Effect of Milk-Based Infant Formula Fortified with PUFAs on Lipid Profile, Growth and Micronutrient Status of Young Children: A Randomized Double-Blind Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Intervention
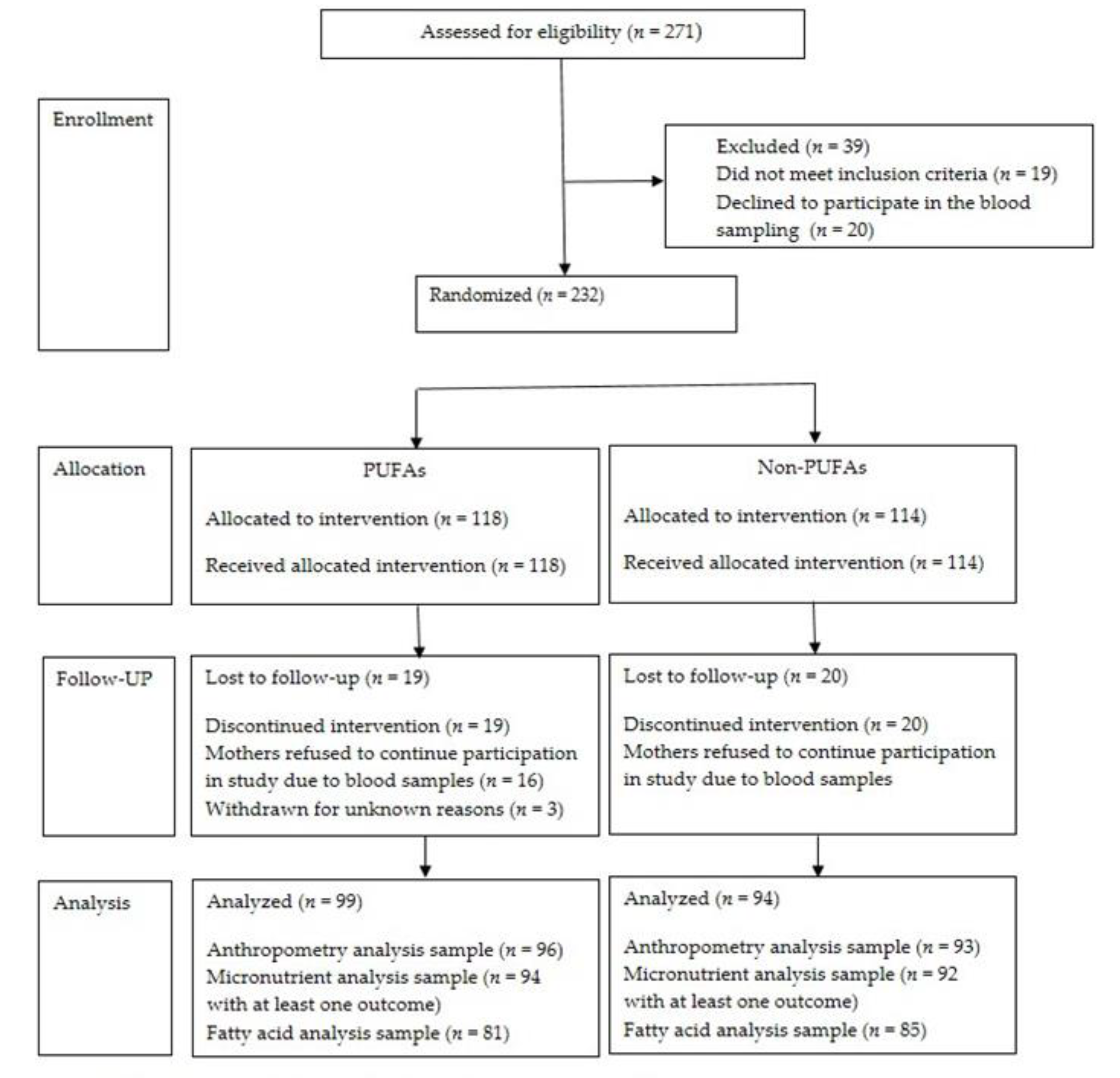
2.2. Population and Setting
2.3. Randomization and Masking
2.4. Product Preparation and Volume Intake
2.5. Outcomes
2.6. Measurement of Blood Samples
2.7. Fatty Acid Profile
2.8. Micronutrients
2.9. Anthropometric Measurements
2.10. Dietary Assessment
2.11. Formula Intake
2.12. Sociodemographic Characteristics
2.13. Morbidity
2.14. Sample Size
2.15. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Fats and Fatty Acids in Human Nutrition. Report on an Expert Consultation; Food and Nutrition Paper; FAO: Geneva, Switzerland, 2008; pp. 10–14. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Gonzalez-Casanova, I.; Schnaas, L.; DiGirolamo, A.; Quezada, A.D.; Pallo, B.C.; Hao, W.; Neufeld, L.M.; Rivera, J.A.; Stein, A.; et al. Prenatal Supplementation with DHA Improves Attention at 5 Y of Age: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 104, 1075–1082. [Google Scholar] [CrossRef]
- Decsi, T.; Lohner, S. Gaps in Meeting Nutrient Needs in Healthy Toddlers. Ann. Nutr. Metab. 2014, 65, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Boey, C.C.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current Information and Asian Perspectives on Long-Chain Polyunsaturated Fatty Acids in Pregnancy, Lactation, and Infancy: Systematic Review and Practice Recommendations from an Early Nutrition Academy Workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cabrera, S.; Stein, A.D.; Wang, M.; Martorell, R.; Rivera, J.; Ramakrishnan, U. Dietary Intakes of Polyunsaturated Fatty Acids among Pregnant Mexican Women. Matern. Child Nutr. 2011, 7, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, G. Essential Fatty Acids in Mothers and Their Neonates. Am. J. Clin. Nutr. 2000, 71, 1262S–1269S. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.R.; Villalpando, S.; Moreno-Saracho, J.E.; Bernal-Medina, D. Fatty Acids Intake in the Mexican Population. Results of the National Nutrition Survey 2006. Nutr. Metab. 2011, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The Epidemiology of Global Micronutrient Deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 22–33. [Google Scholar] [CrossRef]
