[6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
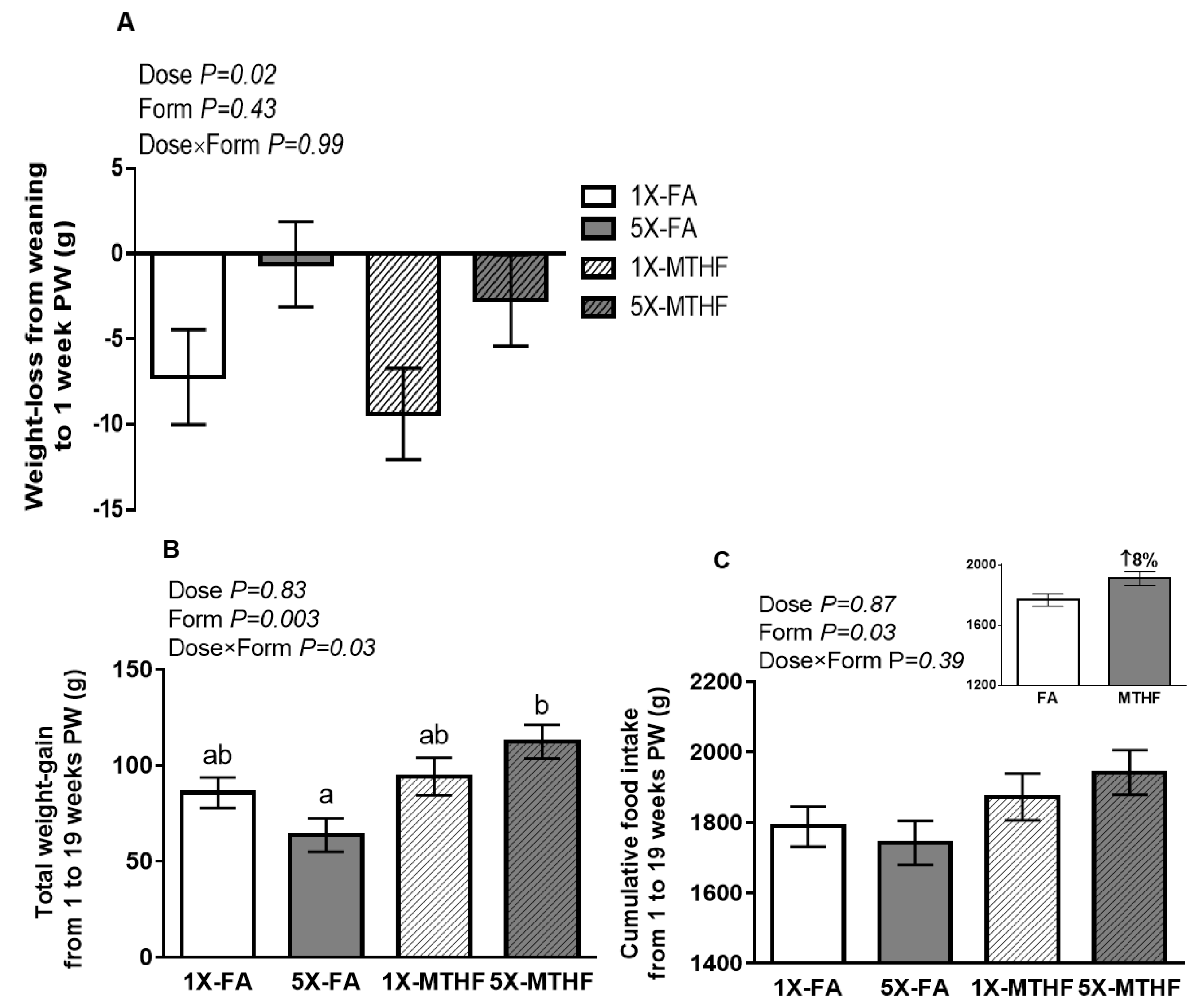
2.2. Body Weight, Food Intake, Body Composition and Plasma Analyses
2.3. Folate and Related 1-Carbon Metabolites
2.4. Plasma Hormones, and Insulin Tolerance Test
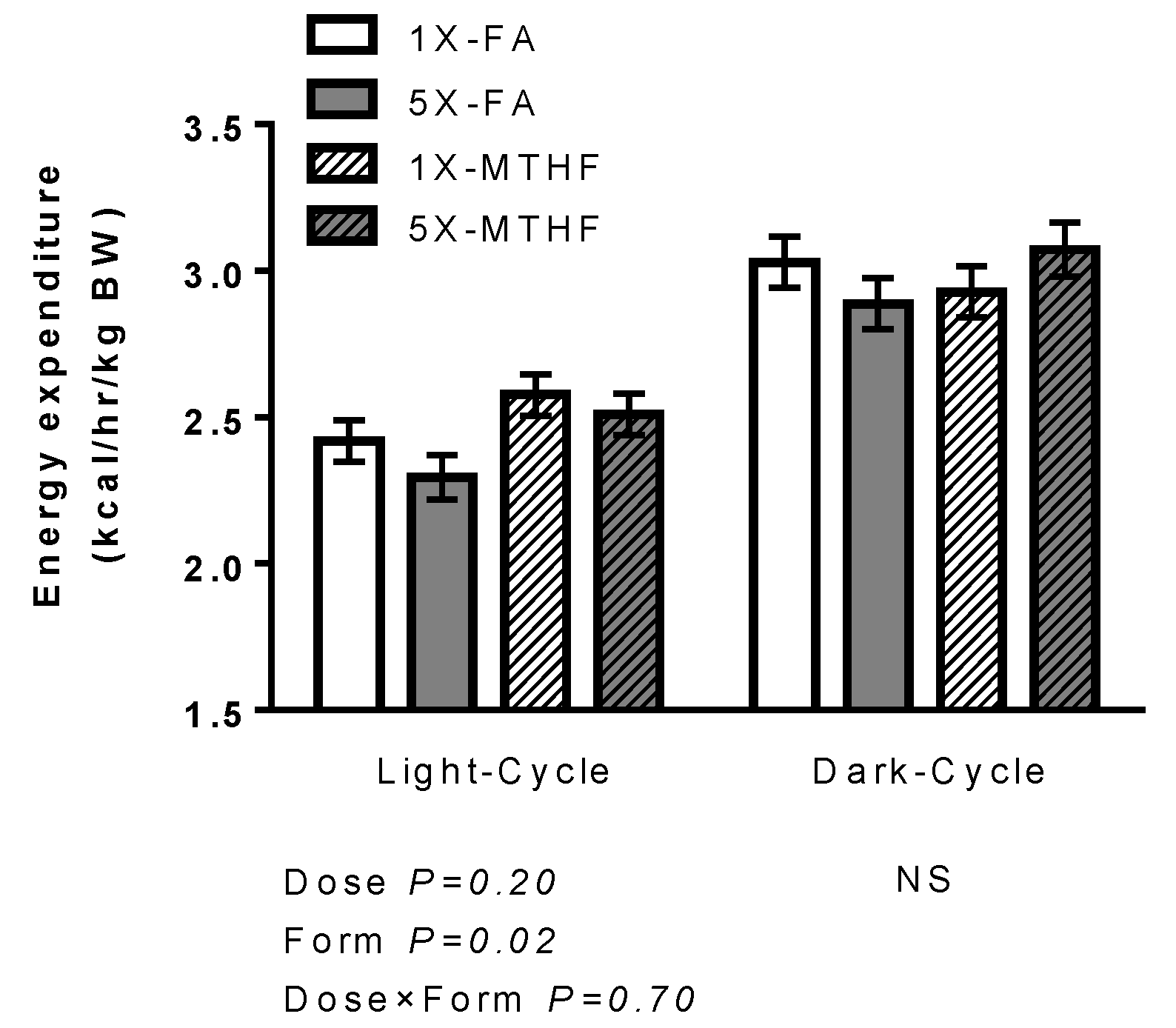
2.5. Energy Expenditure
2.6. Brain Analyses from 5X-FA and 5X-MTHF Dams
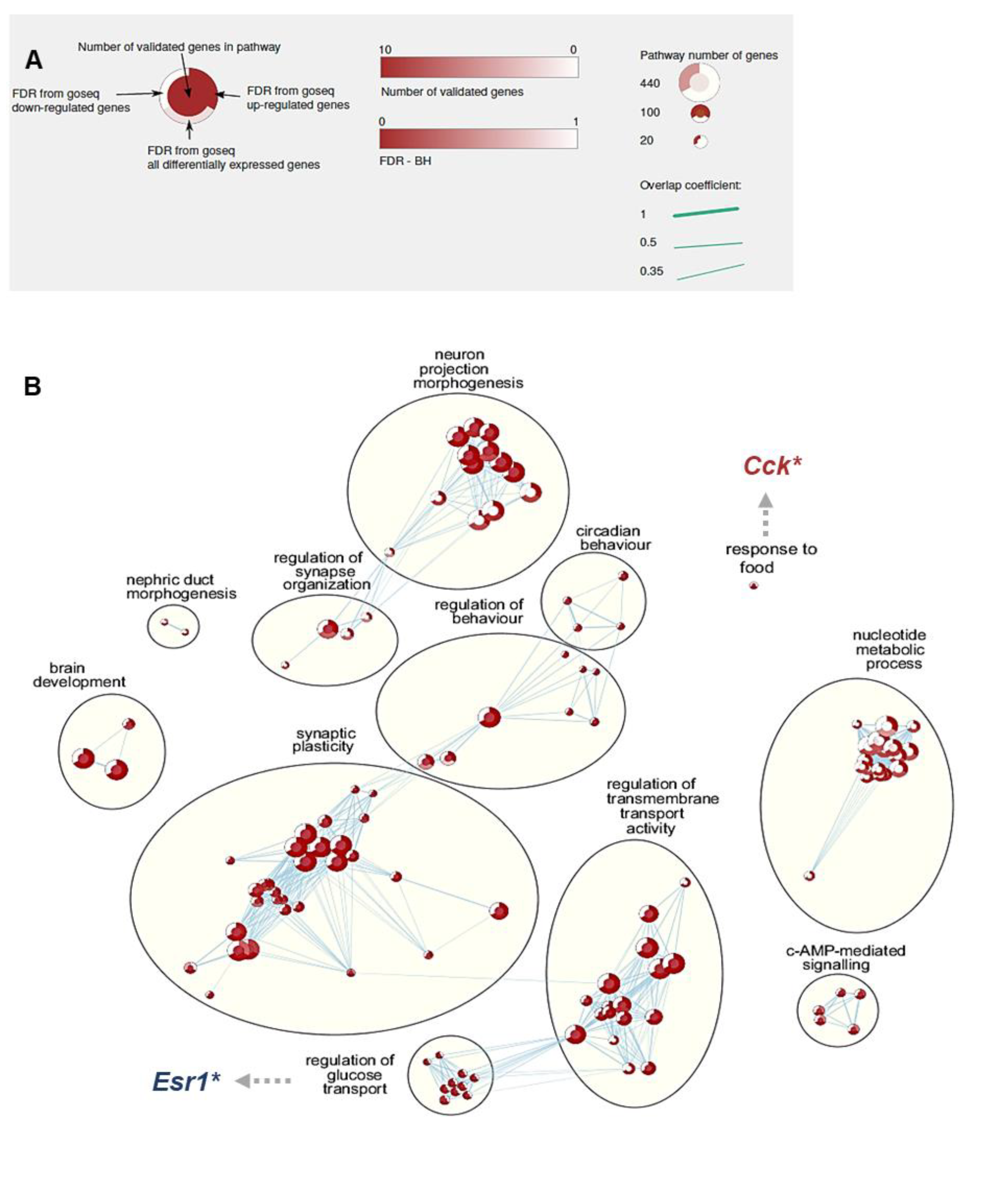
2.6.1. Brain Dissections and RNA-Sequencing
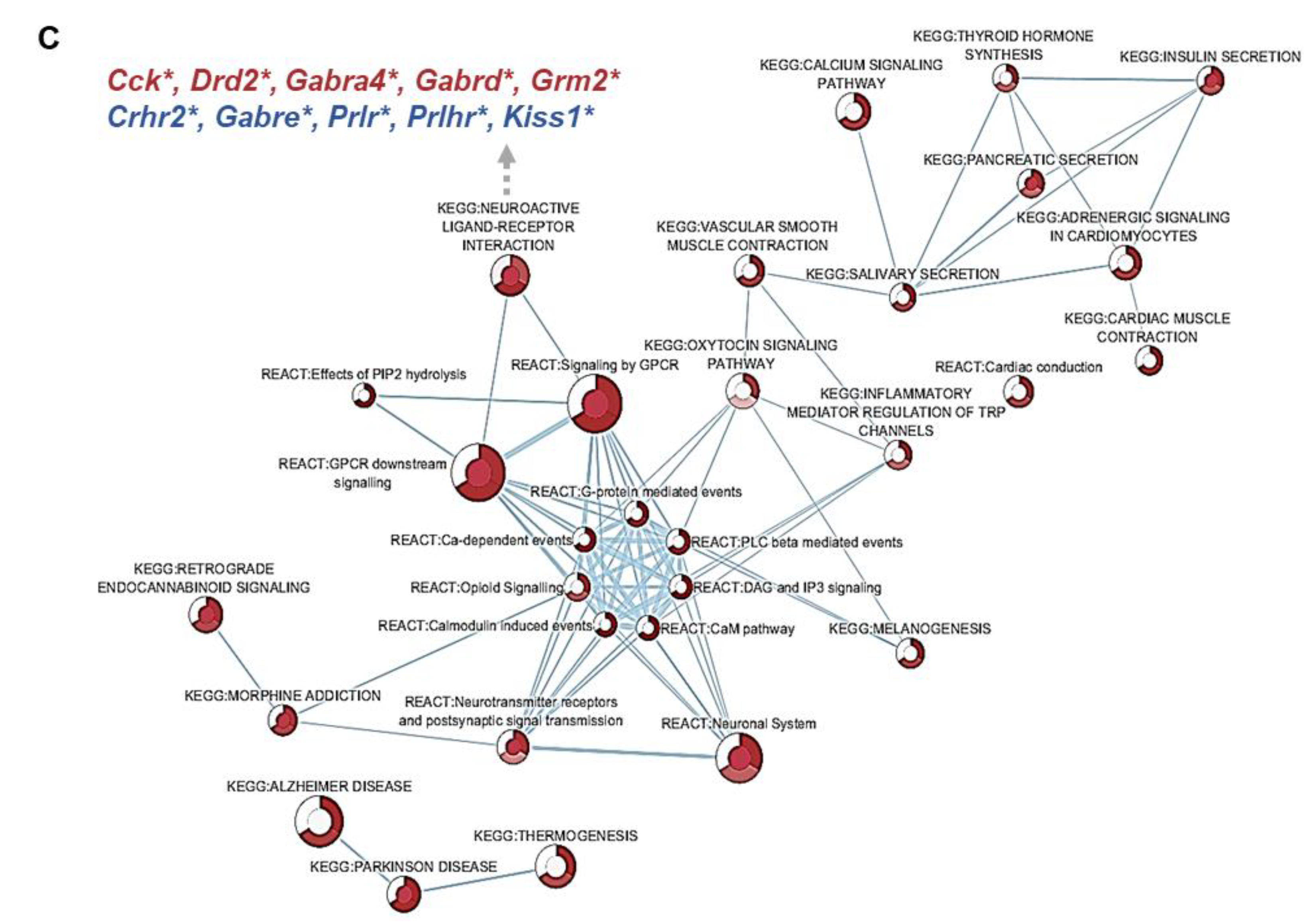
2.6.2. Enrichment Analysis of Differential Expressed Genes (DEGs)
2.6.3. Validation of DEGs: cDNA Synthesis and qRT-PCR
2.7. Statistical Analyses
3. Results
3.1. Effects of Folate Diets on Maternal Plasma and Liver 5-MTHF and 1-Carbon Metabolites at Parturition and 19-Weeks
3.2. Effects of Folate Diets on Energy Expenditure and Locomotor Activity
3.3. Effects of Folate Diets on Plasma Hormones and IR
3.4. Effect of 5X-FA vs. 5X-MTHF Diets on Whole-Transcriptome Gene Expression in the ARC at Parturition
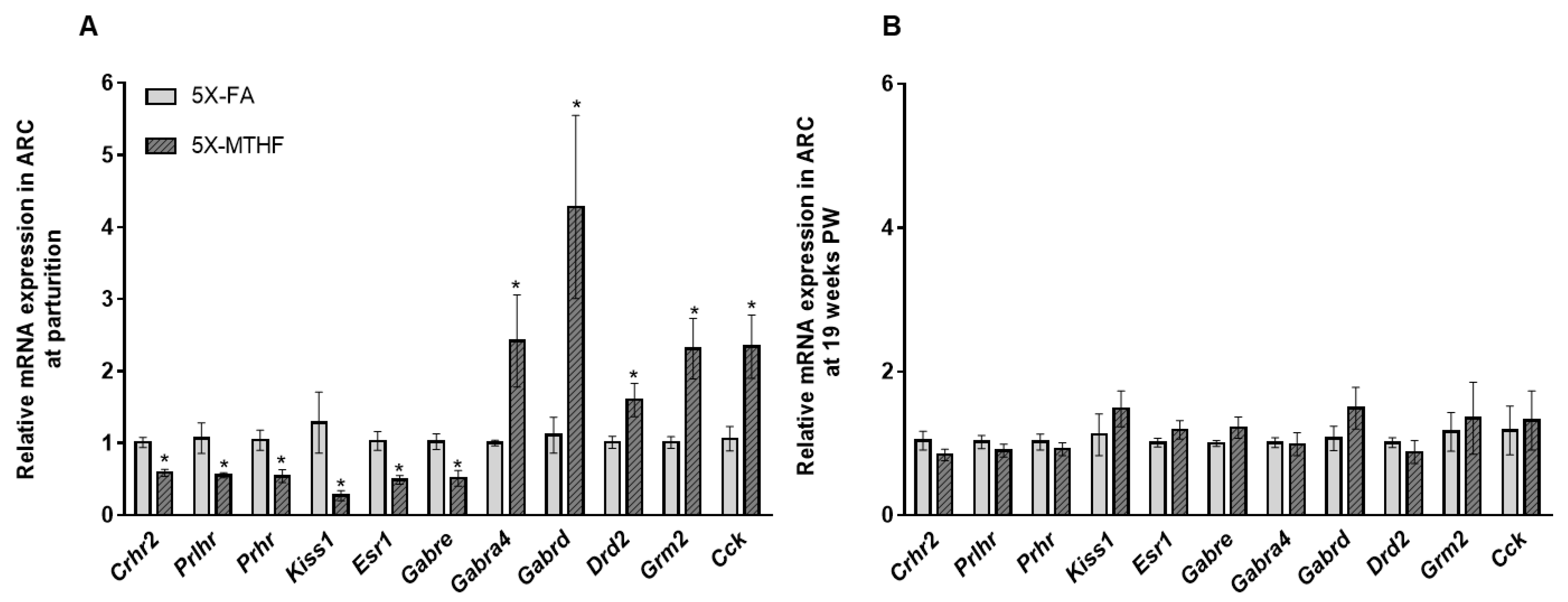
3.5. Validation of RNAseq Data Using qRT-PCR
3.6. Effect of 5X-FA vs. 5X-MTHF Diets on Brain 5-MTHF and 1-Carbon Metabolites in Dams at Parturition and 19-Weeks Post-Weaning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Dietrich, M.O.; Horvath, T.L. Hypothalamic control of energy balance: Insights into the role of synaptic plasticity. Trends Neurosci. 2013, 36, 65–73. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A.; Douglas, A.J. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008, 20, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Barba-Muller, E.; Craddock, S.; Carmona, S.; Hoekzema, E. Brain plasticity in pregnancy and the postpartum period: Links to maternal caregiving and mental health. Arch. Womens Ment. Health 2019, 22, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C. One carbon metabolism in pregnancy: Impact on maternal, fetal and neonatal health. Mol. Cell. Endocrinol. 2016, 435, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J.; Durga, J.; Field, M.S. Folate nutrition and blood-brain barrier dysfunction. Curr. Opin. Biotechnol. 