Eucaloric Balanced Diet Improved Objective Sleep in Adolescents with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design and Procedure
2.3. Baseline Evaluations
2.3.1. Anthropometry and Body Composition
2.3.2. Circadian Typology and Subjective Sleep
2.3.3. Resting Metabolic Rate
2.3.4. Habitual Physical Activity and Energy Expenditure
2.4. Experimental Sessions
2.4.1. Dietary Intervention
2.4.2. Polysomnography
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
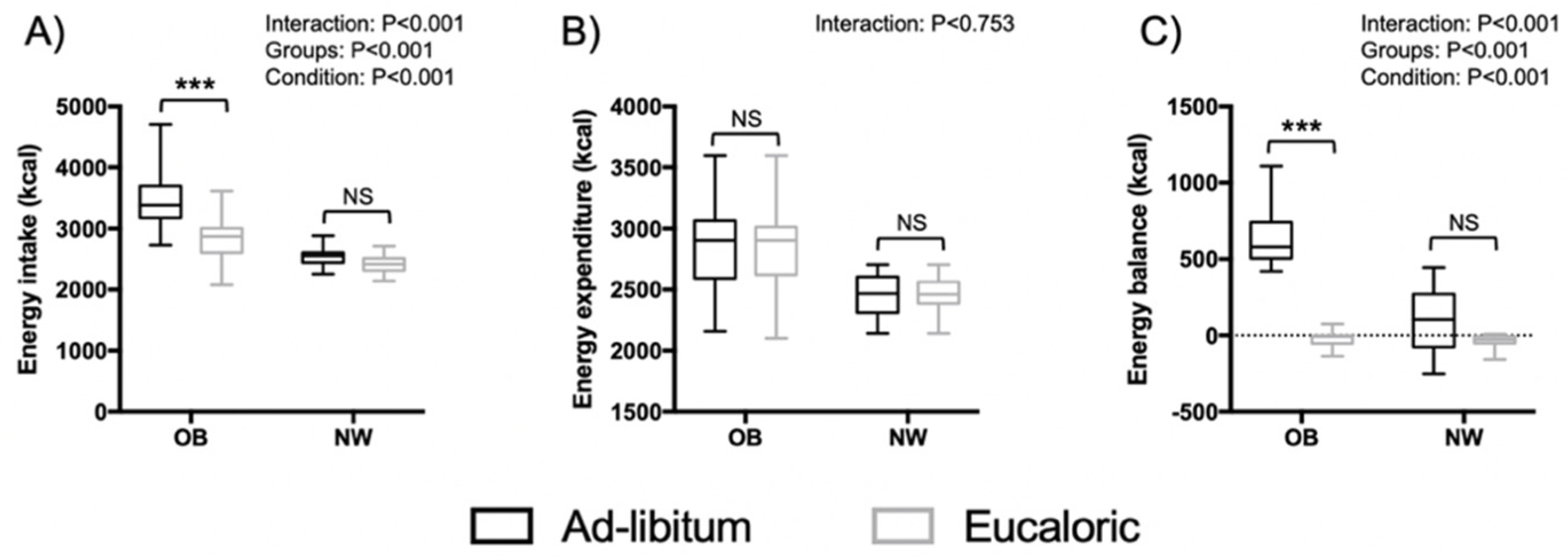
3.2. Energy Balance and Macronutrient Intake Outcomes
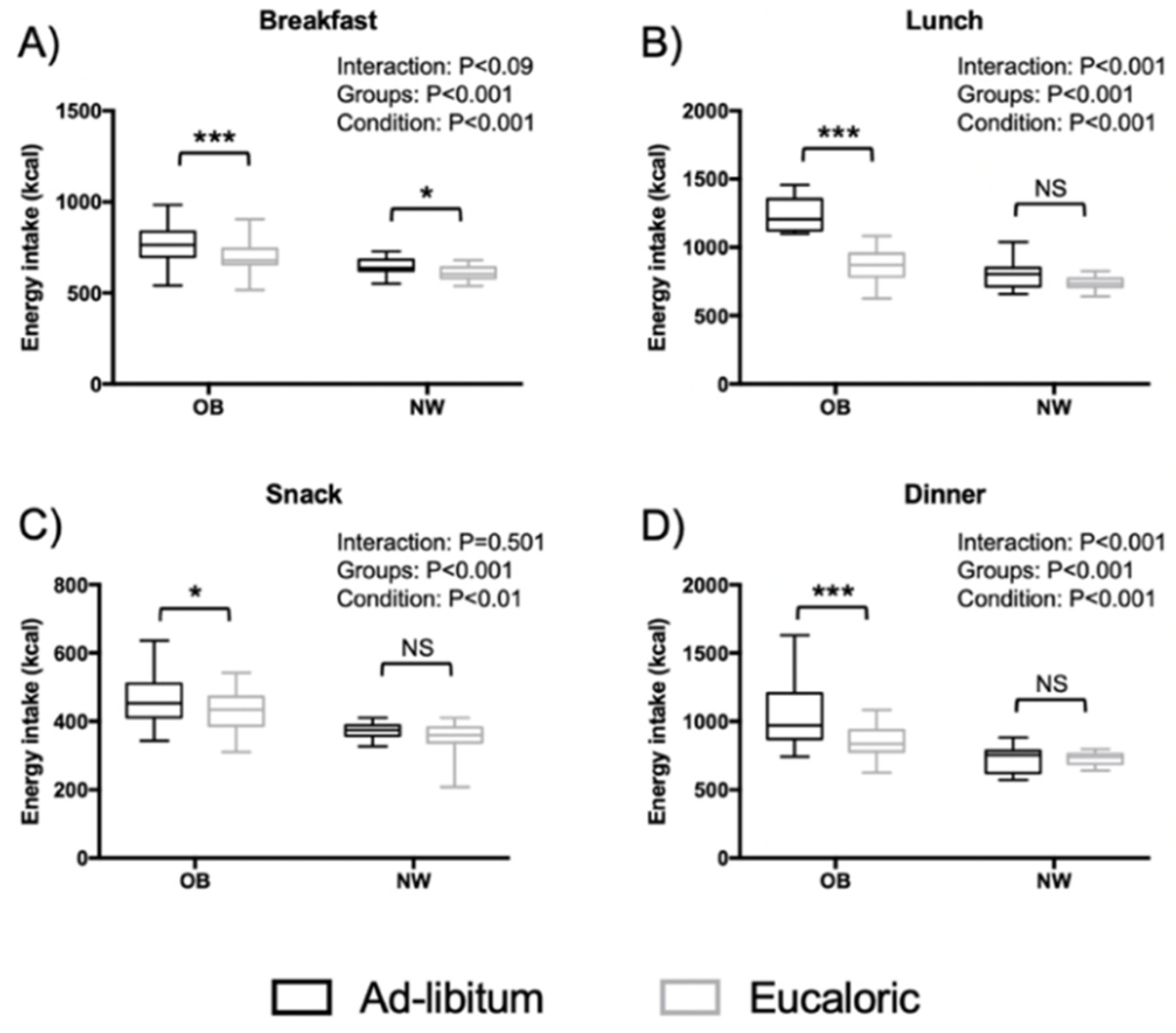
3.3. Distribution of Energy Intake on the Four Meals
3.4. Sleep Outcomes
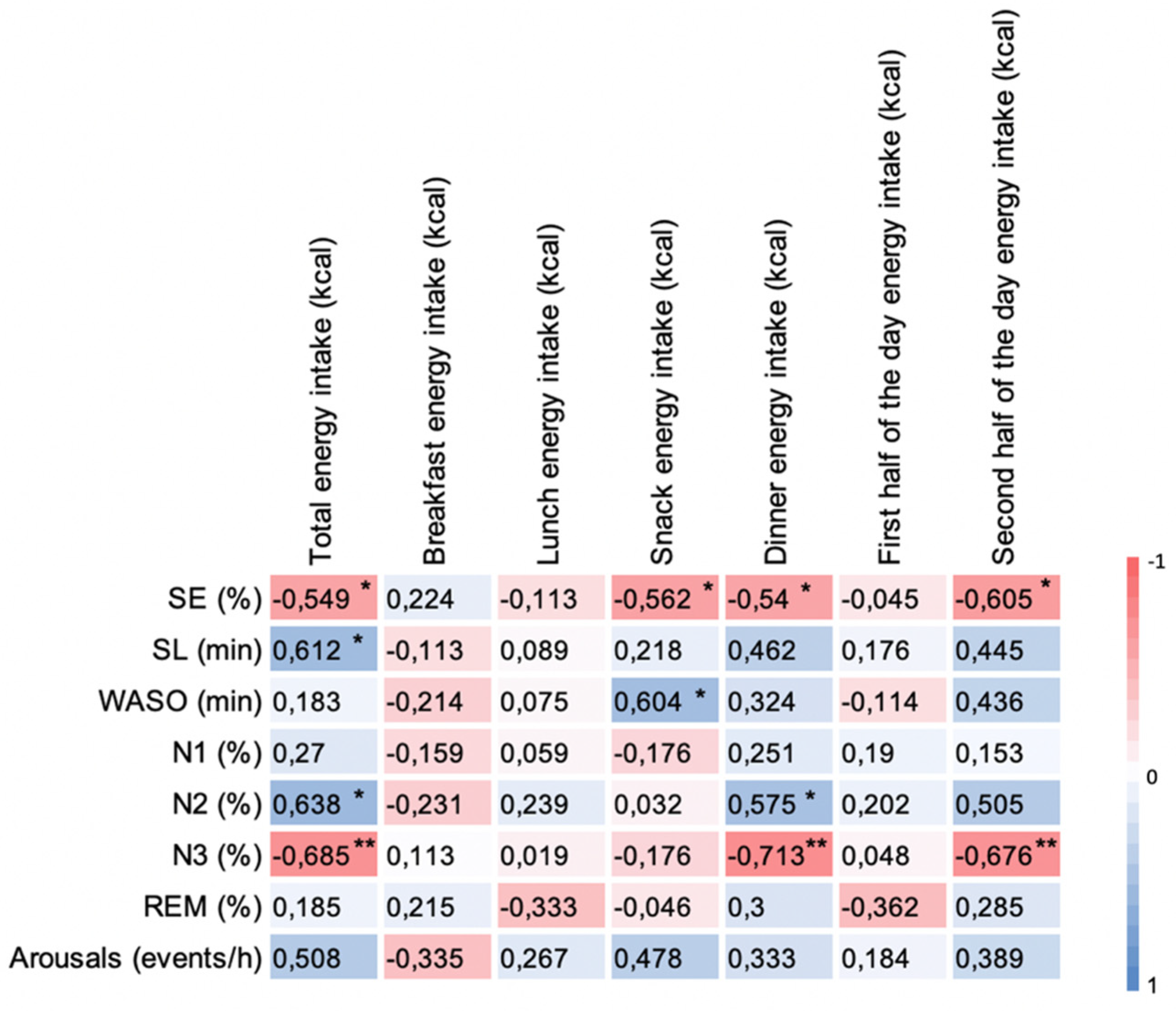
3.5. Correlation of Δ Energy Intake with Δ Sleep Outcomes between Sessions in OB Group
4. Discussion
Limitations, Strengths, and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gradisar, M.; Gardner, G.; Dohnt, H. Recent Worldwide Sleep Patterns and Problems during Adolescence: A Review and Meta-Analysis of Age, Region, and Sleep. Sleep Med. 2011, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Matricciani, L.; Olds, T.; Petkov, J. In Search of Lost Sleep: Secular Trends in the Sleep Time of School-Aged Children and Adolescents. Sleep Med. Rev. 2012, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A. Sleep in Adolescents: The Perfect Storm. Pediatric Clin. 2011, 58, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danisi, J.; Fernandez-Mendoza, J.; Vgontzas, A.N.; Bixler, E.O. Obesity and Sleep Disturbances. In The Behavioral, Molecular, Pharmacological, and Clinical Basis of the Sleep-Wake Cycle; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–142. [Google Scholar]
- Chaput, J.-P. Sleep Patterns, Diet Quality and Energy Balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P. The Role of Sleep Duration in the Regulation of Energy Balance: Effects on Energy Intakes and Expenditure. J. Clin. Sleep Med. 2013, 9, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Morselli, L.; Leproult, R.; Balbo, M.; Spiegel, K. Role of Sleep Duration in the Regulation of Glucose Metabolism and Appetite. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 687–702. [Google Scholar] [CrossRef] [Green Version]
