Risks in Management of Enteral Nutrition in Intensive Care Units: A Literature Review and Narrative Synthesis
Abstract
1. Introduction
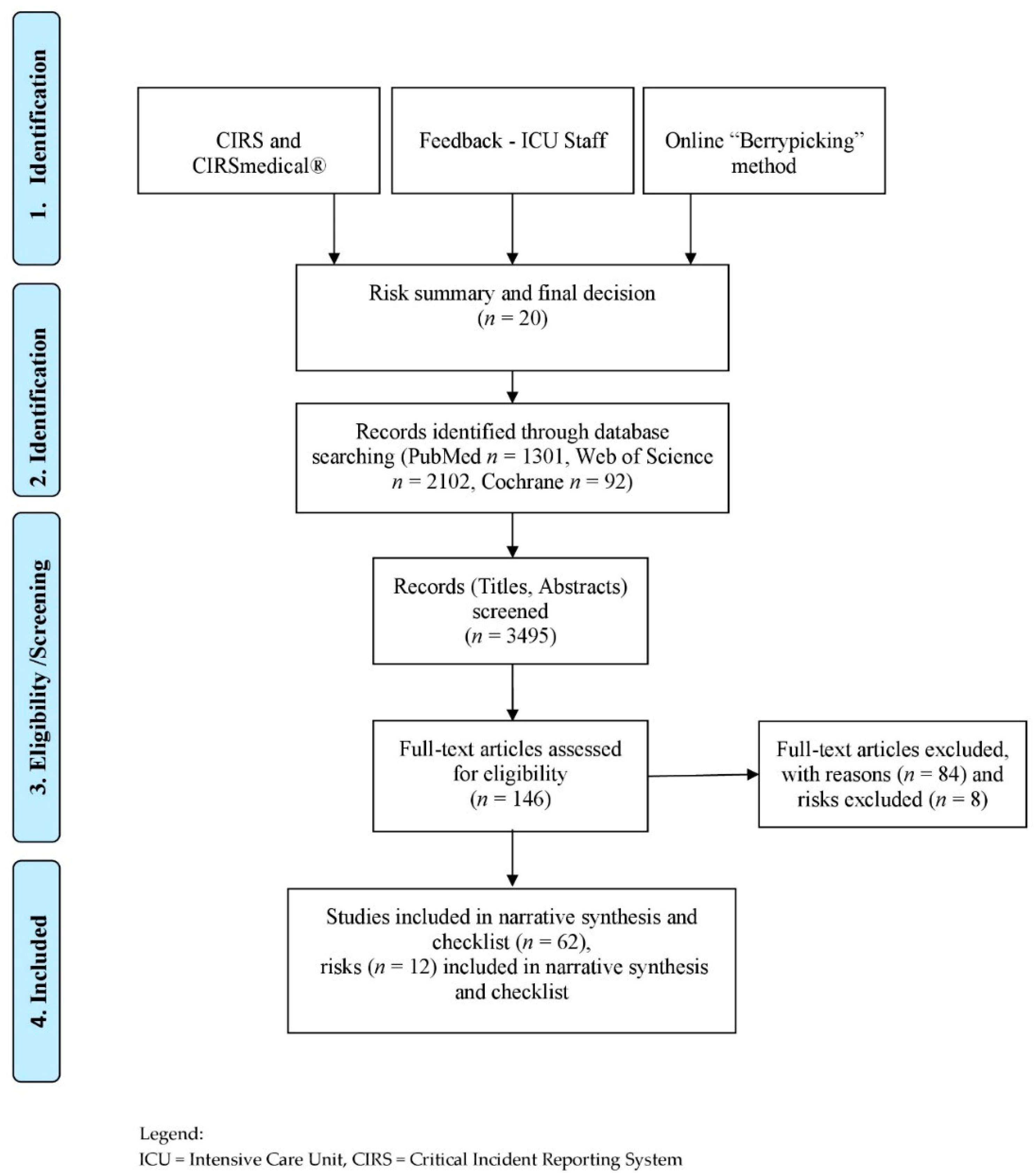
2. Materials and Methods
Search Strategy
3. Results
3.1. Admission
3.1.1. No Use of Clinical Assessment or Screening Nutrition Assessment
3.1.2. Inadequate Tube Management and Position
3.2. Prescription
Missing Energy Target
3.3. Verification
Missing a Nutritionist at the ICU
3.4. Preparation
Insufficient Hygiene and Handling
3.5. Administering
3.5.1. Wrong Time Management, Speed and Route
3.5.2. Nutritional Interruptions
3.5.3. Wrong Body Position
3.6. Monitoring
Gastrointestinal Complication and Infections
3.7. General Risk
3.7.1. Missing or Not Using Existing Guidelines, Standards or Protocols
3.7.2. Understaffing
3.7.3. Lack of Education
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| CIRS | Critical Incident Reporting System |
| DIN EN ISO 80369-3 | Deutsches Institut für Normung and designation of the standard |
| e.g., | exempli gratia: for example |
| EN | Enteral Nutrition |
| ENFit™-technology | Connector for safe feeding |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| GI | gastrointestinal |
| i.e., | id est: that is |
| ICU | Intensive Care Unit |
| MeSH | Medical Subject Headings |
| MNA | Mini Nutritional Assessment |
| mNUTRIC | modified NUTRIC score |
| MTF | modular hospital-built tube feeding systems |
| MUST | Malnutrition Universal Screening Tool (MUST) |
| NRS-2002 | Nutrition Risk Screening 2002 |
| PN | Parenteral Nutrition |
| RCTs | Randomized Controlled Trials |
| RHT systems | “ready-to-hang”-systems |
| SAG | Subjective Global Assessment |
| SOPs | Standard Operating Procedures (SOPs) |
| VCO2 | volume of carbon dioxide |
| VO2 | volume of oxygen |
References
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review. J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Casaer, M.P.; Chapman, M.; Heyland, D.K.; Ichai, C.; Marik, P.E.; Martindale, R.G.; McClave, S.A.; Preiser, J.C.; Reignier, J.; et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med. 2017, 43, 1239–1256. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Daniel, M. Medical Error-the Third Leading Cause of Death in the US. Available online: https://www.bmj.com/content/353/bmj.i2139 (accessed on 20 October 2020).
- Sendlhofer, G.; Brunner, G.; Tax, C.; Falzberger, G.; Smolle, J.; Leitgeb, K.; Kober, B.; Kamolz, L.P. Systematische Einführung vom Klinischen Risikomanagement in einem Universitätsklinikum: Bedeutung von Risikomanagern. Wien. Klin. Wochenschr. 2015, 127, 1–11. [Google Scholar] [CrossRef]
- Chiozza, M.L.; Ponzetti, C. FMEA: A model for reducing medical errors. Clin. Chim. Acta 2009, 404, 75–78. [Google Scholar] [CrossRef]
- Sendlhofer, G.; Leitgeb, K.; Kober, B.; Brunner, G.; Tax, C.; Kamolz, L.P. Neue Wege zur Evaluierung von patientensicherheitsrelevanten Aspekten: Feedback-Patientensicherheit. Z. Evid. Fortbild. Qual. Gesundhwes. 2016, 114, 13–27. [Google Scholar] [CrossRef]
- Kim, H.; Stotts, N.A.; Froelicher, E.S.; Engler, M.M.; Porter, C.; Kwak, H. Adequacy of early enteral nutrition in adult patients in the intensive care unit. J. Clin. Nurs. 2012, 21, 2860–2869. [Google Scholar] [CrossRef]
- Nseir, S.; Le Gouge, A.; Lascarrou, J.-B.; Lacherade, J.-C.; Jaillette, E.; Mira, J.-P.; Mercier, E.; Declercq, P.-L.; Sirodot, M.; Piton, G.; et al. Impact of nutrition route on microaspiration in critically ill patients with shock: A planned ancillary study of the NUTRIREA-2 trial. Crit. Care 2019, 23, 111. [Google Scholar] [CrossRef]
- Sajid, M.S.; Harper, A.; Hussain, Q.; Forni, L.; Singh, K.K. An integrated systematic review and meta-analysis of published randomized controlled trials evaluating nasogastric against postpyloris (nasoduodenal and nasojejunal) feeding in critically ill patients admitted in intensive care unit. Eur. J. Clin. Nutr. 2014, 68, 424–432. [Google Scholar] [CrossRef]
- ISO Small-Bore Connectors for Liquids and Gases in Healthcare Applications—Part 1: General Requirements. Available online: https://www.iso.org/standard/64419.html (accessed on 20 October 2020).