2017, 44, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rey, M.; Iruarrizaga-Lejarreta, M.; Lozano, J.J.; Aransay, A.M.; Fernandez, A.F.; Lavin, J.L.; Mosen-Ansorena, D.; Berdasco, M.; Turmaine, M.; Luka, Z.; et al. S-adenosylmethionine levels regulate the schwann cell DNA methylome. Neuron 2014, 81, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Field, M.S.; Stover, P.J. Safety of folic acid. Ann. N. Y. Acad. Sci. 2018, 1414, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lamers, Y.; MacFarlane, A.J.; O’Connor, D.L.; Fontaine-Bisson, B. Periconceptional intake of folic acid among low-risk women in Canada: Summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am. J. Clin. Nutr. 2018, 108, 1357–1368. [Google Scholar] [CrossRef]
- Pannia, E.; Cho, C.E.; Kubant, R.; Sanchez-Hernandez, D.; Huot, P.S.; Harvey Anderson, G. Role of maternal vitamins in programming health and chronic disease. Nutr. Rev. 2016, 74, 166–180. [Google Scholar] [CrossRef]
- Yang, N.V.; Pannia, E.; Chatterjee, D.; Kubant, R.; Ho, M.; Hammoud, R.; Pausova, Z.; Anderson, G.H. Gestational folic acid content alters the development and function of hypothalamic food intake regulating neurons in Wistar rat offspring post-weaning. Nutr. Neurosci. 2018, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Pannia, E.; Huot, P.S.; Sanchez-Hernandez, D.; Kubant, R.; Dodington, D.W.; Ward, W.E.; Bazinet, R.P.; Anderson, G.H. Methyl vitamins contribute to obesogenic effects of a high multivitamin gestational diet and epigenetic alterations in hypothalamic feeding pathways in Wistar rat offspring. Mol. Nutr. Food Res. 2015, 59, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Sanchez-Hernandez, D.; Reza-Lopez, S.A.; Huot, P.S.; Kim, Y.I.; Anderson, G.H. Obesogenic phenotype of offspring of dams fed a high multivitamin diet is prevented by a post-weaning high multivitamin or high folate diet. Int. J. Obes. 2013, 37, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int. J. Mol. Sci. 2014, 15, 6298–6313. [Google Scholar] [CrossRef] [PubMed]
- Sie, K.K.; Li, J.; Ly, A.; Sohn, K.J.; Croxford, R.; Kim, Y.I. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol. Nutr. Food Res. 2013, 57, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Huot, P.S.; Ly, A.; Szeto, I.M.; Reza-Lopez, S.A.; Cho, D.; Kim, Y.I.; Anderson, G.H. Maternal and postweaning folic acid supplementation interact to influence body weight, insulin resistance, and food intake regulatory gene expression in rat offspring in a sex-specific manner. Appl. Physiol. Nutr. Metab. 2016, 41, 411–420. [Google Scholar] [CrossRef]