- Shechter, A.; Grandner, M.A.; St-Onge, M.-P. The Role of Sleep in the Control of Food Intake. Am. J. Lifestyle Med. 2014, 8, 371–374. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.-P. Sleep–Obesity Relation: Underlying Mechanisms and Consequences for Treatment. Obes. Rev. 2017, 18, 34–39. [Google Scholar] [CrossRef]
- Beebe, D.W.; Simon, S.; Summer, S.; Hemmer, S.; Strotman, D.; Dolan, L.M. Dietary Intake Following Experimentally Restricted Sleep in Adolescents. Sleep 2013, 36, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.N.; Carskadon, M.A.; Considine, R.V.; Fava, J.L.; Lawton, J.; Raynor, H.A.; Jelalian, E.; Owens, J.; Wing, R. Changes in Children’s Sleep Duration on Food Intake, Weight, and Leptin. Pediatrics 2013, 132, e1473–e1480. [Google Scholar] [CrossRef] [Green Version]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The Effects of Physical Activity on Sleep: A Meta-Analytic Review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef]
- Lang, C.; Kalak, N.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U.; Gerber, M. The Relationship between Physical Activity and Sleep from Mid Adolescence to Early Adulthood. A Systematic Review of Methodological Approaches and Meta-Analysis. Sleep Med. Rev. 2016, 28, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Binks, H.; E. Vincent, G.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, S.; Gonzalez, K.; Lee-Ang, L.; Young, M.C.; Tamez, M.; Mattei, J. Diet and Sleep Physiology: Public Health and Clinical Implications. Front. Neurol 2017, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet Promotes Sleep Duration and Quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Zuurbier, L.A.; de Jonge, E.; Voortman, T.; Jacques, P.F.; Lamon-Fava, S.; Scheer, F.A.J.L.; Kiefte-De Jong, J.C.; Hofman, A.; Ordovás, J.M.; et al. Actigraphic Sleep Fragmentation, Efficiency and Duration Associate with Dietary Intake in the Rotterdam Study. J. Sleep Res. 2016, 25, 404–411. [Google Scholar] [CrossRef]
- Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among Dietary Nutrients and Subjective Sleep, Objective Sleep, and Napping in Women. Sleep Med. 2010, 11, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, E.; Yatsuya, H.; Uemura, M.; Murata, C.; Otsuka, R.; Toyoshima, H.; Tamakoshi, K.; Sasaki, S.; Kawaguchi, L.; Aoyama, A. Associations of Protein, Fat, and Carbohydrate Intakes with Insomnia Symptoms among Middle-Aged Japanese Workers. J. Epidemiol. 2013, 23, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Spaeth, A.M.; Dinges, D.F.; Goel, N. Objective Measurements of Energy Balance Are Associated with Sleep Architecture in Healthy Adults. Sleep 2017, 40, zsw018. [Google Scholar] [CrossRef] [Green Version]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between Food Intake and Sleep Pattern in Healthy Individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaghi, A.; O’Connor, H.; Chow, C.M. Acute Effects of the Very Low Carbohydrate Diet on Sleep Indices. Nutr. Neurosci. 2008, 11, 146–154. [Google Scholar] [CrossRef]
- Phillips, F.; Crisp, A.H.; McGuinness, B.; Kalucy, E.C.; Chen, C.N.; Koval, J.; Kalucy, R.S.; Lacey, J.H. Isocaloric Diet Changes and Electroencephalographic Sleep. Lancet 1975, 306, 723–725. [Google Scholar] [CrossRef]
- Yajima, K.; Seya, T.; Iwayama, K.; Hibi, M.; Hari, S.; NAkASHIMA, Y.; Ogata, H.; Omi, N.; Satoh, M.; TOkUYAMA, K. Effects of Nutrient Composition of Dinner on Sleep Architecture and Energy Metabolism during Sleep. J. Nutr. Sci. Vitaminol. 2014, 60, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, Y.; Doi, S.A.R.; Mamun, A.A. Sleep Quality and Obesity in Young Subjects: A Meta-Analysis. Obes. Rev. 2016, 17, 1154–1166. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Horne, J.A.; Östberg, O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Trost, S.G.; Loprinzi, P.D.; Moore, R.; Pfeiffer, K.A. Comparison of Accelerometer Cut Points for Predicting Activity Intensity in Youth. Med. Sci. Sports Exerc. 2011, 43, 1360–1368. [Google Scholar] [CrossRef]
- Levendowski, D.J.; Popovic, D.; Berka, C.; Westbrook, P.R. Retrospective Cross-Validation of Automated Sleep Staging Using Electroocular Recording in Patients with and without Sleep Disordered Breathing. Int. Arch. Med. 2012, 5, 21. [Google Scholar] [CrossRef]