- Dobbins, M. Rapid Review Guidebook. Natl. Collab. Cent. Method Tools 2017, 13, 25. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Kleibel, V.; Mayer, H. Literaturrecherche Für Gesundheitsberufe, 2nd ed.; Maudrich Wien: Wien, Austria, 2011; ISBN 978-3-7089-0309-5. [Google Scholar]
- Canales, C.; Elsayes, A.; Yeh, D.D.; Belcher, D.; Nakayama, A.; McCarthy, C.M.; Chokengarmwong, N.; Quraishi, S.A. Nutrition Risk in Critically Ill Versus the Nutritional Risk Screening 2002: Are They Comparable for Assessing Risk of Malnutrition in Critically Ill Patients? J. Parenter. Enter. Nutr. 2019, 43, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Coruja, M.K.; Cobalchini, Y.; Wentzel, C.; Fink, J.D. Nutrition Risk Screening in Intensive Care Units: Agreement Between NUTRIC and NRS 2002 Tools. Nutr. Clin. Pract. 2020, 35, 567–571. [Google Scholar] [CrossRef]
- Hollis, G.; Robins, E.; Powlesand, A.; Duff, A.; Ziegenfuss, M.; Shekar, K. Evaluation of the nutrition risk in critically ill score against a dietitian led nutrition triage tool in a tertiary ICU. Aust. Crit. Care 2018, 31, 123–124. [Google Scholar] [CrossRef]
- Kenworthy, S.; Agarwal, E.; Farlow, L.; Angus, R.; Marshall, A.P. Feasibility of using the “modified NUTrition Risk In the Critically ill” nutritional risk screening tool to identify nutritionally at-risk patients in an Australian intensive care unit. Aust. Crit. Care 2020, 33, 259–263. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef]
- Zhang, P.; Bian, Y.; Tang, Z.; Wang, F. Use of Nutrition Risk in Critically Ill (NUTRIC) Scoring System for Nutrition Risk Assessment and Prognosis Prediction in Critically Ill Neurological Patients: A Prospective Observational Study. J. Parenter. Enter. Nutr. 2020, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Y.; Mei, Y.; Ruan, H.; Yu, Y. Semi-automated ultrasound guidance applied to nasogastrojejunal tube replacement for enteral nutrition in critically ill adults. Biomed. Eng. Online 2018, 17, 21. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, L.; Zhao, J.; Tian, F.; Sun, H.; Wang, P.; Wang, J.; Wang, Z.; Wang, X. Bedside electromagnetic-guided placement of nasoenteral feeding tubes among critically Ill patients: A single-centre randomized controlled trial. J. Crit. Care 2018, 48, 216–221. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; McMoon, M.M.; Waldron, N.H.; Dye, E.J. Successful Identification of Anatomical Markers and Placement of Feeding Tubes in Critically Ill Patients via Camera-Assisted Technology with Real-Time Video Guidance. J. Parenter. Enter. Nutr. 2019, 43, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Theilla, M.; Hellerman, M.; Singer, P.; Maggiore, U.; Barbagallo, M.; Regolisti, G.; Fiaccadori, E. Energy and protein in critically Ill patients with AKI: A prospective, multicenter observational study using indirect calorimetry and protein catabolic rate. Nutrients 2017, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Reintam-Blaser, A.; Calder, P.C.; Casaer, M.; Hiesmayr, M.J.; Mayer, K.; Montejo, J.C.; Pichard, C.; Preiser, J.C.; van Zanten, A.R.H.; et al. Monitoring nutrition in the ICU. Clin. Nutr. 2019, 38, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.C.; Ashcraft, C.M.; Galvan, D.A. Longitudinal prediction of metabolic rate in critically ill patients. J. Parenter. Enter. Nutr. 2012, 36, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Anbar, R.; Cohen, J.; Shapiro, H.; Shalita-Chesner, M.; Lev, S.; Grozovski, E.; Theilla, M.; Frishman, S.; Madar, Z. The tight calorie control study (TICACOS): A prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011, 37, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ridley, E.J.; Tierney, A.; King, S.; Ainslie, E.; Udy, A.; Scheinkestel, C.; Nyulasi, I. Measured Energy Expenditure Compared With Best-Practice Recommendations for Obese, Critically Ill Patients—A Prospective Observational Study. J. Parenter. Enter. Nutr. 2020, 44, 1144–1149. [Google Scholar] [CrossRef]
- De Waele, E.; Spapen, H.; Honoré, P.M.; Mattens, S.; Rose, T.; Huyghens, L. Bedside calculation of energy expenditure does not guarantee adequate caloric prescription in long-term mechanically ventilated critically ill patients: A quality control study. Sci. World J. 2012, 2012, 909564. [Google Scholar] [CrossRef]
- Soguel, L.; Revelly, J.P.; Schaller, M.D.; Longchamp, C.; Berger, M.M. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: The intensive care unit dietitian can make the difference. Crit. Care Med. 2012, 40, 412–419. [Google Scholar] [CrossRef]
- Heyland, D.K.; Cahill, N.E.; Dhaliwal, R.; Sun, X.; Day, A.G.; McClave, S.A. Impact of enteral feeding protocols on enteral nutrition delivery: Results of a multicenter observational study. J. Parenter. Enter. Nutr. 2010, 34, 675–684. [Google Scholar] [CrossRef]
- Arney, B.D.; Senter, S.A.; Schwartz, A.C.; Meily, T.; Pelekhaty, S. Effect of Registered Dietitian Nutritionist Order-Writing Privileges on Enteral Nutrition Administration in Selected Intensive Care Units. Nutr. Clin. Pract. 2019, 34, 899–905. [Google Scholar] [CrossRef]
- Mistiaen, P.; Van den Heede, K. Nutrition Support Teams: A Systematic Review. J. Parenter. Enter. Nutr. 2020, 44, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Stankorb, S.M.; Salgueiro, M. Microbial contamination of enteral feeding products in thermoneutral and hyperthermal ICU environments. Nutr. Clin. Pract. 2015, 30, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Elke, G.; van Zanten, A.R.H.; Lemieux, M.; McCall, M.; Jeejeebhoy, K.N.; Kott, M.; Jiang, X.; Day, A.G.; Heyland, D.K. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Heighes, P.T.; Simpson, F.; Sweetman, E.A. Early enteral nutrition reduces mortality in trauma patients requiring intensive care: A meta-analysis of randomised controlled trials. Injury 2011, 42, 50–56. [Google Scholar] [CrossRef]
- Tian, F.; Heighes, P.T.; Allingstrup, M.J.; Doig, G.S. Early enteral nutrition provided within 24 hours of ICU admission: A meta-analysis of randomized controlled trials. Crit. Care Med. 2018, 46, 1049–1056. [Google Scholar] [CrossRef]
- Song, J.; Zhong, Y.; Lu, X.; Kang, X.; Wang, Y.; Guo, W.; Liu, J.; Yang, Y.; Pei, L. Enteral nutrition provided within 48 hours after admission in severe acute pancreatitis: A systematic review and meta-analysis. Medicine (United States) 2018, 97, e11871. [Google Scholar] [CrossRef]
- Patel, J.J.; Kozeniecki, M.; Peppard, W.J.; Peppard, S.R.; Zellner-Jones, S.; Graf, J.; Szabo, A.; Heyland, D.K. Phase 3 Pilot Randomized Controlled Trial Comparing Early Trophic Enteral Nutrition With “No Enteral Nutrition” in Mechanically Ventilated Patients With Septic Shock. J. Parenter. Enter. Nutr. 2020, 44, 866–873. [Google Scholar] [CrossRef]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Jiang, L.-X.; Huang, M. Early Versus Delayed Enteral Nutrition in Critically Ill Patients: A Meta-Analysis of Randomized Controlled Trials. Int. J. Clin. Exp. Med. 2019, 12, 4755–4763. [Google Scholar]
- Alkhawaja, S.; Martin, C.; Butler, R.J.; Gwadry-Sridhar, F. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst. Rev. 2015, 1–72. [Google Scholar] [CrossRef]
- Lee, Z.-Y.; Ibrahim, N.A.; Mohd-Yusof, B.-N. Prevalence and duration of reasons for enteral nutrition feeding interruption in a tertiary intensive care unit. Nutrition 2018, 53, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Leslie, G.D.; Leen, T.; Mills, L.; Dobb, G.J. Reducing interruptions to continuous enteral nutrition in the intensive care unit: A comparative study. J. Clin. Nurs. 2013, 22, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, M.; Sanui, M.; Komuro, T.; Iizuka, Y.; Kamio, T.; Koyama, H.; Mouri, H.; Masuyama, T.; Ono, K.; Lefor, A.K. Interruption of enteral nutrition in the intensive care unit: A single-center survey. J. Intensive Care 2017, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Segaran, E.; Barker, I.; Hartle, A. Optimising enteral nutrition in critically ill patients by reducing fasting times. J. Intensive Care Soc. 2016, 17, 38–43. [Google Scholar] [CrossRef]
- Sudenis, T.; Hall, K.; Cartotto, R. Enteral nutrition: What the dietitian prescribes is not what the burn patient gets! J. Burn Care Res. 2015, 36, 297–305. [Google Scholar] [CrossRef]
- Farsi, Z.; Kamali, M.; Butler, S.; Zareiyan, A. The Effect of Semirecumbent and Right Lateral Positions on the Gastric Residual Volume of Mechanically Ventilated, Critically Ill Patients. J. Nurs. Res. 2020, 28, e108. [Google Scholar] [CrossRef]
- Reignier, J.; Dimet, J.; Martin-Lefevre, L.; Bontemps, F.; Fiancette, M.; Clementi, E.; Lebert, C.; Renard, B. Before-after study of a standardized ICU protocol for early enteral feeding in patients turned in the prone position. Clin. Nutr. 2010, 29, 210–216. [Google Scholar] [CrossRef]