- Stevens, G.; E Bennett, J.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; A White, R.; Flaxman, S.R.; et al. Trends and Mortality Effects of Vitamin a Deficiency in Children in 138 Low-Income and Middle-Income Countries between 1991 and 2013: A Pooled Analysis of Population-Based Surveys. Lancet Glob. Heal. 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Flores, A.; Flores, M.; Macias, N.; Hernández-Barrera, L.; Rivera, M.; Contreras, A.; Villalpando, S. Vitamin D Deficiency Is Common and Is Associated with Overweight in Mexican Children Aged 1–11 years. Public Health Nutr. 2017, 20, 1807–1815. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Villalpando, S.; Jáuregui, A.; Rivera, J.A. Overview of the Nutritional Status of Selected Micronutrients in Mexican Children in 2006. Salud Publica Mex. 2012, 54, 146–151. [Google Scholar]
- Keats, E.C.; Neufeld, L.M.; Garrett, G.S.; Mbuya, M.N.N.; Bhutta, Z.A. Improved Micronutrient Status and Health Outcomes in Low- and Middle-Income Countries Following Large-Scale Fortification: Evidence from a Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2019, 109, 1696–1708. [Google Scholar] [CrossRef]
- Villalpando, S.; Shamah, T.; Dommarco, J.A.R.; Lara, Y.; Monterrubio, E. Fortifying Milk with Ferrous Gluconate and Zinc Oxide in a Public Nutrition Program Reduced the Prevalence of Anemia in Toddlers. J. Nutr. 2006, 136, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.L.; Tonascía, S. Clinical Trials. Design; Conduct an Analysis; Oxford University: New York, NY, USA, 1968; pp. 95–112. [Google Scholar]
- Organización Mundial de la Salud en Colaboración con la Organización de las Naciones Unidas Para la Agricultura y la Alimentación. Preparación, Almacenamiento y Manipulación en Condiciones Higiénicas de Preparaciones en Polvo para Lactantes. Directrices; Organización Mundial de la Salud: Geneva, Switzerland, 2007; pp. 1–26. [Google Scholar]
- Hemocue, A.B. Fotómetro de Hemoglobina en Sangre. Manual de Operación. Suecia, Sweden 2003. Available online: https://www.hemocue.com/es-es/soluciones-/hematolog%C3%ADa (accessed on 14 July 2020).
