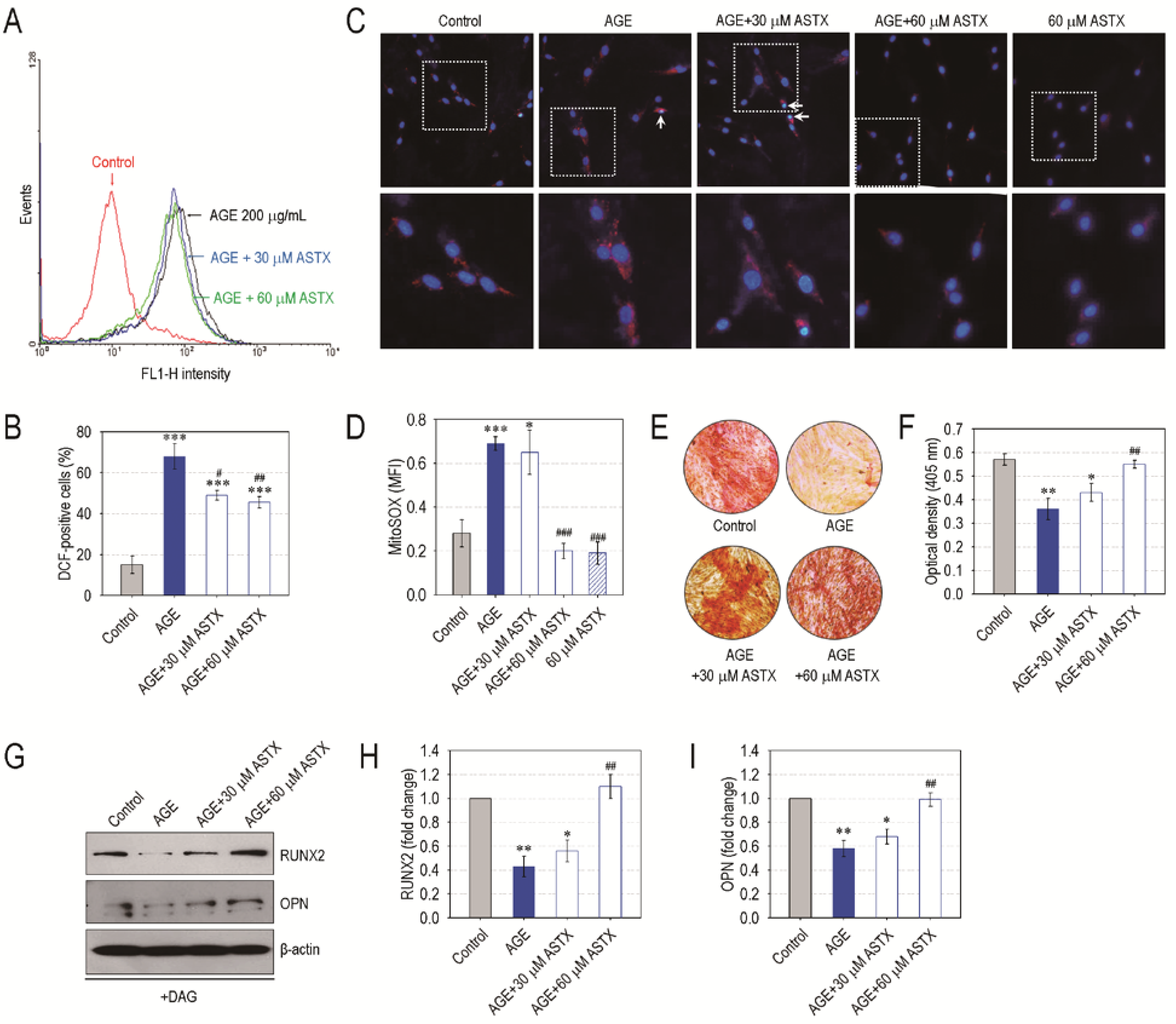
3.6. ASTX Treatment Inhibits ROS Accumulation and Recovers Mineralization in AGE-Exposed hPDLCs
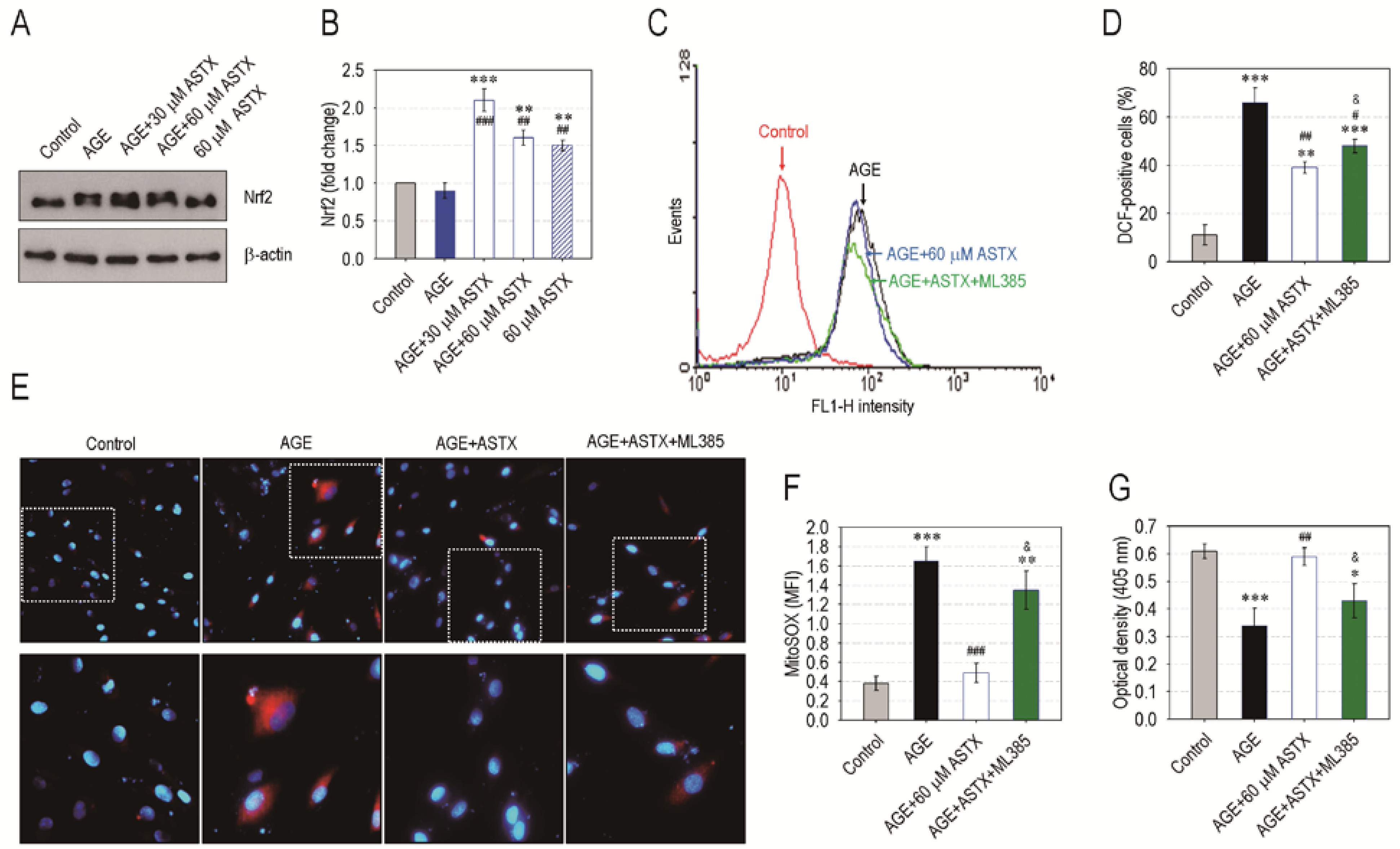
3.7. ASTX Exerts Its Potentials on Oxidative Stress and Proliferation in AGE-Treated hPDLCs via the Upregulation of Nrf2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 3, 193–205. [Google Scholar] [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Marin, C.; Luyten, F.P.; Van der Schueren, B.; Kerckhofs, G.; Vandamme, K. The impact of type 2 diabetes on bone fracture healing. Front. Endocrinol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Liu, R.; Bal, H.S.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D.T. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J. Dent. Res. 2006, 85, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Claudino, M.; Ceolin, D.S.; Alberti, S.; Cestari, T.M.; Spadella, C.T.; Rubira-Bullen, I.R.F.; Garlet, G.P.; de Assis, G.F. Alloxan-induced diabetes triggers the development of periodontal disease in rats. PLoS ONE 2007, 2, e1320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Preshaw, P.M. Periodontal disease and diabetes. J. Dent. 2009, 37, S575–S577. [Google Scholar] [CrossRef]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2013, 40, S113–S134. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling and diabetes. Free. Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajcyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Min, C.K.; Jeon, Y.M.; Bashyal, R.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Oral supplementation with p-coumaric acid protects mice against diabetes-associated spontaneous destruction of periodontal tissue. J. Periodont. Res. 2019, 54, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, Y.; Li, J.; Wang, J. The role of astaxanthin on chronic diseases. Crystals 2021, 11, 505. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Murillo, A.G.; Fernandez, M.L. Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Astaxanthin modulation of signaling pathways that regulate autophagy. Mar. Drugs 2019, 17, 546. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Otton, R.; Marina, D.P.; Bolin, A.P.; dos Santos, R.C.M.; Polotow, T.G.; Sampaio, S.C.; de Barros, M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem.-Biol. Interact. 2010, 186, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, K.J.; Kim, S.J.; Mun, S.K.; Hong, S.G.; Son, Y.J.; Yee, S.T. Suppression effect of astaxanthin on osteoclast formation in vitro and bone loss in vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Kook, S.H.; Kim, J.H.; Poudel, S.B.; Lim, S.S.; Seo, Y.K.; Lee, J.C. COMP-Ang1 prevents periodontitic damages and enhances mandible bone growth in an experimental animal model. Bone 2016, 92, 168–179. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a metanalysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Lu, H.; Kraut, D.; Gerstenfeld, L.C.; Graves, D.T. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003, 144, 346–352. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Innamorati, G.; Valenti, M.T. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev. 2012, 8, 891–897. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Baile, C.A.; Zhu, S.; Shoji, M. Bioactive flavonoid p-hydroxycinnamic acid stimulates osteoblastogenesis and suppresses adipogenesis in bone marrow culture. Cell Tissue Res. 2013, 354, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Kimura, D.; Yamawaki, I.; Noguchi, M.; Yamauchi, N.; Tamura, I.; Tanaka, A.; Umeda, M. High glucose concentrations suppress the proliferation of human periodontal ligament stem cells and their differentiation into osteoblasts. J. Periodontol. 2016, 87, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Naguib, G.; Al-Mashat, H.; Desta, T.; Graves, D.T. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J. Investig. Dermatol. 2004, 123, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Claudino, M.; Gennaro, G.; Cestari, T.M.; Spadella, C.T.; Garlet, G.P.; Assis, G.F. Spontaneous periodontitis development in diabetic rats involves an unrestricted expression of inflammatory cytokines and tissue destructive factors in the absence of major changes in commensal oral microbiota. Exp. Diabetes Res. 2012, 2012, 356841. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, H.; Pan, H.; Zhou, X.; Ruan, Q.; Kong, D.; Chu, Z.; Li, H.; Huang, J.; Huang, X.; et al. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front. Pharmacol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Lidia, I.; Ferrándiz, M.L.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid. Med. Cell. Longev. 2014, 2014, 726590. [Google Scholar]
- Sima, C.; Aboodi, G.M.; Lakschevitz, F.S.; Sun, C.; Goldberg, M.B.; Glogauer, M. Nuclear factor erythroid 2-related factor 2 down-regulation in oral neutrophils is associated with periodontal oxidative damage and severe chronic periodontitis. Am. J. Pathol. 2016, 186, 1417–1426. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Kor. J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Chavakis, E.; Seeger, F.; Tjwa, M.; Zeiher, A.M.; Dimmeler, S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res. Cardiol. 2010, 105, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Kim, J.; Chan, L. Emerging roles of hematopoietic cells in the pathobiology of diabetic complications. Trends Endocrinol. Metab. 2014, 25, 178–187. [Google Scholar] [CrossRef]
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and metabolic regulator of stem cell state and function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, G.; So, H.-S.; Kieu, T.T.T.; Kook, S.-H.; Lee, J.-C.; Jeon, Y.-M. Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System. Nutrients 2021, 13, 3575. https://doi.org/10.3390/nu13103575
Bhattarai G, So H-S, Kieu TTT, Kook S-H, Lee J-C, Jeon Y-M. Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System. Nutrients. 2021; 13(10):3575. https://doi.org/10.3390/nu13103575
Chicago/Turabian StyleBhattarai, Govinda, Han-Sol So, Thi Thu Trang Kieu, Sung-Ho Kook, Jeong-Chae Lee, and Young-Mi Jeon. 2021. "Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System" Nutrients 13, no. 10: 3575. https://doi.org/10.3390/nu13103575
APA StyleBhattarai, G., So, H.-S., Kieu, T. T. T., Kook, S.-H., Lee, J.-C., & Jeon, Y.-M. (2021). Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System. Nutrients, 13(10), 3575. https://doi.org/10.3390/nu13103575