- Machado, L.D.S.; Rizzi, P.; Silva, F.M. Administration of enteral nutrition in the prone position, gastric residual volume and other clinical outcomes in critically ill patients: A systematic review. Rev. Bras. Ter. Intensiva 2020, 32, 133–142. [Google Scholar] [CrossRef]
- Fabiani, A.; Sanson, G.; Bottigliengo, D.; Dreas, L.; Zanetti, M.; Lorenzoni, G.; Gatti, G.; Sacilotto, M.; Pappalardo, A.; Gregori, D. Impact of a natural versus commercial enteral-feeding on the occurrence of diarrhea in critically ill cardiac surgery patients. A retrospective cohort study. Int. J. Nurs. Stud. 2020, 108, 103605. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.B.; Ait Hssain, A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Aldawood, A.S.; Haddad, S.H.; Al-Dorzi, H.M.; Tamim, H.M.; Jones, G.; Mehta, S.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N. Engl. J. Med. 2015, 372, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Atasever, A.G.; Ozcan, P.E.; Kasali, K.; Abdullah, T.; Orhun, G.; Senturk, E. The frequency, risk factors, and complications of gastrointestinal dysfunction during enteral nutrition in critically ill patients. Ther. Clin. Risk Manag. 2018, 14, 385–391. [Google Scholar] [CrossRef]
- Jakob, S.M.; Bütikofer, L.; Berger, D.; Coslovsky, M.; Takala, J. A randomized controlled pilot study to evaluate the effect of an enteral formulation designed to improve gastrointestinal tolerance in the critically ill patient-the SPIRIT trial. Crit. Care 2017, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Gungabissoon, U.; Hacquoil, K.; Bains, C.; Irizarry, M.; Dukes, G.; Williamson, R.; Deane, A.M.; Heyland, D.K. Prevalence, Risk Factors, Clinical Consequences, and Treatment of Enteral Feed Intolerance During Critical Illness. J. Parenter. Enter. Nutr. 2015, 39, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Yeh, D.D.; Quraishi, S.A.; Johnson, E.A.; Kaafarani, H.; Lee, J.; King, D.R.; Demoya, M.; Fagenholz, P.; Butler, K.; et al. Hypophosphatemia in Enterally Fed Patients in the Surgical Intensive Care Unit: Common but Unrelated to Timing of Initiation or Aggressiveness of Nutrition Delivery. Nutr. Clin. Pract. 2017, 32, 252–257. [Google Scholar] [CrossRef]
- Coşkun, R.; Gündoǧan, K.; Baldane, S.; Güven, M.; Sungur, M. Refeeding hypophosphatemia: A potentially fatal danger in the intensive care unit. Turk. J. Med. Sci. 2014, 44, 369–374. [Google Scholar] [CrossRef]
- Ralib, A.M.; Nor, M.B.M. Refeeding hypophosphataemia after enteral nutrition in a Malaysian intensive care unit: Risk factors and outcome. Asia Pac. J. Clin. Nutr. 2018, 27, 329–335. [Google Scholar] [CrossRef]
- Jenkins, B.; Calder, P.C.; Marino, L.V. Evaluation of implementation of fasting guidelines for enterally fed critical care patients. Clin. Nutr. 2019, 38, 252–257. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, X.; Wu, C.; Tang, J.; Li, Q.; Feng, X.; He, T.; Wang, Z.; Gao, J.; Ruan, Z.; et al. The effects of an enteral nutrition feeding protocol on critically ill patients: A prospective multi-center, before-after study. J. Crit. Care 2020, 56, 249–256. [Google Scholar] [CrossRef]
- Friesecke, S.; Schwabe, A.; Stecher, S.S.; Abel, P. Improvement of enteral nutrition in intensive care unit patients by a nurse-driven feeding protocol. Nurs. Crit. Care 2014, 19, 204–210. [Google Scholar] [CrossRef]
- Honda, C.K.Y.; Freitas, F.G.R.; Stanich, P.; Mazza, B.F.; Castro, I.; Nascente, A.P.M.; Bafi, A.T.; Azevedo, L.C.P.; Machado, F.R. Nurse to bed ratio and nutrition support in critically ill patients. Am. J. Crit. Care 2013, 22, e71–e78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darawad, M.W.; Alfasfos, N.; Zaki, I.; Alnajar, M.; Hammad, S.; Samarkandi, O.A. ICU Nurses’ Perceived Barriers to Effective Enteral Nutrition Practices: A Multicenter Survey Study. Open Nurs. J. 2018, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cahill, N.E.; Murch, L.; Cook, D.; Heyland, D.K. Barriers to feeding critically ill patients: A multicenter survey of critical care nurses. J. Crit. Care 2012, 27, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Zhuang, Y.; Qi, H.; Chen, X.; Lv, K. Current status and influencing factors of barriers to enteral feeding of critically ill patients: A multicenter study. J. Clin. Nurs. 2019, 28, 677–685. [Google Scholar] [CrossRef]
- Spear, S.; Sim, V.; Moore, F.A.; Todd, S.R. Just say no to intensive care unit starvation: A nutrition education program for surgery residents. Nutr. Clin. Pract. 2013, 28, 387–391. [Google Scholar] [CrossRef]
- Morphet, J.; Clarke, A.B.; Bloomer, M.J. Intensive care nurses’ knowledge of enteral nutrition: A descriptive questionnaire. Intensive Crit. Care Nurs. 2016, 37, 68–74. [Google Scholar] [CrossRef]
- Kim, H.; Chang, S.J. Implementing an educational program to improve critical care nurses’ enteral nutritional support. Aust. Crit. Care 2019, 32, 218–222. [Google Scholar] [CrossRef]
- Castro, M.G.; Pompilio, C.E.; Horie, L.M.; Verotti, C.C.G.; Waitzberg, D.L. Education program on medical nutrition and length of stay of critically ill patients. Clin. Nutr. 2013, 32, 1061–1066. [Google Scholar] [CrossRef]
- Gonya, S.; Baram, M. Do we really know how much we are feeding our patients? Hosp. Pract. (1995) 2015, 43, 277–283. [Google Scholar] [CrossRef]
- Hurt, R.T.; McClave, S.A.; Evans, D.C.; Jones, C.; Miller, K.R.; Frazier, T.H.; Minhas, M.A.; Lowen, C.C.; Stout, A.; Edakkanambeth Varayil, J.; et al. Targeted Physician Education Positively Affects Delivery of Nutrition Therapy and Patient Outcomes: Results of a Prospective Clinical Trial. J. Parenter. Enter. Nutr. 2015, 39, 948–952. [Google Scholar] [CrossRef]
- Lee, J.M.; Fernandez, F.; Staff, I.; Mah, J.W. Web-based teaching module improves success rates of postpyloric positioning of nasoenteric feeding tubes. J. Parenter. Enter. Nutr. 2012, 36, 323–329. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.; Cahill, N.; Murch, L.; Sinuff, T.; Bray, T.; Tanguay, T.; Heyland, D.K. Lessons learned from implementing a novel feeding protocol: Results of a multicenter evaluation of educational strategies. Nutr. Clin. Pract. 2014, 29, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, B.A.; Gillum, D.R.; Kelly, J.M. Burnout, Moral Distress, and Job Turnover in Critical Care Nurses. Int. J. Stud. Nurs. 2018, 3, 108. [Google Scholar] [CrossRef]
- Xu, E.; Tejada, S.; Solé-Lleonart, C.; Campogiani, L.; Valenzuela-Sanchez, F.; Koulenti, D.; Rello, J. Evaluation of the quality of evidence supporting guideline recommendations for the nutritional management of critically ill adults. Clin. Nutr. ESPEN 2020, 39, 144–149. [Google Scholar] [CrossRef]
| Country | Study Design | Objective of Study/Outcomes | Study Population/Setting | Results/Consequence(s) | Reference |
| Israel | Guideline | ESPEN Guideline “Clinical Nutrition on intensive care unit” | Literature review and expert opinion | Possible complications as well as energy and protein deficit. | [1] |
| Australia | Systematic review | To determine whether malnutrition diagnosed by validated nutrition assessment tools such as the Subjective Global Assessment (SGA) or Mini Nutritional Assessment (MNA) is independently associated with clinical outcomes and if the use of nutrition screening tools demonstrate a similar association. | 20 case-control or cohort studies, adults in the ICU; Outcomes including mortality, length of stay (LOS), incidence of infection (IOI). | Prevalence of malnutrition ranged from 38% to 78%. Malnutrition diagnosed by nutrition assessments was independently associated with increased ICU LOS, ICU readmission, IOI, and the risk of hospital mortality. The SGA had a better predictive validity than the MNA. Compared with nutrition assessment tools the predictive validity of nutrition screening tools was less consistent. | [2] |
| Canada | Post hoc analysis of an existing database derived from a RCT. | To externally validate a modified version of the NUTRIC score. | 1199 ICU patients with multi-organ failure, mechanically ventilated, with expected length of stay >5 days with a primary outcome of 28-day mortality. | Increased nutritional adequacy is associated with increased survival in patients with higher NUTRIC scores (>6) but not in patients with lower NUTRIC scores (<5). There is a strong positive association between nutritional adequacy and 28-day survival in patients with a high NUTRIC score but this association diminishes with decreasing NUTRIC score. Higher NUTRIC scores are significantly associated with higher 6-month mortality (p < 0.0001) and the positive association between nutritional adequacy and 6 months survival was significantly stronger in patients with higher NUTRIC score (p = 0.038). | [3] |
| Australia | Retrospective audit of patients | To evaluate the applicability of a nutrition triage tool against the NUTRIC score. | Patients (n = 151) | The NUTRIC score identified 18 positive responses. (18/49) in the general ICU population and 24 positive responses (24/102) in the cardiac surgical ICU population. Of these positive NUTRIC responses the current nutrition triage tool identified 83% (15/18) and 71% (17/24), respectively. The current tool also identified 39 patients (26%), across both subpopulations which were not identified by the NUTRIC score. | [4] |