- Worldwide Prevalence of Anaemia 1993–2005 WHO Global Database on Anaemia. Available online: https://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf?ua=1 (accessed on 14 July 2020).
- Cohen, J.H.; Haas, J.D. Hemoglobin Correction Factors for Estimating the Prevalence of Iron Deficiency Anemia in Pregnant Women Residing at High Altitudes in Bolivia. Rev. Panam. Salud Pública 1999, 6, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Martínez-Razo, G.; Martínez-Basila, A.; Salas-Fernández, A.; Maldonado-Hernández, J. Association between Metabolic Syndrome and Erythrocyte Fatty Acid Profile in Mexican Adolescents: ATrans Fatty Acid Approach. Food Nutr. Sci. 2013, 4, 51–58. [Google Scholar] [CrossRef]
- del Ayala-Moreno, M.R.; Fernández-Callejas, J.M.S.; Maldonado-Hernández, J. Assessment of Lipid Quality and Composition of Commercial Infant Milk Formulas in Mexico: Emphasis on Trans Fatty Acid Isomers. Food Nutr. Sci. 2016, 7, 273–283. [Google Scholar] [CrossRef]
- Tietz, N.W. (Ed.) Clinical Guide to Laboratory Tests, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 1995; pp. 142–145. [Google Scholar]
- Thorpe, S.J.; Heath, A.; Blackmore, S.; Lee, A.; Hamilton, M.; O’Broin, S.; Nelson, B.C.; Pfeiffer, C. International Standard for Serum Vitamin B12 and Serum Folate: International Collaborative Study to Evaluate a Batch of Lyophilised Serum for B12 and Folate Content. Clin. Chem. Lab. Med. 2007, 45, 380–386. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards & Technology. Certificate of Analysis. Standard Reference Material 968e. Fat-soluble Vitamins, Carotenoids, and Cholesterol in Human Serum. Department of Commerce USA. Available online: https://www-s.nist.gov/m-srmors/certificates/968e.pdf (accessed on 8 December 2020).
