Obesogenic and Ketogenic Diets Distinctly Regulate the SARS-CoV-2 Entry Proteins ACE2 and TMPRSS2 and the Renin-Angiotensin System in Rat Lung and Heart Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Ethics Approval
2.4. Glucose Monitoring and Determination of Plasma Insulin Concentrations
2.5. Western Blotting Analysis of ACE1, ACE2, AT1R, AT2R, and TMPRSS2 Protein Levels in Lung and Cardiac Tissues
2.6. RNA Isolation and Quantitative PCR
2.7. Statistical Analyses
3. Results
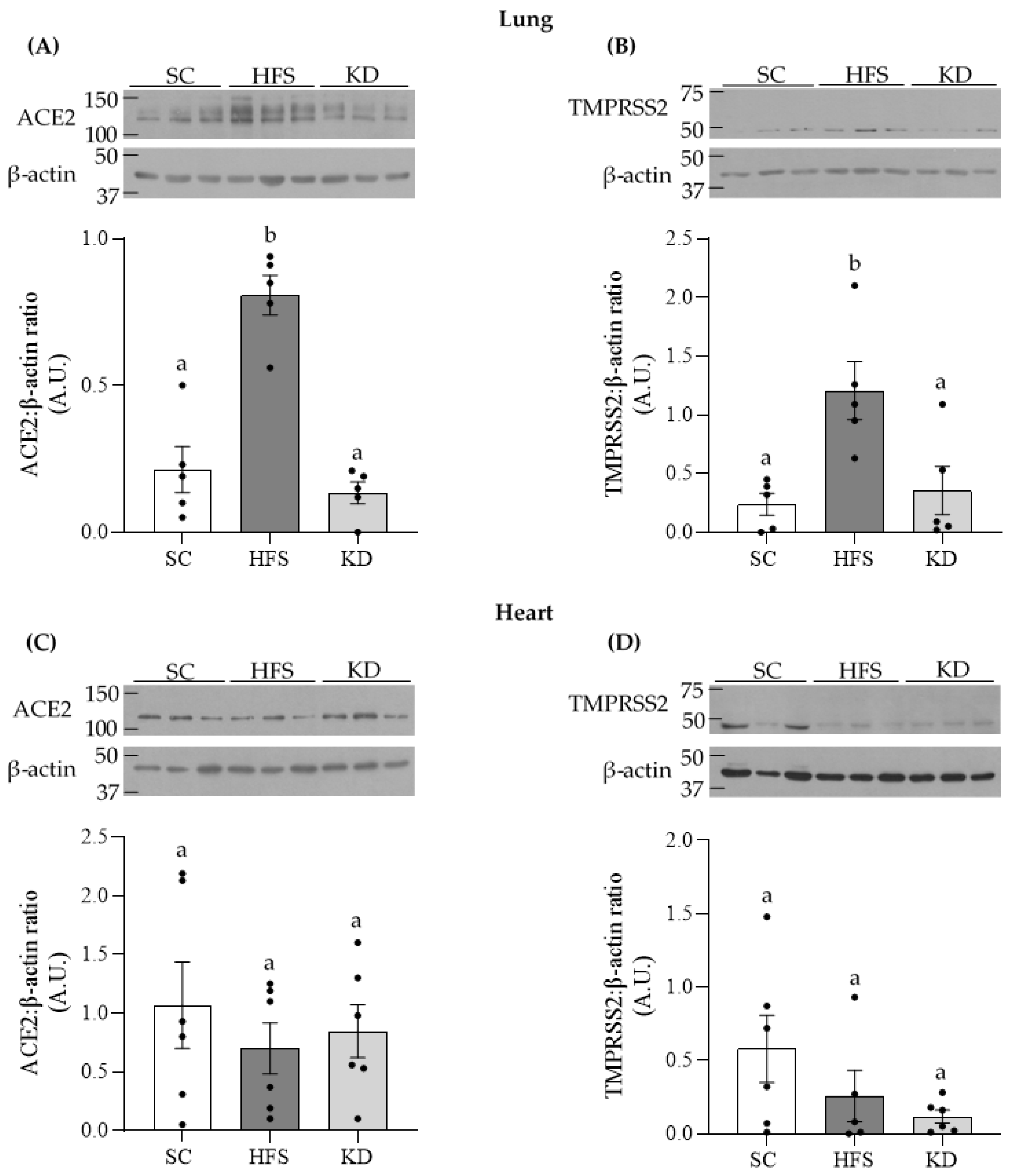
3.1. ACE2 and TMPRSS2 Levels in Lung and Heart Tissues
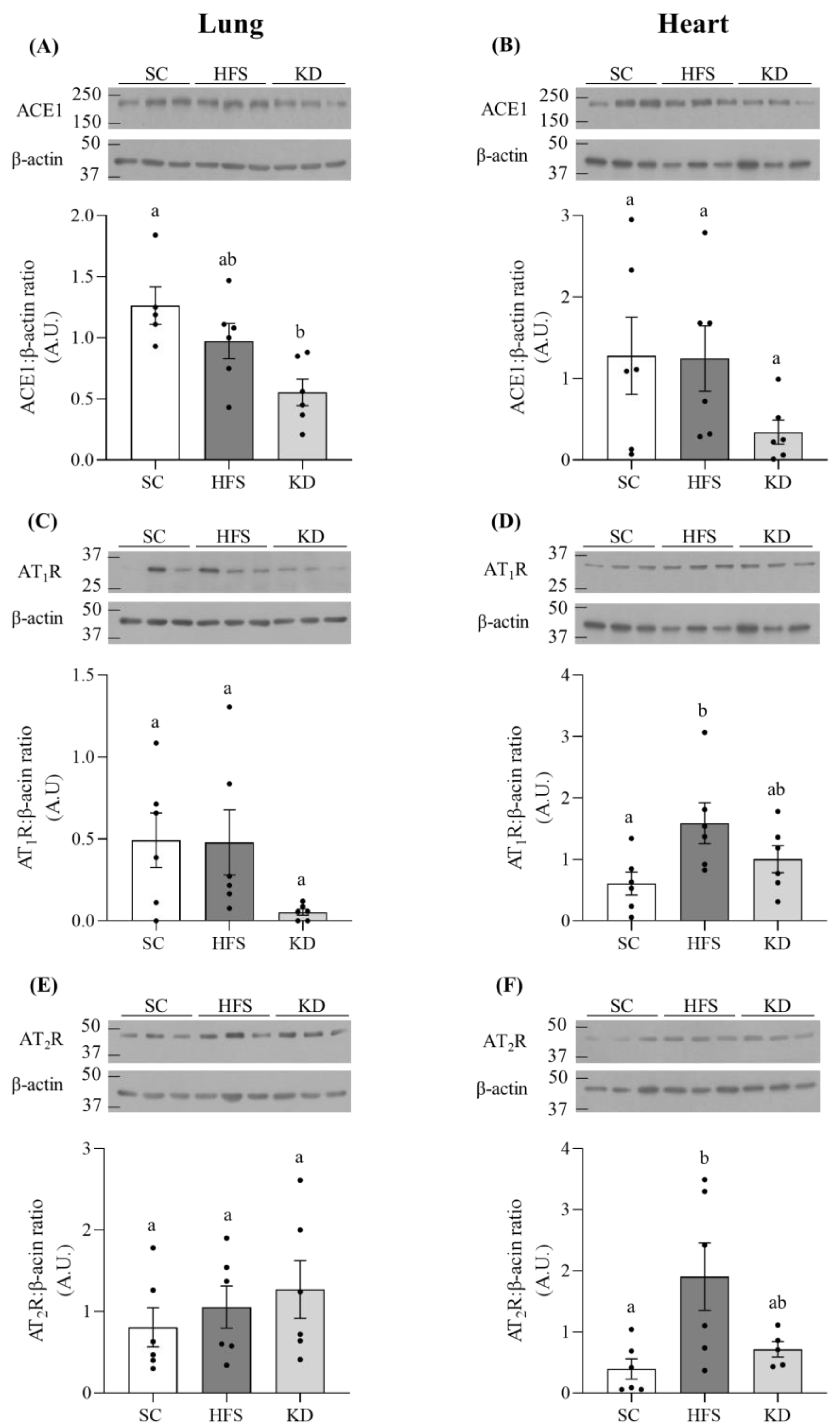
3.2. ACE1, AT1R, and AT2R Levels in Lung and Heart Tissues
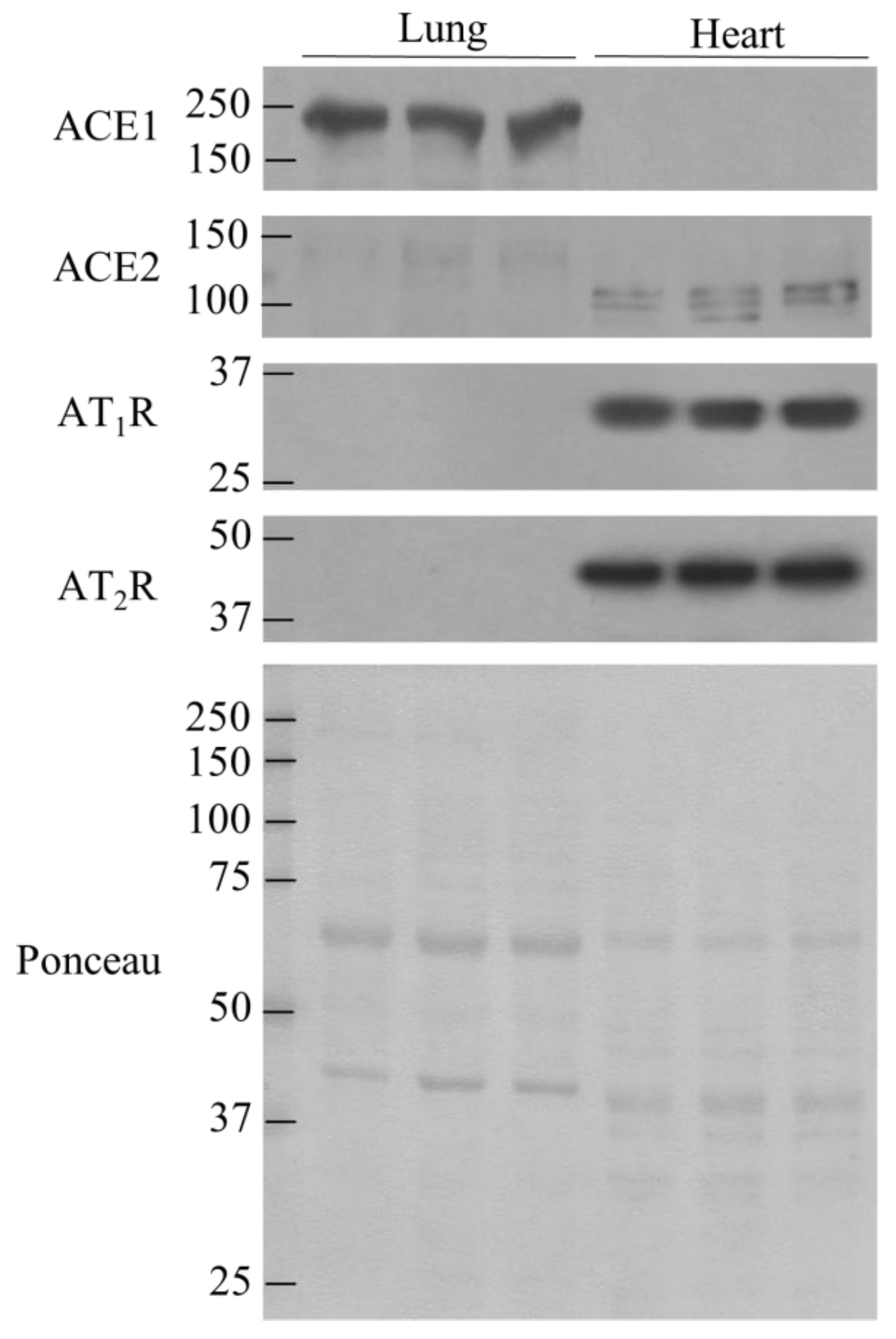
3.3. Comparison of ACE1, ACE2, AT1R, and AT2R in lung and Heart Tissues
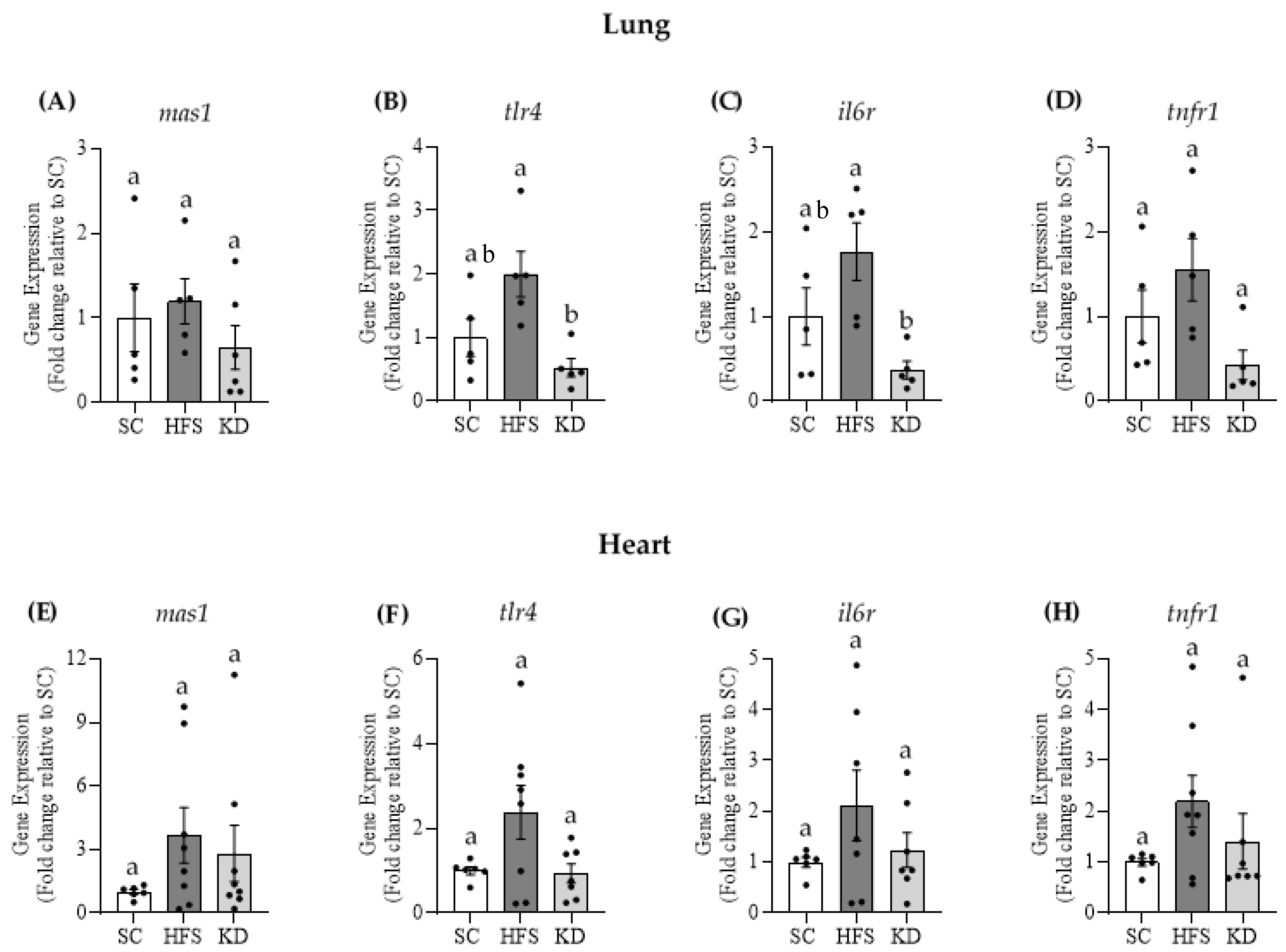
3.4. Gene Expressions of Mas1, tlr4, il6r, and tnfr1 in Lung and Heart Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Luzi, L.; Bucciarelli, L.; Ferrulli, A.; Terruzzi, I.; Massarini, S. Obesity and COVID-19: The Ominous duet affecting the Renin-Angiotensin System Livio. Minerva Endocrinol. 2021, 46, 193–201. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef] [PubMed]