- Huot, P.S.; Dodington, D.W.; Mollard, R.C.; Reza-Lopez, S.A.; Sanchez-Hernandez, D.; Cho, C.E.; Kuk, J.; Ward, W.E.; Anderson, G.H. High Folic Acid Intake during Pregnancy Lowers Body Weight and Reduces Femoral Area and Strength in Female Rat Offspring. J. Osteoporos. 2013, 2013, 154109. [Google Scholar] [CrossRef]
- Pannia, E.; Yang, N.V.; Ho, M.; Chatterjee, D.; Hammoud, R.; Kubant, R.; Anderson, G.H. Folic acid content of diet during pregnancy determines post-birth re-set of metabolism in Wistar rat dams. J. Nutr. Biochem. 2020, 83, 108414. [Google Scholar] [CrossRef]
- Keating, E.; Correia-Branco, A.; Araujo, J.R.; Meireles, M.; Fernandes, R.; Guardao, L.; Guimaraes, J.T.; Martel, F.; Calhau, C. Excess perigestational folic acid exposure induces metabolic dysfunction in post-natal life. J. Endocrinol. 2015, 224, 245–259. [Google Scholar] [CrossRef]
- Mudryj, A.N.; de Groh, M.; Aukema, H.M.; Yu, N. Folate intakes from diet and supplements may place certain Canadians at risk for folic acid toxicity—CORRIGENDUM. Br. J. Nutr. 2016, 116, 1995. [Google Scholar] [CrossRef]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., 3rd; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef] [PubMed]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.W.; Ayling, J.E. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci. Rep. 2018, 8, 4096. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Smith, D.; Hornstra, J.; Rocha, M.; Jansen, G.; Assaraf, Y.; Lasry, I.; Blom, H.; Smulders, Y.M. Folic Acid Impairs the Uptake of 5-Methyltetrahydrofolate in Human Umbilical Vascular Endothelial Cells. J. Cardiovasc. Pharmacol. 2017, 70, 271–275. [Google Scholar] [CrossRef]
- Smith, A.D.; Kim, Y.I.; Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 2008, 87, 517–533. [Google Scholar] [CrossRef]
- Saldanha, L.G.; Dwyer, J.T.; Haggans, C.J.; Mills, J.L.; Potischman, N. Perspective: Time to Resolve Confusion on Folate Amounts, Units, and Forms in Prenatal Supplements. Adv. Nutr. 2020, 11, 753–759. [Google Scholar] [CrossRef]
- Troesch, B.; Demmelmair, J.; Gimpfl, M.; Hecht, C.; Lakovic, G.; Roehle, R.; Sipka, L.; Trisic, B.; Vusurovic, M.; Schoop, R.; et al. Suitability and safety of L-5-methyltetrahydrofolate as a folate source in infant formula: A randomized-controlled trial. PLoS ONE 2019, 14, e0216790. [Google Scholar] [CrossRef]