- Levendowski, D.J.; Ferini-Strambi, L.; Gamaldo, C.; Cetel, M.; Rosenberg, R.; Westbrook, P.R. The Accuracy, Night-to-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers. J. Clin. Sleep Med. 2017, 13, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Lindseth, G.; Lindseth, P.; Thompson, M. Nutritional Effects on Sleep. West. J. Nurs. Res. 2013, 35, 497–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-Glycemic-Index Carbohydrate Meals Shorten Sleep Onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Melo, C.M.; Del Re, M.P.; dos Santos Quaresma, M.V.L.; Antunes, H.K.M.; Togeiro, S.M.; Ribeiro, S.M.L.; Tufik, S.; de Mello, M.T. Relationship of Evening Meal with Sleep Quality in Obese Individuals with Obstructive Sleep Apnea. Clin. Nutr. ESPEN 2019, 29, 231–236. [Google Scholar] [CrossRef]
- Wang, J.B.; Patterson, R.E.; Ang, A.; Emond, J.A.; Shetty, N.; Arab, L. Timing of Energy Intake during the Day Is Associated with the Risk of Obesity in Adults. J. Hum. Nutr. Diet. 2014, 27, 255–262. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric Intake at Breakfast vs. Dinner Differentially Influences Weight Loss of Overweight and Obese Women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Kräuchi, K.; Fattori, E.; Giordano, A.; Falbo, M.; Iadarola, A.; Aglì, F.; Tribolo, A.; Mutani, R.; Cicolin, A. Sleep on a High Heat Capacity Mattress Increases Conductive Body Heat Loss and Slow Wave Sleep. Physiol. Behav. 2018, 185, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kanaly, T.; Shaheen, N.J.; Vaughn, B.V. Gastrointestinal Physiology and Digestive Disorders in Sleep. Curr. Opin. Pulm. Med. 2009, 15, 571–577. [Google Scholar] [CrossRef]
- Jarosz, M.; Taraszewska, A. Risk Factors for Gastroesophageal Reflux Disease: The Role of Diet. Prz. Gastroenterol. 2014, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Grunwald, G.K.; Johnson, S.L.; Bessesen, D.H. Effects of Short-Term Overfeeding on Hunger, Satiety, and Energy Intake in Thin and Reduced-Obese Individuals. Appetite 2004, 43, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Metz, L.; Julien, A.; Morio, B.; Duché, P. Obese but Not Lean Adolescents Spontaneously Decrease Energy Intake after Intensive Exercise. Physiol. Behav. 