- Sarver, D.C.; Wong, G.W. Obesity alters Ace2 and Tmprss2 expression in lung, trachea, and esophagus in a sex-dependent manner: Implications for COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Batchu, S.N.; Kaur, H.; Yerra, V.G.; Advani, S.L.; Golam Kabir, M.; Liu, Y.; Klein, T.; Advani, A. Lung and Kidney ACE2 and TMPRSS2 in Renin-Angiotensin System Blocker–Treated Comorbid Diabetic Mice Mimicking Host Factors That Have Been Linked to Severe COVID-19. Diabetes 2021, 70, 759–771. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Filippatos, T. The renin-angiotensin-aldosterone system as a link between obesity and coronavirus disease 2019 severity. Obes. Rev. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Marcus, Y.; Shefer, G.; Stern, N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol. Cell. Endocrinol. 2013, 378, 1–14. [Google Scholar] [CrossRef]
- Schulman, I.H.; Raij, L. The angiotensin II type 2 receptor: What is its clinical significance? Curr. Hypertens. Rep. 2008, 10, 188–193. [Google Scholar] [CrossRef]
- Walton, C.M.; Perry, K.; Hart, R.H.; Berry, S.L.; Bikman, B.T. Improvement in Glycemic and Lipid Profiles in Type 2 Diabetics with a 90-Day Ketogenic Diet. J. Diabetes Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Asrih, M.; Altirriba, J.; Rohner-Jeanrenaud, F.; Jornayvaz, F.R. Ketogenic diet impairs FGF21 signaling and promotes differential inflammatory responses in the liver and white adipose tissue. PLoS ONE 2015, 10, e0126364. [Google Scholar] [CrossRef] [Green Version]
- Pinho, R.A.; Sepa-Kishi, D.M.; Bikopoulos, G.; Wu, M.V.; Uthayakumar, A.; Mohasses, A.; Hughes, M.C.; Perry, C.G.R.; Ceddia, R.B. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic. Biol. Med. 2017, 110. [Google Scholar] [CrossRef]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wijnant, S.R.A.; Jacobs, M.; Van Eeckhoutte, H.; Lapauw, B.; Joos, G.F.; Bracke, K.R.; Brusselle, G.G. Expression of ACE2, the SARS-CoV-2 Receptor, in Lung Tissue of Patients with Type 2 Diabetes. Diabetes 2020, 69, 2691–2699. [Google Scholar] [CrossRef]
- Fondevila, M.F.; Mercado-Gómez, M.; Rodríguez, A.; Gonzalez-Rellan, M.J.; Iruzubieta, P.; Valentí, V.; Escalada, J.; Schwaninger, M.; Prevot, V.; Dieguez, C.; et al. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J. Hepatol. 2021, 74, 469–471. [Google Scholar] [CrossRef]