- Pietrzik, K.; Bailey, L.; Shane, B. Folic acid and L-5-methyltetrahydrofolate: Comparison of clinical pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2010, 49, 535–548. [Google Scholar] [CrossRef]
- Servy, E.J.; Jacquesson-Fournols, L.; Cohen, M.; Menezo, Y.J.R. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: A key to pregnancy outcome: A case series. J. Assist. Reprod. Genet. 2018, 35, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Savella, G.M.; Church, T.R.; Goez-Mogollon, L.; Sosinsky, A.Z.; Noe, O.B.; Kaimal, A.; Cohen, L.S. A prenatal supplement with methylfolate for the treatment and prevention of depression in women trying to conceive and during pregnancy. Ann. Clin. Psychiatry 2019, 31, 4–16. [Google Scholar] [PubMed]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127 (Suppl. 5), 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Prinz-Langenohl, R.; Bramswig, S.; Tobolski, O.; Smulders, Y.M.; Smith, D.E.; Finglas, P.M.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C-->T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar] [CrossRef]
- Lamers, Y.; Prinz-Langenohl, R.; Moser, R.; Pietrzik, K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am. J. Clin. Nutr. 2004, 79, 473–478. [Google Scholar] [CrossRef]
- Cochrane, K.M.; Mayer, C.; Devlin, A.M.; Elango, R.; Hutcheon, J.A.; Karakochuk, C.D. Is natural (6S)-5-methyltetrahydrofolic acid as effective as synthetic folic acid in increasing serum and red blood cell folate concentrations during pregnancy? A proof-of-concept pilot study. Trials 2020, 21, 380. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient requirements of the laboratory rat. In Nutrient Requirements of Laboratory Animals, 4th ed.; National Academy Press: Washington, DC, USA, 1995; pp. 11–79. [Google Scholar]
- Medicine, I.O. Folate. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998; pp. 196–305. [Google Scholar]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Calcium l-methylfolate as a source of folate added for nutritional purposes to infant and follow-on formula, baby food and processed cereal-based food. EFSA J. 2020, 18, e05947. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Rich-Edwards, J.W. The reset hypothesis: Lactation and maternal metabolism. Am. J. Perinatol. 2009, 26, 81–88. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Arning, E.; Bottiglieri, T. Quantitation of 5-Methyltetrahydrofolate in Cerebrospinal Fluid Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Methods Mol. Biol. 2016, 1378, 175–182. [Google Scholar] [CrossRef]