| China | Prospective observational study, single center | Evaluation of nutrition risk assessment and prognosis with prediction tools (NRS-2002, NUTRIC, and mNUTRIC scores) in neurological ICU (NICU) patients. Primary outcome: 28-day-mortality. | 140 critically ill neurological patients. | Based on their mNUTRIC scores, a high nutrition risk (mNUTRIC score ≥ 5) was observed in 28.6% of patients, whereas a low nutrition risk (mNUTRIC score < 5) existed in 71.4% of patients. NUTRIC and mNUTRIC score are both able to predict 28-day-mortality. Odds ratios of 3.30 (95% CI, 1.01–10.80), 3.54 (95% CI, 1.04–12.02), 10.88 (95% CI, 3.33–35.57), and 13.95 (95% CI, 4.32–45.01), respectively. | [5] |
| Australia | Retrospective observational study, single-center | To assess the feasibility of using the mNUTRIC tool to screen for patients at increased nutrition risk and to determine the proportion of those high-risk patients who were reviewed by a dietitian. | 260 critically ill patients. | The median time required to complete a full mNUTRIC screen was 4 min and 54 s. During the study period, 160 patients admitted to the ICU were screened as being at low nutrition risk (mNUTRIC < 5). Of these patients, 63% (n = 101) were not reviewed formally by a dietitian. Eighty-one patients were flagged as high nutrition risk (mNUTRIC < 6); of these, 45 (56%) were formally reviewed by a dietitian and 36 (44%) did not have a dietetic consultation during their ICU stay. | [6] |
| USA | Retrospective analysis, single-center | To compare the Nutrition Risk in Critically Ill (NUTRIC) to the Nutritional Risk Screening (NRS) 2002 in terms of their associations with macronutrient deficit in ICU patients | 312 adult critically ill patients | Mean NUTRIC and NRS 2002 scores were 4 ± 2 and 4 ± 1, respectively. Linear regression demonstrated that each increment in NUTRIC score was associated with a 49 g higher protein deficit (β = 48.70: 95% confidence interval [CI] 29.23–68.17) and a 752 kcal higher caloric deficit (β = 751.95; 95% CI 447.80–1056.09). Logistic regression demonstrated that NUTRIC scores > 4 had over twice the odds of protein deficits ≥ 300 g (odds ratio (OR) 2.35; 95% CI 1.43–3.85) and caloric deficits ≥ 6000 kcal (OR 2.73; 95% CI 1.66–4.50) compared with NUTRIC scores ≥ 4. No association of NRS 2002 scores with macronutrient deficit was observed. | [7] |
| Brazil | Retrospective cohort study, multicenter | To compare the nutrition risks detected by NUTRIC with those by NRS 2002 in order to identify whether both tools are concordant for equivalent use in clinical practice in the ICU. | 208 critically ill patients | The comparison between both nutrition screening tools showed fair agreement (κ = 0.39). Nearly half of the patients were classified at high nutrition risk by NUTRIC (47.6%), whereas only approximately one-third of the sample was classified similarly by NRS 2002 (35.6%). | [8] |
| USA | Single center study, prospective case series | To identify the esophagus and the stomach, tube positioning via real-time video guidance with integrated real-time imaging system (IRIS). | 49 critically ill patients hospitalized in the ICU or step-down unit, minimal feeding for 72-hours, 42 completed study. | After training, the tube placement was simple and safe. 44 subjects (92%) successfully placed. Median time 8 minutes till placement. | [9] |
| China | Single center study, case series | Enteral nutrition support to critically ill patients via the nasogastrojejunal approach guided by semi-automated ultrasound. | 41 critically ill patients | The application of saline can be taken as sound window and the metal wire as the tracking target, the bedside nasogastrojejunal tube guided by semi-automated ultrasound is an effective feeding tube placement method. The total nursing service satisfaction of patients was 90.24%, and the total incidence of adverse reactions was 17.07%. | [10] |
| China | Single-center, randomized controlled trial | To compare the effectiveness of EM (electromagnetic)-guided and endoscopic nasoenteral feeding tube placement among critically ill patients. The primary end point was the total success rate of correct jejunal placement. | 161 adult patients admitted to ICUs requiring nasoenteral feeding. Patients were randomly assigned to EM-guided or endoscopic nasoenteral feeding tube placement (1:1) | Success rate was achieved in 74/81 and 76/80 patients who underwent EM-guided and endoscopic jejunal tube placements, respectively (91.4% vs. 95%; relative risk, 0.556; (CI), 0.156–1.980; p = 0.360). The EM-guided group had more placement attempts, longer placement time, and shorter inserted nasal intestinal tube length. They had shorter total placement procedure duration and physician’s order–tube placement and order–start of feeding intervals. The EM-guided group had higher discomfort level and recommendation scores and lesser patient costs. | [11] |
| Israel | Pilot RCT (TICACOS), single center | To determine whether nutritional support guided by repeated measurements of resting energy requirements improves the outcome. | Mechanically ventilated patients (n = 130) Patients were randomized to receive enteral nutrition (EN) with an energy target determined either by indirect calorimetry measurements (study group, n = 56), or according to 25 kcal/kg/day (control group, n = 56). | The study group had a higher mean energy (2086 ± 460 vs. 1480 ± 356 kcal/day, p = 0.01) and protein intake (76 ± 16 vs. 53 ± 16 g/day, p = 0.01). There was a trend towards an improved hospital mortality in the intention to treat group (21/65 patients, 32.3% vs. 31/65 patients, 47.7%, p = 0.058) whereas length of ventilation (16.1 ± 14.7 vs. 10.5 ± 8.3 days, p = 0.03) and ICU stay (17.2 ± 14.6 vs. 11.7 ± 8.4, p = 0.04) were increased. A Kaplan–Meier curve for the ‘‘per protocol’’ group shows that hospital mortality was significantly lower in the study group (16/56 patients, 28.5% vs. 27/56 patients, 48.2%; p = 0.023. Survival at 60 days was 57.9 ± 9.9% in the study group and 48.1 ± 7.6% in the control group (p = 0.023). | [12] |
| Italy | Prospective, multicenter observational study | To evaluate the validity of predictive formulas and equations for the calculation of energy expenditure and protein needs, by using indirect calorimetry (IC) and the protein catabolite rate; and to compare prescribed and actually received nutrients with estimated and measured needs. | 42 critically ill adult patients hospitalized with acute kidney injury. | There were 654 days of artificial nutrition. Average energy and protein prescribed were respectively 1551 ± 644 kcal and 70.5 ± 38.2 g, while energy and protein actually delivered were 1408 ± 651 kcal and 63.4 ± 35.3 g (p < 0.0001 for both comparisons). In general, average energy needs measured by IC were significantly higher than both the prescribed and delivered nutrient amounts (IC 1724 kcal ± 431; prescribed 1575 ± 672; received 1439 ± 680, p < 0.0001). No predictive formula was precise enough, and Bland–Altman plots wide limits of agreement for all equations highlight the potential to under- or overfeed individual patients. | [13] |
| Belgium | Prospective quality control study, single center | Whether feeding prescriptions were translated into adequate caloric intake within the scope of a “real-life,” guideline-oriented nutritional approach. | 50 patients older than 18 years admitted to ICU when intubated and expected to receive mechanical ventilation for at least seven days. | 24.6% of the 350 nutritional prescriptions correctly estimated the need. In 40.0% of cases, nutritional needs were insufficiently covered. Overestimation occurred in the remaining 35.4%. Caloric prescription resulted in accurate delivery in 56.0% of cases. Effective feeding was not met in 32.6% of prescriptions, and in 9.14% actual feeding surpassed the prescribed amount by more than 10%. This study demonstrated a dissimilarity between the amount of calories prescribed according to current nutritional guidelines and the caloric need calculated by a stress-corrected Harris–Benedict equation. | [14] |
| USA | Single center, comparative, longitudinal predictive study | To quantify estimation errors against indirect calorimetry measurements indirect calorimetry was used to measure resting metabolic rate for 7 days. Three estimation methods were compared with the cumulative measurement. Cumulative energy expenditure was the primary end point. | 13 mechanically ventilated, ICU patients, more than 18 years old. | The actual mean 7-day cumulative difference of the sample was −618 kcal with a standard deviation of 774 kcal. This difference was equivalent to −4.7% ± 6.2% of the cumulative measured value over 7 days. The difference between measured and estimated cumulative resting metabolic rate was not statistically significant (p = 0.079). Among the 7 patients in group 1 (standard Penn State equation), the cumulative error of the extrapolated value compared with the measured value was −1423 ± 1524 kcal (p = 0.049 vs. measurement) representing −8.8% ± 9.1% of the cumulative measurement. On average, the Penn State equations predict resting metabolic rate over time within 5% of the measured value. This performance is similar to the practice of making 1 measurement and extrapolating it over 1 week. | [15] |