- D’Abbondanza, M.; Ministrini, S.; Pucci, G.; Nulli Migliola, E.; Martorelli, E.E.; Gandolfo, V.; Siepi, D.; Lupattelli, G.; Vaudo, G. Very Low-Carbohydrate Ketogenic Diet for the Treatment of Severe Obesity and Associated Non-Alcoholic Fatty Liver Disease: The Role of Sex Differences. Nutrients 2020, 12, 2748. [Google Scholar] [CrossRef]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; Hu, Y.; Kong, J.; Yin, H.; Wang, X.; et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021, 1–3. [Google Scholar] [CrossRef]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: New perspectives for neuroprotection in alzheimer’s disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streijger, F.; Plunet, W.T.; Lee, J.H.T.; Liu, J.; Lam, C.K.; Park, S.; Hilton, B.J.; Fransen, B.L.; Matheson, K.A.J.; Assinck, P.; et al. Ketogenic diet improves forelimb motor function after spinal cord injury in rodents. PLoS ONE 2013, 8, e78765. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12. [Google Scholar] [CrossRef]
| Week | SC | HFS | KD | |
|---|---|---|---|---|
| Energy intake (kcal/rat/day) | 0 | 69.23 ± 3.8 | 66.49 ± 2.3 | 66.49 ± 2.9 |
| 4 | 72.60 ± 4.2 | 68.37 ± 4.0 | 63.83 ± 0.76 | |