- Nandania, J.; Kokkonen, M.; Euro, L.; Velagapudi, V. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1092, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.; Bottiglieri, T.; Wasek-Patterson, B.; McMahon, A.; Hughes, C.F.; McCann, A.; Horigan, G.; Strain, J.J.; McNulty, H.; Ward, M. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie 2020, 173, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ducros, V.; Belva-Besnet, H.; Casetta, B.; Favier, A. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin. Chem. Lab. Med. 2006, 44, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Kubant, R.; Poon, A.N.; Sanchez-Hernandez, D.; Domenichiello, A.F.; Huot, P.S.; Pannia, E.; Cho, C.E.; Hunschede, S.; Bazinet, R.P.; Anderson, G.H. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutr. Diabetes 2015, 5, e188. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, R.; Pannia, E.; Kubant, R.; Liao, C.S.; Ho, M.; Yang, N.V.; Chatterjee, D.; Caudill, M.A.; Malysheva, O.V.; Pausova, Z.; et al. Maternal Choline Intake Programs Hypothalamic Energy Regulation and Later-Life Phenotype of Male Wistar Rat Offspring. Mol. Nutr. Food Res. 2020, 64, e1901178. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2017. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 November 2020).
- Krueger, F. Trim Galore: A Wrapper Tool around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files, with Some Extra Functionality for MspI-Digested RRBS-Type (Reduced Representation Bisufite-Seq) Libraries. 2018. Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 1 November 2020).
- Wingett, S. FastQ Screen: FastQ Screen Allows You to Screen a Library of Sequences in FastQ Format against a Set of Sequence Databases so You Can See If the Composition of the Library Matches with What You Expect. 2017. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastq_screen/ (accessed on 1 November 2020).
- Wang, L. RSeQC: An RNA-seq Quality Control Package. 2019. Available online: http://rseqc.sourceforge.net/ (accessed on 1 November 2020).
- Dobin, A. STAR: RNA-seq aligner. 2019. Available online: https://github.com/alexdobin/STAR (accessed on 1 November 2020).