| Australia | Prospective observational study, single-center | To measure energy expenditures | 20 patients within the ICU, BMI >30, >18 years old, mechanically ventilated. After enrollment, measured energy expenditure was attempted at baseline and twice weekly to extubation or day 14. | Median measured energy expenditure was 2439 (1806–2703) kcal, the study estimate was 2247 (1986–2502) kcal (or −156 (−328 to 18) kcal lower than the measured expenditure), and the guideline estimate of 11–14 kcal/kg was 1444 (1259–1500) kcal (or −950 (−1254 to −595) kcal lower than the measured expenditure). Bland–Altman bias and 95% limits of agreement between the study estimate and measured expenditure was −8% (±46%) and between the guideline estimate, −49%. Poor clinical utility; and that, furthermore, measured energy expenditure increased over time with large individual variation | [16] |
| Country | Study Design | Objective of Study/Outcomes | Study Population/Setting | Effect/Consequence(s) | Reference |
| Switzerland | Prospective interventional study over three periods (A, baseline; B and C, intervention periods). Single-center | To measure the clinical impact of a two-step interdisciplinary quality nutrition program. | 572 critically ill patients, 49% on enteral nutrition (EN), 7% on EN+ parenteral nutrition (PN), Two-step quality program: (1) bottom-up implementation of feeding guideline; and (2) additional presence of an ICU dietitian. | The daily energy balance difference was significant between periods A and C with a dietitian (p = 0.0012), whereas it was not significant between periods A and B. The normalized daily energy delivery (kcal day ≥ 1 or kcal kg ≥ 1 day ≥ 1) improved significantly in both periods B and C. The cumulated energy balance on day 7 improved progressively over the three periods, becoming significantly less negative. The cumulated ICU stay energy balances also improved significantly. The dietitian interventions significantly improved the day 7 energy balances. | [17] |
| Canada | International, prospective, observational, cohort study | To develop, validate, and implement a system to reward top performers in ICU nutrition practice and to illuminate characteristics of top-performing ICUs. | 81 ICUs from 18 countries. Patients: 2956 consecutively enrolled mechanically ventilated adult patients. Interventions: To qualify for the “Best of the Best” (BOB) award, sites had to have implemented a nutrition proto- col and contributed complete data on a minimum of 20 patients BOB-scoring criteria: Patients receiving enteral nutrition (EN), overall adequacy of EN plus appropriate PN, patients with EN initiated within 48 hours, patients with high gastric residual volume receiving promotility drugs or small bowel tubes, glucose measurements greater than 10 mmol/L. | The BOB award ranking ranged from 1 for the best site to 81 for the worst site. There were significant correlations between the overall BOB score and nutrition adequacy (r = 0.94). Regression analysis of the categorical variables suggested that the presence of a dietitian in the ICU was associated with a high BOB award ranking. After controlling for region, hospital size, and ICU structure, compared with ICUs without dietitians, the overall rank of ICUs with dietitians was 23.6 better. | [18] |
| USA | Retrospective, single-center, performance improvement project | The purpose was to evaluate the effect of registered dietitian nutritionist (RDN) order-writing privileges on enteral nutrition (EN) order compliance and nutrition delivery in ICUs. | 50 critically ill patients, 150 EN days, data collection retrospectively via electronic health record. | Nonsignificant increase in EN order compliance occurred after implementation of RDN order-writing privileges, as measured by cumulative and component EN order parameters. Compliance increased by 17% for the cumulative EN order and 15% for the tube feed infusion rate order post-RDN order- writing privileges. RDN order-writing privileges improved EN order compliance and significantly improved protein delivery in selected ICUs The percent of protein needs delivered significantly increased from a mean (±SD) of 72.1% (±28.6) to 89.1% (±24.8) after implementation of RDN order- writing privileges p < 0.001). | [19] |
| Belgium | Systematic review | The review compared mainly reviews and RCTs in western countries with Nutrition Support Teams (NST) vs. a non-NST control group. Primary outcome was the prevalence of enteral nutrition vs. parenteral nutrition. | 27 reviews and studies including quite heterogenic groups of patients. | There is weak evidence of that NSTs might increase appropriate EN use in ICU patients. The decrease of duration of PN could not be shown. Although almost all studies were in favor of NSTs, the evidence is weak. | [20] |
| USA | Single center performance improvement project | Comparison of microbial growth on different feeding product in normothermal ICU vs. hyperthermal Burn-ICU setting. | 60 EN systems in normothermal (23 °C) and hyper thermal environment (32 °C), they compared open systems, “ready-to-hang”-systems (RTH) and modular, hospital-build tube feeding (MTF). | In the hyperthermal group the quantity of microbial colonization soon exceeded FDA recommendations in the MTF-group. | [21] |
| USA | Systematic review and meta-analysis | To assess the potential effect of methodologic bias on nutrition trials. | 15 RCT, Primary (mortality, morbidity) and secondary (time on ventilator or in intensive care unit/hospital, cost) outcomes were abstracted from each trial comparing early enteral nutrition (EEN) to no/delayed enteral nutrition. | EEN had a favorable effect on mortality (RR 0.61, 95% CI 0.41, 0.89) and infectious morbidity (RR 0.80, 95% CI 0.72, 0.89), but not on non-infectious morbidity or any secondary outcome. Mortality benefit was observed only in trials with more risks of bias; infectious morbidity benefit was observed in some analyses of trials with fewer bias risks. | [22] |
| China | Systematic review and meta-analysis | To evaluate the efficacy and safety of enteral nutrition (EN) within 48 h after admission in the patients with severe acute pancreatitis (SAP) or predicted severe acute pancreatitis (pSAP). | 10 RCT containing 1051 patients were included. | Comparing early enteral nutrition (EEN) to late EN or total parental nutrition in SAP or pSAP, the pooled risk ratios were 0.53 (95% confidence interval (CI) 0.35–0.81, p = 0.003) for mortality, 0.58 (95% CI 0.43–0.77, p = 0.0002) for multiple organ failure (MOF), 0.50 (95% CI 0.33–0.75, p = 0.0008) for operative intervention, 0.75 (95% CI 0.61–0.93, p = 0.009) for systemic infection, 0.42 (95% CI 0.26–0.69, p = 0.0005) for local septic complications, 0.84 (95% CI 0.74–0.96, p = 0.01) for gastrointestinal symptoms. 0.87 (95% CI 0.74–1.02, p = 0.08) for systemic inflammatory response syndrome (SIRS), and 1.24 (95% CI 0.66–2.31, p = 0.50) for other local complications. | [23] |
| Estonia | Systematic review/Delphi | To determine whether early enteral nutrition (EEN) is advisable in the heterogeneous cohort of critically ill patients and to provide evidence-based guidelines for EEN (EN started within 48 h of admission) during critical illness. | 30 RCTs were analyzed. 5 meta-analyzes were performed: in unselected critically ill patients, and specifically in traumatic brain injury, severe acute pancreatitis, gastrointestinal (GI) surgery and abdominal trauma. | EEN reduced infectious complications in unselected critically ill patients, in patients with severe acute pancreatitis, and after GI surgery. Did not detect any evidence of superiority for early parenteral nutrition or delayed EN over EEN. | [24] |
| Australia | Meta-analysis | To determine whether the provision of early standard enteral nutrition (EN) confers treatment benefits to adult trauma patients who require intensive care. | RCTs conducted in adult trauma patients requiring intensive care that compared the delivery of standard EN, provided within 24 h of injury, to standard care were included. Outcomes included mortality, functional status and quality of life. Secondary analyses considered vomiting/regurgitation, pneumonia, bacteriaemia, sepsis and multiple organ dysfunction syndrome. | Three RCTs with 126 participants were found to be free from major flaws and were included in the primary analysis. The provision of early EN was associated with a significant reduction in mortality (OR = 0.20, 95% confidence interval 0.04–0.91, I2 = 0). No other outcomes could be pooled. | [25] |