- Bedner, M.; Lippa, K.A.; Tai, S.S.-C. An Assessment of 25-hydroxyvitamin D Measurements in Comparability Studies Conducted by the Vitamin D Metabolites Quality Assurance Program. Clin. Chim. Acta 2013, 426, 6–11. [Google Scholar] [CrossRef]
- Bieri, J.G.; Tolliver, T.J.; Catignani, G.L. Simultaneous Determination of α-Tocopherol and Retinol in Plasma or Red Cells by High Pressure Liquid Chromatography. Am. J. Clin. Nutr. 1979, 32, 2143–2149. [Google Scholar] [CrossRef]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Silva, I.; Jiménez-Aguilar, A.; Valenzuela-Bravo, D.; Martinez-Tapia, B.; Rodríguez-Ramírez, S.; Gaona-Pineda, E.B.; Angulo-Estrada, S.; Shamah-Levy, T. Methodology for Estimating Dietary Data from the Semi-Quantitative Food Frequency Questionnaire of the Mexican National Health and Nutrition Survey 2012. Salud Pública México 2016, 58, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ávila, J.E.; González-Avilés, L.; Rosales-Mendoza, E. Manual de Usuario. SNUT Sistema de Evaluación de Hábitos Nutricionales y Consumo de Nutrimentos; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2013. [Google Scholar]
- Gutiérrez, J.P. Clasificación Socioeconómica de los Hogares en la ENSANUT 2012. Salud Publica Mexica 2013, 55, S341–S346. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. Better Bootstrap Confidence Intervals. In An Introduction to the Bootstrap; Chapman & Hall/CRC: London, UK, 1993; pp. 178–201. [Google Scholar]
- Giannì, M.L.; Roggero, P.; Baudry, C.; Fressange-Mazda, C.; Galli, C.; Agostoni, C.; Le Ruyet, P.; Mosca, F. An Infant Formula Containing Dairy Lipids Increased Red Blood Cell Membrane Omega 3 Fatty Acids in 4 Month-Old Healthy Newborns: A Randomized Controlled Trial. BMC Pediatr. 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.N.; Drover, J.R.; Castañeda, Y.S.; Minns, L.; Wheaton, D.K.H.; Mundy, D.; et al. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: A Double-Masked, Randomized Controlled Clinical Trial of the Maturation of Infant Visual Acuity as a Function of the Dietary Level of Docosahexaenoic Acid. Am. J. Clin. Nutr. 2010, 91, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Seifert, J.; Szabo, N.J.; Clare-Salzler, M.; Rewers, M.; Norris, J.M. Erythrocyte Membrane Fatty Acid Content in Infants Consuming Formulas Supplemented with Docosahexaenoic Acid (DHA) and Arachidonic Acid (ARA): An Observational Study. Matern. Child Nutr. 2010, 6, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Chatoor, I. Infantile Anorexia Nervosa: A Developmental Disorder or Separation and Individuation. J. Am. Acad. Psychoanal. 