- Dobin, A. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Merico, D.; Isserlin, R.; Stueker, O.; Emili, A.; Bader, G.D. Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 2010, 5, e13984. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Friedman, J.M. The function of leptin in nutrition, weight, and physiology. Nutr. Rev. 2002, 60, S1–S14, discussion S68–S84. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. 2019, 12, 191–198. [Google Scholar] [CrossRef]
- Tschop, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.; Han, S.; Englander, E.W.; Greeley, G.H., Jr. Influence of a long-term high-fat diet on ghrelin secretion and ghrelin-induced food intake in rats. Regul. Pept. 2012, 173, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obesity 2018, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Beerens, S.; Adan, R.A.H. Role of leptin in energy expenditure: The hypothalamic perspective. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R938–R947. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Flint, A.; Field, A.E.; Kraft, P.; Han, J.; Rimm, E.B.; van Dam, R.M. A genome-wide investigation of food addiction. Obesity 2016, 24, 1336–1341. [Google Scholar] [CrossRef]
- Fan, T.; Hu, Y.; Xin, J.; Zhao, M.; Wang, J. Analyzing the genes and pathways related to major depressive disorder via a systems biology approach. Brain Behav. 2020, 10, e01502. [Google Scholar] [CrossRef]
- Ray, S.; Tzeng, R.Y.; DiCarlo, L.M.; Bundy, J.L.; Vied, C.; Tyson, G.; Nowakowski, R.; Arbeitman, M.N. An Examination of Dynamic Gene Expression Changes in the Mouse Brain During Pregnancy and the Postpartum Period. G3: Genes Genomes Genet. 2015, 6, 221–233. [Google Scholar] [CrossRef]
- Kokay, I.C.; Petersen, S.L.; Grattan, D.R. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology 2011, 152, 526–535. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Kokay, I.C.; Grattan, D.R. Dissociation of prolactin secretion from tuberoinfundibular dopamine activity in late pregnant rats. Endocrinology 2001, 142, 2719–2724. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Kokay, I.C.; Phillipps, H.R.; Yip, S.H.; Gustafson, P.; Wyatt, A.; Larsen, C.M.; Knowles, P.; Ladyman, S.R.; LeTissier, P.; et al. Conditional Deletion of the Prolactin Receptor Reveals Functional Subpopulations of Dopamine Neurons in the Arcuate Nucleus of the Hypothalamus. J. Neurosci. 2016, 36, 9173–9185. [Google Scholar] [CrossRef] [PubMed]
- De Gortari, P.; Mancera, K.; Cote-Velez, A.; Amaya, M.I.; Martinez, A.; Jaimes-Hoy, L.; Joseph-Bravo, P. Involvement of CRH-R2 receptor in eating behavior and in the response of the HPT axis in rats subjected to dehydration-induced anorexia. Psychoneuroendocrinology 2009, 34, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Geddes, B.J.; Zhang, C.; Foley, K.P.; Stricker-Krongrad, A. The prolactin-releasing peptide receptor (GPR10) regulates body weight homeostasis in mice. J. Mol. Neurosci. 2004, 22, 93–103. [Google Scholar] [CrossRef]
- Talbi, R.; Navarro, V.M. Novel insights into the metabolic action of Kiss1 neurons. Endocr. Connect. 2020, 9, R124–R133. [Google Scholar] [CrossRef]
- Frank, A.; Brown, L.M.; Clegg, D.J. The role of hypothalamic estrogen receptors in metabolic regulation. Front. Neuroendocrinol. 2014, 35, 550–557. [Google Scholar] [CrossRef]
- Delgado, T.C. Glutamate and GABA in Appetite Regulation. Front. Endocrinol. 2013, 4, 103. [Google Scholar] [CrossRef]
- Naef, L.; Woodside, B. Prolactin/Leptin interactions in the control of food intake in rats. Endocrinology 2007, 148, 5977–5983. [Google Scholar] [CrossRef]
- Billes, S.K.; Simonds, S.E.; Cowley, M.A. Leptin reduces food intake via a dopamine D2 receptor-dependent mechanism. Mol. Metab. 2012, 1, 86–93. [Google Scholar] [CrossRef]
- Merino, B.; Cano, V.; Guzman, R.; Somoza, B.; Ruiz-Gayo, M. Leptin-mediated hypothalamic pathway of cholecystokinin (CCK-8) to regulate body weight in free-feeding rats. Endocrinology 2008, 149, 1994–2000. [Google Scholar] [CrossRef][Green Version]
- Henderson, L.P. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: Effects on reproductive function. Neuropharmacology 2007, 52, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Clyne, M.; Khoury, M.J.; Gwinn, M. Phenopedia and Genopedia: Disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics 2010, 26, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Venn, B.J.; Green, T.J.; Moser, R.; Mann, J.I. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: A randomized placebo-controlled study. Am. J. Clin. Nutr. 2003, 77, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Mills, J.L.; Cox, C.; Daly, S.F.; Conley, M.; Brody, L.C.; Kirke, P.N.; Scott, J.M.; Ueland, P.M. Choline and homocysteine interrelations in umbilical cord and maternal plasma at delivery. Am. J. Clin. Nutr. 2005, 82, 836–842. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Niculescu, M.D. Perinatal choline influences brain structure and function. Nutr. Rev. 2006, 64, 197–203. [Google Scholar] [CrossRef]