| USA | RCT, single-center | Comparing early trophic enteral nutrition (EN) with “no EN” in mechanically ventilated adults with septic shock | 31 patients, adults who were at least 18 years of age, admitted to the medical ICU with a primary diagnosis of septic shock, and mechanically ventilated within 24 h of ICU admission. | 31 patients, 15 received early EN, 16 randomized to receive no EN. Early trophic EN group started EN with a 1.2-kcal/mL formula within 16 hours (interquartile range (IQR) 9–21) from ICU admission, and the “no EN” group started EN 48 hours (IQR 13–61) after ICU admission. Twenty percent of early EN patients had a vomiting episode over the first 7 days, as compared with 56% in the “no EN” group (p = 0.038). No patient had bowel obstruction, or ileus. One patient in the “no EN” group had VAP, compared with 0 in the early trophic EN group. Candida was isolated in subsequent urine or respiratory culture in 6/16 (38%) patients in the “no EN” group and in 1/15 (7%) patient from the early EN group (p = 0.083). | [26] |
| China | Systematic review and meta-analysis | To evaluate the effect of early enteral nutrition (EEN) on the outcome of critically ill patients. | 1725 patients, RCTs conducted in critically ill patients that compared the EEN, provided within 48 h of ICU admission or post-operation, to delayed enteral nutrition (DEN) were included. | Although no significant difference was observed in the risk of mortality, EEN within 48 h can improve the clinical outcomes of critically ill patients compared to DEN. This study showed that EEN within 48 h of admission is associated with a reduced risk of complications, infection, pneumonia, and length of stay compared to DEN. | [27] |
| Australia | Meta-Analysis of Randomized Controlled Trials | To identify, appraise, and synthesize the most current evidence to determine whether early enteral nutrition (EN) alters patient outcomes from critical illness. | 699 full-text articles were retrieved and screened. Sixteen randomized controlled trials enrolling 3225 critically ill participants were included. | Compared with all other types of nutrition support, commencing EN within 24 hours of ICU admission did not result in a reduction in mortality (odds ratio, 1.01; 95% CI, 0.86–1.18; p = 0.91; I2 = 32%). However, there was a differential treatment effect between a priori identified subgroups (p = 0.032): early EN reduced mortality compared with delayed enteral intake (odds ratio, 0.45; 95% CI, 0.21–0.95; p = 0.038; I2 = 0%), whereas a mortality difference was not detected between early EN and PN (odds ratio, 1.04; 95% CI, 0.89–1.22; p = 0.58; I2 = 30%). Overall, patients who were randomized to receive early EN were less likely to develop pneumonia (odds ratio, 0.75; 95% CI, 0.60–0.94; p = 0.012; I2 = 48%). | [28] |
| Country | Study Design | Objective of Study/Outcomes | Study Population/Setting | Results/Consequence(s) | Reference |
| Australia | Prospective before and after study, single-center study | Number of interruptions and the reasons for interrupting, development of recommendations for nursing practice | Before (338 patients), after (315 patients) Development of strategies to improve practice and minimize the effect to practice. | Number of interruptions decreased from 907 to 662. Interruptions due to gastrointestinal issues decreased (14 vs. 10%). Time lost to feeding because of interruptions was similar in both groups. | [29] |
| Japan | Single-center retrospective chart review | Duration of interruption; reason for each interruption, presence of written orders for interruptions. | Retrospective chart review of 100 patients | There were 567 episodes of enteral nutrition (EN) interruption over a median ICU length of stay of 17.1 days. There were a median of three EN interruption episodes per patient. Median duration of EN interruption in all patients was 5.5 h. | [30] |
| Malaysia | Prospective observational study, single-center | Prevalence, causes, and duration of such interruption were investigated. | 154 ICU patients | About 332 episodes of interruptions were recorded. For each patient, feeding interruptions occurred for a median of 3 days. Total duration of feeding interruptions for the entire ICU stay: 24.5 h. Median energy and protein deficits per patient due to feeding interruptions for the entire ICU stay were 1780.23 kcal and 100.58 g. | [31] |
| Canada | Retrospective review | To examine differences between prescribed and actual enteral nutrition (EN) delivery and to identify the specific causes of EN interruption and to quantify these. | Adult regional American Burn Association-verified burn center, total of 90 subjects were studied. On postburn days 0 to 14 the daily volume of EN prescribed by the dietitian was compared with the actual volume received by the patient. | Enterally fed burn patients received significantly less nutrition than prescribed. Interruptions for surgery accounted for 24% of total discrepancy time. Other causes of discrepancies were physician- or nurse-directed interruptions (16% of time), planned extubation (7%), feed intolerance (11%), tube malfunction (2%), bedside procedures (2%), and dressing changes (3%). | [32] |
| UK | A service improvement project | To evaluate the effectiveness of a fasting guideline in a general/trauma ICU. | A general/trauma ICU in a London teaching hospital. The unit takes approximately 700 admissions a year, with 30–50% of admissions being trauma. There are eight intensive care medicine consultants, 100 nursing staff (50% band 5, 38% band 6, 10% band 7 and 2% band 8) and full-time critical care specialist dietitian, pharmacist and physiotherapists. | There were 62 interruptions to enteral nutrition delivery with the first data collection and 64 in the second. Prolonged fasting before and after surgery and airway procedures were initially identified as the two most important causes of delays. | [33] |
| Iran | RCT, single center | Comparison of gastric residual volume (GRV) by position (supine, semirecumbent (SR), and right lateral (RL)) and by group (A and B). Groups A and B were in the supine position in Stage 1. Group A was in the SR position in Stage 2 and in the RL position in Stage 3. Group B was in the RL position in Stage 2 and in the SR position in Stage 3. | 36 mechanically ventilated patients. GRV was measured 3 h after feeding in supine and then in right-lateral and semi-recumbent position. | No significant difference in the GRV between groups while in the supine position (p = 0.085), SR position (p = 0.106), or RL position (p = 0.059). The effect of group (A vs. B) and position (supine, SR, or RL) on GRV was statistically significant for both groups (both at p = 0.001). GRV was significantly lower in the SR position compared with the supine position in both groups (p < 0.05), and GRV in the RL position was significantly lower than in the supine position in both groups (p < 0.05). GRVs in the SR and RL positions, although significantly and respectively different from the supine position, were not significantly different from each other (p > 0.05). | [34] |
| Brazil | Systematic review | To evaluate the effect of enteral feeding of critically ill adult and pediatric patients in the prone position on gastric residual volume and other clinical outcomes. | Four studies with adult patients and one with preterm patients were included. Main outcome = gastric residual volume | Three studies did not show differences in the gastric residual volume between the prone and supine positions (p > 0.05), while one study showed a higher gastric residual volume during enteral feeding in the prone position and another group observed a greater gastric residual volume in the supine position (reduction of the gastric residual volume by 23.3% in the supine position versus 43.9% in the prone position; p < 0.01). Two studies evaluated the frequency of vomiting; one found that it was higher in the prone position (30 versus 26 episodes; p < 0.001), the other study no significant difference (p > 0.05). | [35] |
| France | Before–after study, single-center | To evaluate an intervention for improving the delivery of early enteral nutrition (EEN) in patients receiving mechanical ventilation with prone positioning (PP). | Eligible patients receiving EEN and mechanical ventilation in PP were included within 48 h after intubation in a before–after study. Patients were semi-recumbent when supine. | An intervention including PP with 25° elevation, an increased acceleration to target rate of EN, and erythromycin improved EN delivery. Compared to the before group, larger feeding volumes were delivered in the intervention group (median volume per day with PP, 774 ml (IQR 513–925) vs. 1170 mL (IQR 736–1417); p < 0.001) without increases in residual gastric volume, vomiting, or ventilator-associated pneumonia. | [36] |