2014, 123, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Genin, P.M.; Mathieu, M.-E.; Pereira, B.; Metz, L. Reproducibility of an In-Laboratory Test Meal to Assess Ad Libitum Energy Intake in Adolescents with Obesity. Appetite 2016, 105, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Rochette, E.; Bovet, M.; Merlin, E.; Duché, P. Acute Intense Exercise Improves Sleep and Decreases next Morning Consumption of Energy-Dense Food in Adolescent Girls with Obesity and Evening Chronotype. Pediatr. Obes. 2020, 15, e12613. [Google Scholar] [CrossRef] [PubMed]

| OB Mean (SD) | NW Mean (SD) | p Value | |
|---|---|---|---|
| Anthropometry & body composition | |||
| Age (year) | 14.0 (0.9) | 14.0 (0.9) | - |
| Height (cm) | 163.0 (11.7) | 164.7 (7.8) | 0.635 |
| Weight (kg) | 88.7 (23.8) | 56.27 (6.14) | <0.001 |
| BMI (kg·m−2) | 33.1 (7.1) | 20.71 (1.58) | <0.001 |
| FM (%) | 36.9 (5.7) | 14.0 (3.2) | <0.001 |
| FFM (kg) | 53.4 (12.3) | 48.2 (4.7) | 0.164 |
| Circadian typology and subjective sleep | |||
| Circadian typology (MEQscore) | 29.7 (3.4) | 32.9 (5.9) | 0.210 |
| Sleep quality (PSQIscore) | 8.4 (3.0) | 4.2 (1.9) | <0.001 |
| Sleepiness (ESSscore) | 9.3 (3.8) | 4.3 (2.0) | <0.001 |
| Physical activity, RMR and TEE | |||
| Sedentary (min·day−1) | 441 (46) | 436 (62) | 0.667 |
| Light (min·day−1) | 240 (36) | 235 (43) | 0.603 |
| MVPA (min·day−1) | 50 (27) | 63 (39) | 0.454 |
| RMR (kcal·day−1) | 2035 (364) | 1631 (106) | <0.001 |
| TEE (kcal·day−1) | 2864 (369) | 2460 (148) | <0.001 |
| OB | NW | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad libitum | Eucaloric | Ad libitum | Eucaloric | Interaction (Weight Status × Condition) | Weight Status Effect | Condition Effect | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | p Value | F | p Value | F | p Value | |
| SE (%) | 77.0 (9.6) | 78.0 (10.4) | 90.5 (6.3) | 91.1 (6.7) | 0.367 | 0.550 ES = 0.24 | 17.310 | <0.001 ES = 1.64 | 5.591 | 0.026 ES = 0.92 |
| SL (min) | 32.0 (24.8) | 23.5 (21.6) ** | 19.7 (11.8) | 16.1 (13.0) | 3.699 | 0.065 ES = 0.76 | 2.012 | 0.168 ES = 0.56 | 22.576 | <0.001 ES = 1.86 |