1989, 17, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Wang, M.; Martorell, R.; Neufeld, L.M.; Flores-Ayala, R.; Rivera, J.A.; Ramakrishnan, U. Growth to Age 18 Months Following Prenatal Supplementation with Docosahexaenoic Acid Differs by Maternal Gravidity in Mexico. J. Nutr. 2011, 141, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Casanova, I.; Stein, A.D.; Hao, W.; Garcia-Feregrino, R.; Barraza-Villarreal, A.; Romieu, I.; Rivera, J.A.; Martorell, R.; Ramakrishnan, U. Prenatal Supplementation with Docosahexaenoic Acid Has No Effect on Growth through 60 Months of Age. J. Nutr. 2015, 145, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.M.; Tolley, E.A.; Thodosoff, J.M.; Kerling, E.H.; Sullivan, D.K.; Colombo, J.; Carlson, S.E. Long Chain Polyunsaturated Fatty Acid Supplementation in Infancy Increases Length- and Weight-for-Age but not BMI to 6 Years When Controlling for Effects of Maternal Smoking. Prostaglandins Leukot. Essent. Fatty Acids 2015, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huffman, S.L.; Harika, R.K.; Eilander, A.; Osendarp, S.J.M. Essential Fats: How Do They Affect Growth and Development of Infants and Young Children in Developing Countries? A Literature Review. Matern. Child Nutr. 2011, 7 (Suppl. 3), 44–65. [Google Scholar] [CrossRef] [PubMed]
- Strand, T.A.; Taneja, S.; Kumar, T.; Manger, M.S.; Refsum, H.; Yajnik, C.S.; Bhandari, N. Vitamin B-12, Folic Acid, and Growth in 6- to 30-Month-Old Children: A Randomized Controlled Trial. Pediatrics 2015, 135, e918–e926. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Polyunsaturated Fatty Acids (PUFAs) (100 g Powder) | PUFAs Per Serving * | NON-PUFAs (100 g Powder) | NON-PUFAs Per Serving * |
|---|---|---|---|---|
| Energy (kj/kcal) | 1894/447 | 757.6 /178.8 | 2193/524 | 877.2/209.6 |
| Carbohydrates (g) | 58.5 | 23.4 | 37.9 | 15.16 |
| Prebiotics (Dietary fiber) (g) | 3.0 | 1.2 | --- | --- |
| Total Lipids (g) | 16.6 | 6.64 | 29.6 | 11.84 |
| Linoleic Acid (mg) | 1550.0 | 620 | --- | --- |
| Alpha-Linolenic Acid (mg) | 195.0 | 78 | --- | --- |
| DHA a (mg) | 40.5 | 16.2 | --- | --- |
| Protein (g) | 17.9 | 7.16 | 26.7 | 10.68 |
| Vit. A (µg retinol eq) | 267.0 | 106.8 | 522.67 | 209.068 |
| Vit. D3 (µg, cholecalciferol) | 3.65 | 1.46 | 20.33 | 8.132 |
| Vit. E (µg, tocopherol eq) | 5.37 | 2.15 | 9.75 | 3.9 |
| Vit. C (mg, ascorbic acid) | 50.0 | 20 | 35.41 | 14.164 |
| Vit. B1 (µg, thiamin) | 763.0 | 305.2 | 370 | 148.0 |
| Vit. B2 (µg, riboflavin) | 1070.0 | 428 | 1500 | 600 |
| Vit. B3 (µg, niacin) | 5200.0 | 2080 | 720 | 288 |
| Vit. B5 (µg, panthotenic acid) | 3060.0 | 1224 | 3500 | 1400 |
| Vit. B6 (µg, piridoxin) | 494.0 | 197.6 | 220 | 88 |
| Vit. B8 (µg, biotin) | 19.50 | 7.8 | 20.1 | 8.04 |
| Vit. B9 (µg, folic acid) | 85.8 | 34.32 | 17.5 | 7.0 |