| France | RCT, multicenter, open-label, parallel-group study | To evaluate the outcome of early first-line enteral nutrition versus parenteral nutrition | 2410 patients on 44 ICU | Higher rate of digestive complication in enteral nutrition. Vomiting (406 (34%) vs. 246 (20%)); HR 1.89 ((1.62–2.20); p < 0.0001), diarrhoea (432 (36%) vs. 393 (33%)); 1.20 ((1.05–1.37); p = 0.009), bowel ischaemia (19 (2%) vs. five (<1%)); 3.84 ((1.43–10.3); p = 0.007), and acute colonic pseudo-obstruction (11 (1%) vs. three (<1%)); 3.7 ((1.03–13.2); p = 0.04). | [37] |
| Saudi Arabia | Prospective, randomized, multi-center study | Primary outcome was 90-day all-cause mortality. | 894 ICU patients. Randomly assigned to either permissive underfeeding or standard enteral feeding group. | No significant difference of 90-day mortality between the groups. Of patients in the standard enteral feeding group, 17.7% developed feeding intolerance, which summarizes vomiting, abdominal distention, or a gastric residual volume of more than 200 mL. | [38] |
| Turkey | Prospective observational study, single-center study | To evaluate the frequency, risk factors and complications of gastrointestinal dysfunction during enteral nutrition (EN) in the first 2 weeks of the ICU stay and to identify precautions to prevent the development of gastrointestinal dysfunction and avoid complications. | 137 ICU patients | Gastrointestinal dysfunction can cause inadequate nutrition. Incidence of gastrointestinal dysfunction was 63% (diarrhea 26%, constipation 29%, upper digestive intolerance 36%, vomiting 19%). Negative fluid balance and MDR bacteria positivity were independent risk factors for gastrointestinal dysfunction. | [39] |
| Switzerland | Prospective double-blind, RCT, single-center pilot study | Assessment of incidence and frequency of diarrhea and the respective effects of a modified enteral diet compared to a standard diet. | 90 ICU patients with enteral tube feeding. 1:1 randomization, receiving either a standard formula or Peptamen® AF, rich in proteins, medium chain triglycerides and fish oil. (intervention, n = 46; control, n = 44). | The incidence of diarrhea was 64% in the intervention and 70% in the control group. Diarrhea was associated with length of mechanical ventilation (9.5 (6.0–13.1) vs. 3.9 (3.2–4.6) days; p = 0.006) and length of ICU stay (11.0 (8.9–13.1) vs. 5.0 (3.8–6.2) days; p = 0.001) | [40] |
| UK | Retrospective, multi-center study | Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance on ICU. | 1888 patients, 167 ICU’s | Incidence of intolerance was 30.5% after a median 3 days from enteral nutrition (EN) initiation and led to prolonged ventilation (2.5 vs. 11.2, p < 0.0001), increased ICU stay (14.4 vs. 11.3 days, p < 0.0001), and increased mortality (30.8% vs. 26.2, p = 0.04). | [41] |
| Malaysia | Prospective, observational study, single-center study | Incidence, risk factors, and outcome of refeeding syndrome. | 109 ICU patients | 42.6% developed refeeding hypophosphatemia. | [42] |
| Turkey | Retrospective, single-center study | Incidence of refeeding hypophosphatemia. | 117 ICU patients with enteral nutrition (EN), parenteral nutrition (PE) or EN + PN | Overall incidence was 52.14%. Refeeding hypophosphatemia was found in 47.5% of the patients with PN, in 55.17% of the patients with EN, and in 52.6% of the patients with EN + PN. Mortality rate was significantly higher in patients with hypophosphatemia than without. (p = 0.037). | [43] |
| USA | Retrospective, case-control, single-center study | Incidence of enteral nutrition (EN) induced hypophosphatemia | 213 patients, surgical ICU | 59% incidence of hypophosphatemia of any cause. 33% refeeding hypophosphatemia 39% non-refeeding hypophosphatemia Not associated with worse clinical outcome. | [44] |
| UK | Systematic review and meta-analysis | Effectiveness of nasogastric versus post-pylorus feeding in ICU. | 20 RCT’s 1496 patients | Lower rate of aspiration pneumonia in post-pyloric feeding (nasojejunal tube) (OR, 1.41; 95% CI, 1.01, 1.98; z = 2.03; p < 0.04; I-2 = 10%) | [45] |
| Italy | Retrospective cohort study | To compare the occurrence of diarrhea in patients fed with blenderized natural food diet compared to commercial enteral feeding preparation. | 215 patients on cardiac-surgery ICU. | Commercial enteral feeding was administered by continuous pump infusion. (n = 112). Natural enteral feeding was administered by bolus 3 times per day (n = 103). Development of diarrhea was significantly lower in the blenderized natural food diet group compared to the commercial enteral feeding preparation group. (27.2% versus 48.2%, p = 0.002) | [46] |
| Canada | Systematic review and meta-analysis | To evaluate the effectiveness and safety of post-pyloric feeding versus gastric feeding for critically ill adults who require enteral tube feeding. | 14 eligible studies including 1109 ICU patients | There was no difference in mortality or duration of mechanical ventilation between the groups. Post-pyloric feeding is associated with lower rates of pneumonia compared with gastric tube feeding. (moderate quality of evidence; RR 0.65, 95% confidence interval (CI) 0.51 to 0.84. | [47] |
| Israel | Guideline | ESPEN Guideline “Clinical Nutrition on intensive care unit” | Literature review and Expert opinion. | Possible complications as well as energy and protein deficit. | [1] |
| UK | Retrospective single center study | To study the effect of implementation of fasting guideline | 74 ICU patients | 77% of staff were familiar with the guidelines, whilst 42% requested further education. The main barriers to guideline compliance were delays and unpredictable timing of procedures, and differing guidance from senior staff and non-ICU teams. Significant improvement in enteral nutrition (EN) delivery and reduced duration of feed breaks when using a protocol. | [48] |
| Germany | Before and after design, single-center study | To examine whether early enteral nutrition (EN) of critically ill patients could be improved by a nurse-driven implementation of an existing feeding protocol. | A total of 101 and 97 patients were included, respectively, before and after the intervention. | Following intervention, EN started significantly earlier (28 ± 20 h versus 47 ± 34 h, p < 0.001), within 24 h in 64% versus 25% (p < 0.0001). For each of the first 5 days, the proportion of patients meeting their nutritional goal was significantly higher. | [49] |
| Canada | International, prospective, observational, cohort studies, multicenter study | To evaluate the effect of enteral feeding protocols on key indicators of enteral nutrition in the critical care setting. | 5497 consecutively enrolled, mechanically ventilated, adult patients, 269 intensive care units (ICUs) in 28 countries. | Protocolized sites used more enteral nutrition (EN) alone (70.4% of patients vs. 63.6%, p = 0.0036), started EN earlier (41.2 hours from admission to ICU vs. 57.1, p = 0.0003), and used more motility agents in patients with high gastric residual volumes (64.3% of patients vs. 49.0%, p = 0.0028) compared with sites that did not use a feeding protocol. Overall nutritional adequacy (61.2% of patients’ caloric requirements vs. 51.7%, p = 0.0003) and adequacy from EN were higher in protocolized sites compared with non-protocolized sites (45.4% of requirements vs. 34.7%, p < 0.0001). | [18] |
| China | Prospective multi-center before-after study, conducted in 15 ICU’s of general hospitals in China | To explore the effects of an enteral nutrition (EN) feeding protocol (simplified-five-step SFS) in critically ill patients. Primary endpoint was the percentage of patients receiving EN within 7 days after ICU admission. | 439 (209 control, 230 intervention) patients in ICU for more than 3 d | SFS implementation did not increase percentage of patients receiving EN within 7 days (p = 0.65). EN related Adverse Events: 31.5 of 1000 ICU patient days in the control group and 19.1 of 1000 in the intervention group with statistical difference (p = 0.004). EN feeding protocol might be associated with increase of hospital survival (OR: 0.74, 95% CI: 0.55–1.00, p = 0.046) Intervention group reached significantly higher percentage of estimated calorie targets on day 6 (p = 0.01) and 7 (p = 0.002) than control. | [50] |
| Brazil | Single-center prospective cohort study | To determine how often daily calorie goals are met and the factors responsible for inadequate nutrition support. | 262 daily evaluations in 40 patients | Most patients did not achieve the prescribed daily calorie goal→ associated with the use of midazolam and assistance by a reduced nursing staff. Daily calorie goal was achieved in only 46.2% of the evaluations (n = 121) Risk factors for inadequate nutrition support were the use of midazolam (odds ratio, 1.58; 95% CI, 1.18–2.11) and fewer nursing professionals per bed (odds ratio, 2.56; 95% CI, 1.43–4.57). | [51] |