- Knight, L.S.; Piibe, Q.; Lambie, I.; Perkins, C.; Yancey, P.H. Betaine in the Brain: Characterization of Betaine Uptake, its Influence on Other Osmolytes and its Potential Role in Neuroprotection from Osmotic Stress. Neurochem. Res. 2017, 42, 3490–3503. [Google Scholar] [CrossRef]
- Kempson, S.A.; Zhou, Y.; Danbolt, N.C. The betaine/GABA transporter and betaine: Roles in brain, kidney, and liver. Front. Physiol. 2014, 5, 159. [Google Scholar] [CrossRef]
- Suyama, S.; Yada, T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Li, Y.; Sun, C. Integration of metabolomics and proteomics to highlight altered neural development related pathways in the adult offspring after maternal folic acid supplement. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Wieske, F.; Dirnagl, U.; Winter, C. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Mol. Genet. Metab. Rep. 2015, 3, 1–4. [Google Scholar] [CrossRef]
| FA | MTHF | Two-Way ANOVA p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X-FA | 5X-FA | 1X-MTHF | 5X-MTHF | Dose | Form | Dose × Form | |||||||||
| Parturition | |||||||||||||||
| 5-MTHF (nmol/L) | 103.83 | ± | 18.89 | 188 | ± | 20.69 | 117.2 | ± | 18.89 | 152.6 | ± | 20.69 | 0.007 | 0.58 | 0.24 |
| Methionine (µmol/L) | 65 | ± | 4.43 | 79.2 | ± | 4.85 | 60.4 | ± | 4.85 | 76.6 | ± | 4.85 | 0.005 | 0.46 | 0.84 |
| SAM (nmol/L) | 297.2 | ± | 19.94 | 294.3 | ± | 25.74 | 285.8 | ± | 18.2 | 281 | ± | 19.94 | 0.86 | 0.57 | 0.96 |
| SAH (nmol/L) | 73.2 | ± | 15.09 | 79.5 | ± | 16.87 | 123.8 | ± | 15.09 | 88 | ± | 15.09 | 0.35 | 0.07 | 0.2 |
| Hcy (µmol/L) | 14.55 | ± | 3.02 | 11.9 | ± | 3.31 | 29.12 | ± | 3.31 | 20.8 | ± | 3.31 | 0.11 | 0.002 | 0.39 |
| Cys (nmol/L) | 1304 | ± | 156.2 | 1494 | ± | 156.18 | 1917 | ± | 142.6 | 1504 | ± | 156.2 | 0.48 | 0.06 | 0.07 |
| Choline (µmol/L) | 13.7 | ± | 1.32 | 12.28 | ± | 1.18 | 14.42 | ± | 1.08 | 10.72 | ± | 1.18 | 0.048 | 0.73 | 0.36 |
| Betaine (µmol/L) | 34.72 ab | ± | 2.48 | 43.12 a | ± | 2.73 | 38.72 ab | ± | 2.49 | 30.7 b | ± | 3.04 | 0.943 | 0.14 | 0.007 |
| 19-weeks PW | |||||||||||||||
| 5-MTHF (nmol/L) | 116.9 | ± | 10.29 | 122.1 | ± | 10.29 | 131.1 | ± | 11 | 132.15 | ± | 10.29 | 0.77 | 0.26 | 0.84 |
| Methionine (µmol/L) | 76.65 | ± | 4.19 | 66.9 | ± | 4.84 | 67.1 | ± | 4.84 | 74.7 | ± | 4.19 | 0.81 | 0.84 | 0.07 |
| SAM (nmol/L) | 255.2 | ± | 22.77 | 254.6 | ± | 22.77 | 298.9 | ± | 22.77 | 279.4 | ± | 22.77 | 0.66 | 0.14 | 0.68 |
| SAH (nmol/L) | 72.4 | ± | 8.6 | 79.77 | ± | 9.19 | 86.84 | ± | 8.6 | 75.73 | ± | 8.6 | 0.82 | 0.57 | 0.29 |
| Hcy (µmol/L) | 3.69 | ± | 0.98 | 6.21 | ± | 0.92 | 4.93 | ± | 0.92 | 4.51 | ± | 0.92 | 0.27 | 0.81 | 0.13 |
| Cys (nmol/L) | 765.47 | ± | 80.91 | 752.9 | ± | 86.5 | 733.3 | ± | 80.91 | 776.36 | ± | 80.91 | 0.85 | 0.96 | 0.74 |
| Choline (µmol/L) | 11.23 | ± | 0.97 | 10.31 | ± | 0.97 | 11.98 | ± | 0.97 | 10.44 | ± | 0.97 | 0.22 | 0.67 | 0.75 |
| Betaine (µmol/L) | 84.61 | ± | 5.7 | 78.71 | ± | 5.7 | 64.44 | ± | 5.7 | 76.81 | ± | 6.1 | 0.58 | 0.07 | 0.13 |
| FA | MTHF | Two-Way ANOVA p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X-FA | 5X-FA | 1X-MTHF | 5X-MTHF | Dose | Form | Dose × Form | |||||||||
| Parturition | |||||||||||||||
| 5-MTHF (nmol/g) | 26.13 | ± | 2.92 | 25.67 | ± | 2.92 | 23.73 | ± | 2.92 | 26.69 | ± | 2.92 | 0.68 | 0.81 | 0.56 |
| Met (nmol/g) | 181.5 | ± | 14.65 | 212.67 | ± | 14.66 | 181.2 | ± | 16.05 | 220.25 | ± | 17.95 | 0.04 | 0.82 | 0.81 |
| SAM (nmol/g) | 55.5 | ± | 4.27 | 51.55 | ± | 3.48 | 54.08 | ± | 3.82 | 51.77 | ± | 4.27 | 0.44 | 0.88 | 0.83 |
| SAH (nmol/g) | 31.76 | ± | 1.56 | 30.68 | ± | 1.56 | 34.7 | ± | 1.71 | 35.52 | ± | 1.92 | 0.94 | 0.03 | 0.58 |
| Cys (nmol/g) | 20.2 ab | ± | 3.81 | 18.82 ab | ± | 3.81 | 13.25 a | ± | 4.26 | 31.42 b | ± | 4.26 | 0.06 | 0.49 | 0.03 |
| Choline (nmol/g) | 1759 a | ± | 154.86 | 1124.17 b | ± | 141.37 | 787.5 b | ± | 154.86 | 988.75 b | ± | 173.14 | 0.2 | 0.004 | 0.02 |
| Betaine (nmol/g) | 1078 | ± | 163.18 | 1247.5 | ± | 148.96 | 1156 | ± | 163.18 | 793.73 | ± | 182.4 | 0.57 | 0.27 | 0.13 |
| 19-weeks PW | |||||||||||||||
| 5-MTHF (nmol/g) | 26.13 | ± | 2.92 | 25.67 | ± | 2.92 | 23.73 | ± | 2.92 | 26.69 | ± | 2.92 | 0.68 | 0.81 | 0.56 |
| Met (nmol/g) | 424.75 | ± | 50.01 | 419.63 | ± | 50.01 | 475 | ± | 50.01 | 489.75 | ± | 50.01 | 0.92 | 0.24 | 0.84 |
| SAM (nmol/g) | 19.21 ab | ± | 3.02 | 28.07 a | ± | 2.61 | 26.96 ab | ± | 2.79 | 18.1 b | ± | 2.62 | 0.99 | 0.69 | 0.004 |