| Vit. B12 (µg, cianocobalamin) | 1.65 | 0.66 | --- | --- |
| Vit. K (µg) | 31.0 | 12.4 | 33.0 | 13.2 |
| Choline (mg) | 81.0 | 32.4 | --- | --- |
| Taurine (mg) | --- | 25.6 | ||
| Calcium (mg) | 660.0 | 264.0 | 186.2 | 74.48 |
| Phosphorus (mg) | 600.0 | 240.0 | 690.0 | 276.00 |
| Iron (mg) | 6.9 | 2.76 | 0.3 | 0.12 |
| Magnesium (mg) | 57.5 | 23.0 | 74.0 | 29.60 |
| Zinc (mg) | 5.3 | 2.12 | 2.65 | 1.06 |
| Iodine (µg) | 125.0 | 50.0 | 1.36 | 0.544 |
| Copper (µg) | 360.0 | 144.0 | --- | --- |
| Sodium (mg) | 255.0 | 102.0 | 340.0 | 136.0 |
| Potassium(mg) | 020.0 | 408.0 | 1080.0 | 432.0 |
| Selenium (µg) | 10.5 | 4.2 | --- | --- |
| Inositol (mg) | 58.5 | 23.4 | --- | --- |
| Variables | PUFAs (n = 99) | Non-PUFAS (n = 94) | ||
|---|---|---|---|---|
| N | P50 (P25, P75) or % | N | P50 (P25, P75) or % | |
| Age, months | 96 | 21.27 (16.59, 27.88) | 93 | 22.97 (16.53, 27.14) |
| <24 | 60 | 62.5 | 55 | 59.1 |
| 24–30 | 36 | 37.5 | 38 | 40.9 |
| Sex | ||||
| Male | 60 | 60.6 | 45 | 47.9 |
| Female | 39 | 39.4 | 49 | 52.1 |
| Anthropometry | ||||
| Birth weight, kg | 98 | 3.19 (2.70, 3.45) | 89 | 3.10 (2.78, 3.50) |
| Weight, kg | 96 | 11.20 (10.40,12.25) | 93 | 11.50 (10.10, 12.80) |
| Height, cm | 96 | 82.40 (78.15, 86.90) | 93 | 83.70 (78.30, 87.60) |
| HAZ | 96 | −0.77 (−1.63, -0.04) | 93 | −0.58 (−1.58, 0.02) |
| Stunting a | 12 | 12.5 | 13 | 14 |
| WAZ | 96 | −0.07 (−0.86, 0.55) | 93 | −0.04 (−0.63, 0.58) |
| Low weight b | 1 | 1 | 0 | 0 |
| WHZ | 96 | 0.35 (−0.34, 0.92) | 93 | 0.34 (−0.20, 0.85) |
| Wasting c | 1 | 1 | 0 | 0 |
| BMIZ | 96 | 0.47 (−0.25, 1.08) | 93 | 0.54 (−0.11, 1.00) |
| Overweight d | 4 | 4.2 | 7 | 7.5 |
| Socioeconomic score | 98 | 0.21 (−0.82, 0.89) | 94 | 0.21 (−0.79, 0.89) |
| Biochemical indicators | ||||
| Hemoglobin, g/dL | 96 | 12.2 (11.2, 13.0) | 93 | 11.9 (11.0, 2.6) |
| <11 | 21 | 21.9 | 23 | 24.7 |
| <10 | 10 | 10.4 | 9 | 9.7 |
| Vitamin A, µg/dL | 89 | 31.58 (26.83, 35.78) | 88 | 32.12 (28.49, 35.25) |
| Vitamin D, nmol/L | 92 | 37.60 (30.75, 58.44) | 88 | 39.87 (31.06, 54.89) |
| Zinc, µg/dL | 77 | 119.2 (111.7, 133.5) | 71 | 120.6 (109.2,132.3) |
| Ferritin, µg/L | 78 | 20.15 (12.85, 29.58) | 72 | 17.99 (9.27, 24.86) |
| Folate, ng/mL | 90 | 15.40 (12.60, 16.70) | 89 | 14.90 (12.10, 16.70) |
| Fatty acids e | ||||
| Estearic | 81 | 7.74 (7.14, 8.37) | 85 | 7.90 (7.24, 8.42) |
| Lauric | 81 | 0.20 (0.11, 0.31) | 85 | 0.25 (0.15, 0.31) |
| Linoleic | 81 | 30.68 (28.29, 32.96) | 85 | 28.19 (25.39, 30.52) |
| Alpha-Linolenic | 81 | 0.55 (0.45, 0.65) | 85 | 0.55 (0.49, 0.62) |
| Miristic | 81 | 1.26 (0.95, 1.61) | 85 | 1.57 (1.11, 2.19) |
| Oleic | 81 | 25.64 (23.44, 27.45) | 85 | 26.16 (24.63, 27.79) |