| Jordan | Multicenter Survey Study | To explore Jordanian ICU nurses’ perceived barriers for enteral nutrition that hinders them from utilizing the recommended enteral nutrition (EN) guidelines. | 131 ICU nurses | Most patients did not achieve the prescribed daily calorie goal, associated with the use of midazolam and assistance by a reduced nursing staff. The most important barrier was “Not enough nursing staff to deliver adequate nutrition” (M = 4.80, SD = 1.81, 60%), followed by “Fear of adverse events due to aggressively feeding patients” (M = 4.59, SD = 1.50, 56%). | [52] |
| USA | Cross-sectional survey, multicenter study | To describe the barriers to enterally feeding critically ill patients from a nursing perspective and to examine whether these barriers differ across centers. | A total of 138 of 340 critical care nurses completed the questionnaire. | No or not enough dietitian coverage during weekends and holidays. The 5 most important barriers to nurses were (1) other aspects of patient care taking priority over nutrition, (2) not enough feeding pumps available, (3) enteral formula not available on the unit, (4) difficulties in obtaining small bowel access in patients not tolerating enteral nutrition, and (5) no or not enough dietitian coverage during weekends and holidays. | [53] |
| China | Cross-sectional descriptive multicenter study | To investigate the barriers in administering enteral feeding to critically ill patients from the nursing perspective. | 808 nurses from 10 comprehensive hospitals | Frequency of enteral nutrition (EN)-related training, full-time ICU nutritionist, hospital level, specific protocols for enteral feeding and position were significantly influencing the enteral feeding of ICU patients. | [54] |
| USA | Prospective single-center observational pilot study | Nutrition education program would improve our residents’ knowledge of ICU nutrition. | 8 surgery residents completed the nutrition education program. Pre- and post-testing were performed to assess short-term comprehension. | The nutrition education program improved both short-term and long-term ICU nutrition knowledge of surgery residents (p < 0.01). | [55] |
| Australia | Online questionnaire | To explore Australian nurses’ enteral nutrition (EN) knowledge and sources of information. | 359 responses of registered nurses | Most respondents reported their EN knowledge was good (n = 205, 60.1%) or excellent (n = 35, 10.3%), but many lacked knowledge regarding the effect of malnutrition on patient outcomes. Dietitians and hospital protocols were the most valuable sources of enteral nutrition information, but were not consistently utilized. | [56] |
| South Korea | Quasi-experimental, one-group study with a pre- and post-test design, multicenter study | To evaluate the effects of an education program to improve critical care nurses’ perceptions, knowledge, and practices towards providing enteral nutritional support for ICU patients. | Nurses (n = 205) were recruited from nine ICUs. | Nurses’ overall perception significantly improved after the program (mean change = 3.18, p < 0.001). Nurses’ knowledge about enteral nutritional support showed a significant improvement after the education program (mean change = 11.2%, p < 0.001). Nurses’ total practice score significantly improved after the program (mean change = 2.54, p < 0.001). | [57] |
| Brazil | Prospective, non-blinded single-center study | To evaluate the impact of a multifaceted nutritional educational intervention on the quality of nutritional therapy and clinical outcomes in critically ill patients. | 16-bed ICU Phase 1: the quality of NT was evaluated in 50 newly admitted ICU patients in a pre-educational program (Pre-EP). Phase 2: nutritional protocols were created and an education program was implemented. Phase 3: another 50 patients were enrolled and observed in a post-educational program (Post-EP) using phase. | The mean ± SD duration of fasting decreased (Pre-EP 3.8 ± 3.1 days vs. Post-EP: 2.2 ± 2.6 days; p = 0.002), the adequacy of nutritional therapy improved (Pre-EP 74.2% ± 33.3% vs. Post-EP 96.2% ± 23.8%; p < 0.001), and enteral nutrition was initiated earlier than 48 h more commonly (Pre-EP 24% vs. Post-E 60%; p = 0.001). Median ICU length of stay decreased (Pre-EP: 18.5 days vs. Post-EP: 9.5 days; p < 0.001) although hospital length of stay did not. | [58] |
| UK | Prospective, pre- and post-intervention single-center study | To assess accuracy of enteral feeding records, to increase nursing education and to improve nutritional documentation. | 188 patient electronic medical records (EMR) | The intervention of an education program reduced the documented discrepancy between the pump readings and charted volumes from 44 to 33%. A correlation analysis also showed a tighter relationship post-intervention (rpost = 0.84 vs. rpre = 0.76, both had a p < 0.01). | [59] |
| USA | Prospective clinical trial | The experimental group (EG) received targeted education consisting of strategies to increase delivery of early enteral nutrition. Strategies included early enteral access, avoidance of nil per os (NPO) and clear liquid diets (CLD), volume-based feeding, early resumption of feeds post procedure, and charting caloric deficits. The control group (CG) did not receive targeted education but was allowed to practice in a standard ad hoc fashion. | Patients (n = 121) assigned to 1 of 2 trauma groups | EG received a higher percentage of measured goal calories (30.1 ± 18.5%, 22.1 ± 23.7%, p = 0.024) compared with the CG. Mean caloric deficit was not significantly different between groups (−6796 ± 4164 kcal vs. −8817 ± 7087 kcal, p = 0.305). CLD days per patient (0.1 ± 0.5 vs. 0.6 ± 0.9), length of stay in the intensive care unit (3.5 ± 5.5 vs. 5.2 ± 6.8 days), and duration of mechanical ventilation (1.6 ± 3.7 vs. 2.8 ± 5.0 days) were all reduced in the EG compared with the CG (p < 0.05). EG patients had fewer nosocomial infections (10.6% vs. 23.6%) and less organ failure (10.6% vs. 18.2%) than did the CG, but these differences did not reach statistical significance. | [60] |
| Canada | Questionnaire Multicenter Evaluation, enquiry study | This study describes the results of an evaluation of educational strategies used to implement a novel enteral feeding protocol. | The response rate to the questionnaire was 166 of 434 or 38.2%. | More than 70% of respondents rated 5 of the educational strategies as very useful or somewhat useful. The percentage of nurses who found the bedside protocol tools of the enteral feeding order set, gastric feeding flowchart, and volume-based feeding schedule either “very easy” or “somewhat easy” to use were 64.0%, 60.5%, and 59.1%, respectively. | [61] |
| USA | Creation of a Web-Based Teaching Module | The authors created a self-directed Web-based teaching module (WBTM) to educate and standardize placement of postpyloric nasoenteric tube (NET). | Forty-three first-, second-, or third-year residents or medical or physician assistant students took pretests for knowledge and confidence surveys, viewed the WBTM, placed NET at the bedside, then took a posttest and confidence survey while awaiting confirmation of tube position by abdominal radiograph. | Knowledge and confidence significantly improved. Overall success rate of postpyloric NET placement for all participants on first attempt was 74.4% vs. 46.7% in the control (p = 0.005). Improvement occurred in all subgroups, including those with no prior experience, who were successful 70.4% of the time (p = 0.009). | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, M.; Schwarz, C.M.; Fürst, S.; Starchl, C.; Lobmeyr, E.; Sendlhofer, G.; Jeitziner, M.-M. Risks in Management of Enteral Nutrition in Intensive Care Units: A Literature Review and Narrative Synthesis. Nutrients 2021, 13, 82. https://doi.org/10.3390/nu13010082
Hoffmann M, Schwarz CM, Fürst S, Starchl C, Lobmeyr E, Sendlhofer G, Jeitziner M-M. Risks in Management of Enteral Nutrition in Intensive Care Units: A Literature Review and Narrative Synthesis. Nutrients. 2021; 13(1):82. https://doi.org/10.3390/nu13010082
Chicago/Turabian StyleHoffmann, Magdalena, Christine Maria Schwarz, Stefan Fürst, Christina Starchl, Elisabeth Lobmeyr, Gerald Sendlhofer, and Marie-Madlen Jeitziner. 2021. "Risks in Management of Enteral Nutrition in Intensive Care Units: A Literature Review and Narrative Synthesis" Nutrients 13, no. 1: 82. https://doi.org/10.3390/nu13010082
APA StyleHoffmann, M., Schwarz, C. M., Fürst, S., Starchl, C., Lobmeyr, E., Sendlhofer, G., & Jeitziner, M.-M. (2021). Risks in Management of Enteral Nutrition in Intensive Care Units: A Literature Review and Narrative Synthesis. Nutrients, 13(1), 82. https://doi.org/10.3390/nu13010082