| 8 | 65.83 ± 2.9 | 66.94 ± 3.2 | 59.82 ± 1.1 | |
| 16 | 69.65 ± 2.1 | 69.75 ± 6.0 | 57.15 ± 5.0 * |
| Forward | Reverse | |
|---|---|---|
| mas1 | 5′-CGCCAACCCTTTCATCTACT-3′ | 5′-CCTAGGTTGCATCTCGTCTTT-3′ |
| tlr4 | 5′-ACCTAAGGAGAGGAGGCTAAG-3′ | 5′-GGTAACTGCAGCACACTACA-3′ |
| il6r | 5′-TGGAGCAGACAGAGAGACTT-3′ | 5′-AGCTTACAGGTAACAGAGCATAAA-3′ |
| tnfr1 | 5′-CCCTGTGAACCTCCTCTTTG-3′ | 5′-CTATGTACACCAAGTCGGTAGC-3′ |
| β-actin | 5′-GTGAAAAGATGACCCAGATC-3′ | 5′-CACCGCCTGGATGGCTACGT-3′ |
| Duration of Study (Weeks) | ||||||
|---|---|---|---|---|---|---|
| Groups | 0 | 4 | 8 | 12 | 16 | |
| Body mass (g) | SC | 231.97 ± 7.1 | 317.21 ± 10.4 | 370.21 ± 14.3 | 400.47 ± 15.0 | 421.29 ± 15.7 |
| HFS | 236.18 ± 5.4 | 345.97 ± 10.7 | 409.77 ± 14.6 * | 452.3 ± 15.7 * | 470.81 ± 17.1 * | |
| KD | 230.12 ± 3.6 | 321.21 ± 4.5 | 374.24 ± 6.6 | 404.69 ± 6.5 | 425.54 ± 4.7 | |
| Duration of Study (Weeks) | |||
|---|---|---|---|
| Blood Parameters | Groups | 0 | 16 |
| Glucose (mmol/L) | SC | 6.85 ± 0.12 | 5.72 ± 0.18 # |
| HFS | 6.77 ± 0.21 | 5.91 ± 0.10 # | |
| KD | 6.59 ± 0.12 | 5.71 ± 0.07 # | |
| Insulin (ng/mL) | SC | 2.16 ± 0.50 | 4.30 ± 0.43 |
| HFS | 1.90 ± 0.33 | 7.68 ± 1.54 *# | |