| SAH (nmol/g) | 25.98 | ± | 2.87 | 33.5 | ± | 2.48 | 36.07 | ± | 2.65 | 28.92 | ± | 2.48 | 0.99 | 0.30 | 0.01 |
| Cys (nmol/g) | 21.4 | ± | 1.59 | 19.65 | ± | 1.59 | 20.07 | ± | 1.7 | 20.21 | ± | 1.59 | 0.62 | 0.81 | 0.56 |
| Choline (nmol/g) | 557.75 | ± | 66.24 | 635.25 | ± | 66.62 | 746.12 | ± | 66.62 | 626.25 | ± | 66.62 | 0.75 | 0.19 | 0.14 |
| Betaine (nmol/g) | 2938.75 | ± | 338.44 | 2552.5 | ± | 338.44 | 2185.71 | ± | 361.81 | 2440.88 | ± | 338.44 | 0.85 | 0.22 | 0.36 |
| FA | MTHF | Two-Way ANOVA p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X-FA | 5X-FA | 1X-MTHF | 5X-MTHF | Dose | Form | Dose × Form | |||||||||
| Parturition | |||||||||||||||
| AI (%) | 2.84 | ± | 0.33 | 2.81 | ± | 0.5 | 3.63 | ± | 0.68 | 2.75 | ± | 0.41 | 0.39 | 0.48 | 0.41 |
| Liver (%) | 4.48 ab | ± | 0.14 | 4.18 ab | ± | 0.11 | 4.09 a | ± | 0.08 | 4.66 b | ± | 0.17 | 0.35 | 0.76 | 0.006 |
| 19-weeks PW | |||||||||||||||
| AI (%) | 9.6 | ± | 0.7 | 8.9 | ± | 0.7 | 9.9 | ± | 0.6 | 9 | ± | 0.5 | 0.19 | 0.85 | 0.83 |
| Liver (%) | 2.41 | ± | 0.12 | 2.21 | ± | 0.09 | 2.33 | ± | 0.09 | 2.29 | ± | 0.11 | 0.23 | 0.81 | 0.33 |
| Total liver lipids (%) | 5.32 | ± | 0.26 | 5.12 | ± | 0.25 | 5.7 | ± | 0.25 | 5.25 | ± | 0.25 | 0.21 | 0.32 | 0.63 |
| FA | MTHF | Two-Way ANOVA p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X-FA | 5X-FA | 1X-MTHF | 5X-MTHF | Dose | Form | Dose × Form | |||||||||
| Parturition | |||||||||||||||
| Leptin ng/mL | 5.68 | ± | 0.74 | 6.13 | ± | 0.74 | 4.39 | ± | 0.81 | 3.88 | ± | 0.81 | 0.97 | 0.03 | 0.55 |
| Leptin/VAT | 0.63 | ± | 0.12 | 0.88 | ± | 0.12 | 0.43 | ± | 0.13 | 0.5 | ± | 0.13 | 0.23 | 0.03 | 0.46 |
| Insulin ng/mL | 1.36 | ± | 0.34 | 1.88 | ± | 0.31 | 1.14 | ± | 0.31 | 1.46 | ± | 0.31 | 0.20 | 0.33 | 0.77 |
| Glucose mg/dL | 116.8 | ± | 6.54 | 122.4 | ± | 5.85 | 101 | ± | 5.85 | 115.1 | ± | 5.85 | 0.12 | 0.08 | 0.50 |
| HOMA-IR | 1.67 | ± | 0.37 | 2.31 | ± | 0.33 | 1.19 | ± | 0.33 | 1.71 | ± | 0.33 | 0.11 | 0.13 | 0.86 |
| 19-weeks PW | |||||||||||||||
| Leptin ng/mL | 9.9 | ± | 2.06 | 9.66 | ± | 1.71 | 15.02 | ± | 1.71 | 14.96 | ± | 1.86 | 0.94 | 0.008 | 0.96 |
| Leptin/VAT | 0.23 | ± | 0.06 | 0.26 | ± | 0.05 | 0.39 | ± | 0.06 | 0.44 | ± | 0.06 | 0.53 | 0.007 | 0.88 |
| Ghrelin ng/mL | 146.6 | ± | 16 | 120.1 | ± | 14.3 | 78.73 | ± | 16 | 96.96 | ± | 15.1 | 0.79 | 0.006 | 0.16 |
| Insulin ng/mL | 1.75 | ± | 0.22 | 1.62 | ± | 0.23 | 1.98 | ± | 0.23 | 1.71 | ± | 0.26 | 0.40 | 0.52 | 0.77 |
| Glucose mg/dL | 129.9 | ± | 2.39 | 128.5 | ± | 2.53 | 128.2 | ± | 2.71 | 123.9 | ± | 2.71 | 0.27 | 0.21 | 0.57 |
| HOMA-IR | 2.56 | ± | 0.3 | 2.35 | ± | 0.32 | 2.64 | ± | 0.3 | 2.2 | ± | 0.35 | 0.30 | 0.89 | 0.76 |
| 5X-FA | 5X-MTHF | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Parturition | |||||||
| 5-MTHF (nmol/g) | 0.24 | ± | 0.02 | 0.29 | ± | 0.05 | 0.43 |
| Met (nmol/g) | 48.9 | ± | 1.21 | 44.66 | ± | 2.07 | 0.14 |
| SAM (nmol/g) | 22.57 | ± | 0.5 | 21.86 | ± | 1.06 | 0.59 |
| SAH (nmol/g) | 3.4 | ± | 0.34 | 3 | ± | 0.19 | 0.34 |
| Cys (nmol/g) | 47.95 | ± | 4.87 | 46.12 | ± | 5.84 | 0.82 |
| Choline (nmol/g) | 811 | ± | 51.9 | 706 | ± | 38.02 | 0.14 |
| Betaine (nmol/g) | 13.65 | ± | 2.25 | 6.36 * | ± | 1.15 | 0.02 |
| 19-weeks PW | |||||||
| 5-MTHF (nmol/g) | 0.26 | ± | 0.03 | 0.23 | ± | 0.03 | 0.59 |
| Met (nmol/g) | 41.4 | ± | 3.66 | 36.5 | ± | 4.61 | 0.43 |
| SAM (nmol/g) | 21.07 | ± | 0.81 | 22.1 | ± | 1.63 | 0.86 |
| SAH (nmol/g) | 2.77 | ± | 0.18 | 2.58 | ± | 0.12 | 0.42 |
| Cys (nmol/g) | 42.2 | ± | 1.92 | 44.46 | ± | 2.82 | 0.53 |
| Choline (nmol/g) | 408.83 | ± | 40.72 | 375 | ± | 22.27 | 0.14 |
| Betaine (nmol/g) | 8.94 | ± | 1.08 | 7.16 | ± | 0.46 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannia, E.; Hammoud, R.; Simonian, R.; Arning, E.; Ashcraft, P.; Wasek, B.; Bottiglieri, T.; Pausova, Z.; Kubant, R.; Anderson, G.H. [6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth. Nutrients 2021, 13, 48. https://doi.org/10.3390/nu13010048
Pannia E, Hammoud R, Simonian R, Arning E, Ashcraft P, Wasek B, Bottiglieri T, Pausova Z, Kubant R, Anderson GH. [6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth. Nutrients. 2021; 13(1):48. https://doi.org/10.3390/nu13010048
Chicago/Turabian StylePannia, Emanuela, Rola Hammoud, Rebecca Simonian, Erland Arning, Paula Ashcraft, Brandi Wasek, Teodoro Bottiglieri, Zdenka Pausova, Ruslan Kubant, and G. Harvey Anderson. 2021. "[6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth" Nutrients 13, no. 1: 48. https://doi.org/10.3390/nu13010048
APA StylePannia, E., Hammoud, R., Simonian, R., Arning, E., Ashcraft, P., Wasek, B., Bottiglieri, T., Pausova, Z., Kubant, R., & Anderson, G. H. (2021). [6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth. Nutrients, 13(1), 48. https://doi.org/10.3390/nu13010048