| WASO (min) | 92.0 (46.3) | 95.0 (49.8) | 31.5 (23.8) | 31.9 (25.3) | 1.216 | 0.280 ES = 0.44 | 18.420 | <0.001 ES = 1.68 | 1.962 | 0.173 ES = 0.54 |
| Wake (min) | 124.0 (51.8) | 118.6 (56.5) | 51.2 (34.3) | 48.0 (36.6) | 0.367 | 0.550 ES = 0.24 | 17.310 | <0.001 ES = 1.64 | 5.591 | 0.026 ES = 1.92 |
| Stage REM (min) | 74.1 (26.9) | 76.0 (26.5) | 103.5 (40.8) | 95.8 (38.3) | 3.149 | 0.088 ES = 0.70 | 3.873 | 0.060 ES = 0.78 | 1.199 | 0.280 ES = 0.42 |
| Stage N1 (min) | 36.6 (9.4) | 30.6 (11.8) * | 30.7 (16.3) | 29.7 (17.8) | 5.508 | 0.027 ES = 0.92 | 0.421 | 0.522 ES = 0.24 | 10.797 | 0.003 ES = 1.28 |
| Stage N2 (min) | 209.0 (29.1) | 209.7 (33.1) | 256.7 (46.1) | 258.2 (48.6) | 0.883 | 0.356 ES = 0.36 | 10.838 | 0.003 ES = 1.3 | 0.044 | 0.836 ES = 0.08 |
| Stage N3 (min) | 96.0 (45.0) | 105.0 (54.5) | 97.7 (47.7) | 108.0 (50.3) | 0.034 | 0.856 ES = 0.06 | 0.002 | 0.961 ES < 0.01 | 6.434 | 0.018 ES = 1.00 |
| Arousals (events/h) | 17.3 (2.3) | 15.4 (3.7) | 13.3 (5.4) | 12.34 (3.68) | 0.637 | 0.432 ES = 0.32 | 6.502 | 0.017 ES = 1.00 | 7.099 | 0.013 ES = 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, O.; Rochette, E.; Del Sordo, G.; Doré, É.; Merlin, É.; Walrand, S.; Duché, P. Eucaloric Balanced Diet Improved Objective Sleep in Adolescents with Obesity. Nutrients 2021, 13, 3550. https://doi.org/10.3390/nu13103550
Saidi O, Rochette E, Del Sordo G, Doré É, Merlin É, Walrand S, Duché P. Eucaloric Balanced Diet Improved Objective Sleep in Adolescents with Obesity. Nutrients. 2021; 13(10):3550. https://doi.org/10.3390/nu13103550
Chicago/Turabian StyleSaidi, Oussama, Emmanuelle Rochette, Giovanna Del Sordo, Éric Doré, Étienne Merlin, Stéphane Walrand, and Pascale Duché. 2021. "Eucaloric Balanced Diet Improved Objective Sleep in Adolescents with Obesity" Nutrients 13, no. 10: 3550. https://doi.org/10.3390/nu13103550
APA StyleSaidi, O., Rochette, E., Del Sordo, G., Doré, É., Merlin, É., Walrand, S., & Duché, P. (2021). Eucaloric Balanced Diet Improved Objective Sleep in Adolescents with Obesity. Nutrients, 13(10), 3550. https://doi.org/10.3390/nu13103550