| KD | 2.18 ± 0.34 | 5.85 ± 0.66 # | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Eira, D.; Jani, S.; Ceddia, R.B. Obesogenic and Ketogenic Diets Distinctly Regulate the SARS-CoV-2 Entry Proteins ACE2 and TMPRSS2 and the Renin-Angiotensin System in Rat Lung and Heart Tissues. Nutrients 2021, 13, 3357. https://doi.org/10.3390/nu13103357
Da Eira D, Jani S, Ceddia RB. Obesogenic and Ketogenic Diets Distinctly Regulate the SARS-CoV-2 Entry Proteins ACE2 and TMPRSS2 and the Renin-Angiotensin System in Rat Lung and Heart Tissues. Nutrients. 2021; 13(10):3357. https://doi.org/10.3390/nu13103357
Chicago/Turabian StyleDa Eira, Daniel, Shailee Jani, and Rolando B. Ceddia. 2021. "Obesogenic and Ketogenic Diets Distinctly Regulate the SARS-CoV-2 Entry Proteins ACE2 and TMPRSS2 and the Renin-Angiotensin System in Rat Lung and Heart Tissues" Nutrients 13, no. 10: 3357. https://doi.org/10.3390/nu13103357
APA StyleDa Eira, D., Jani, S., & Ceddia, R. B. (2021). Obesogenic and Ketogenic Diets Distinctly Regulate the SARS-CoV-2 Entry Proteins ACE2 and TMPRSS2 and the Renin-Angiotensin System in Rat Lung and Heart Tissues. Nutrients, 13(10), 3357. https://doi.org/10.3390/nu13103357