| Palmitic | 81 | 26.65 (25.18, 28.99) | 85 | 28.27 (26.34, 29.54) |
| Palmitoleic | 81 | 2.03 (1.53, 2.52) | 85 | 2.29 (1.82, 2.77) |
| EPA f | 81 | 0.26 (0.16, 0.32) | 85 | 0.30 (0.22, 0.36) |
| DHAg | 81 | 0.56 (0.42, 0.76) | 85 | 0.59 (0.44, 0.77) |
| Dietary indicators | ||||
| Energy intake, kcal/day | 95 | 1421.4 (901.49,1927.93) | 92 | 1383.1 (993.99, 1719.59) |
| Carbohydrates, g/day | 95 | 216.9 (150.3, 307.7) | 92 | 217.5 (151.5, 286.8) |
| Proteins, g/day | 95 | 43.2 (28.5, 60.5) | 92 | 41.3 (27.9, 54.8) |
| Lipids, g/day | 95 | 40.2 (23.2,57.2) | 92 | 38.6 (23.7, 51.4) |
| Alpha-Linolenic (omega-3), g/day | 95 | 0.31 (0.17, 0.48) | 92 | 0.28 (0.18,0.43) |
| Gamma-Linolenic, g/day | 95 | 0.02 (0.009, 0.02) | 92 | 0.01 (0.008, 0.20) |
| EPA f, g/day | 95 | 0.007 (0.003, 0.018) | 92 | 0.005 (0.002, 0.11) |
| DHA g, g/day | 95 | 0.02 (0.01, 0.05) | 92 | 0.02 (0.005, 0.04) |
| Outcome | PUFAs (n = 81) | Non-PUFAs (n = 85) | PUFAs vs. Non-PUFAs | ||
|---|---|---|---|---|---|
| N | Median Change (95% CI) | N | Median Change (95% CI) | Difference (95% CI) | |
| Arachidonic | 81 | −0.06 (−0.27, 0.14) | 85 | 0.16 (−0.12, 0.66) | −0.22 (−0.69, 0.19) |
| Estearic | 81 | 0.17 (−0.08, 0.41) | 85 | 0.19 (−0.10, 0.38) | −0.02 (−0.36, 0.40) |
| Lauric | 81 | 0.02 (−0.03, 0.05) | 85 | 0.02 (−0.04, 0.04) | 0.00 (0.00, 0.06) |
| Linoleic | 81 | −1.38 (−2.27, -0.43) | 85 | -0.94 (−1.72, 0.20) | −0.43 (−1.93, 0.78) |
| Alpha-Linolenic | 81 | 0.08 (0.04, 0.12) | 85 | 0.02 (−0.01, 0.04) | 0.06 (0.02, 0.12) |
| Miristic | 81 | 0.01 (−0.18, 0.19) | 85 | 0.20 (0.00, 0.44) | −0.19 (−.50, 0.08) |
| Oleic | 81 | 0.65 (−0.10, 1.41) | 85 | −0.54 (−1.27, 0.54) | 1.18 (−0.35, 2.08) |
| Palmitic | 81 | −0.36 (−1.37, 0.29) | 85 | 0.55 (−0.21, 1.19) | −0.91 (−2.16, 0.06) |
| Palmitoleic | 81 | 0.08 (−0.13, 0.22) | 85 | 0.14 (0.00, 0.28) | −0.06 (−0.36, 0.13) |
| EPA b | 81 | 0.05 (0.01, 0.08) | 85 | 0.02 (−0.01, 0.04) | 0.02 (−0.02, 0.07) |
| DHA c | 81 | 0.22 (0.17, 0.35) | 85 | −0.07 (−0.12, −0.03) | 0.29 (0.22, 0.40) |
| Outcome | PUFAs | Non-PUFAs | PUFAs vs. Non-PUFAs | ||
|---|---|---|---|---|---|
| N | Median Change (95% CI) | N | Median Change (95% CI) | Difference (95% CI) | |
| Anthropometry | |||||
| Height for age, Z | 96 | 0.16 (0.08, 0.28) | 93 | 0.23 (0.14, 0.33) | −0.07 (−0.19, 0.07) |
| Weight for age, Z | 96 | 0.04 (-0.03, 0.12) | 93 | 0.10 (−0.04, 0.19) | −0.05 (−0.16, 0.10) |
| Weight for height, Z | 96 | −0.03 (−0.14, 0.09) | 93 | 0.00 (−0.14, 0.13) | −0.03 (−0.20, 0.16) |
| BMI for age, Z | 96 | −0.04 (−0.18, 0.08) | 93 | 0.00 (−0.15, 0.15) | −0.04 (−0.25, 0.14) |
| Vitamins & minerals | |||||
| Vitamin A, µg/dL | 89 | 1.19 (−0.77, 2.40) | 88 | −0.54 (−1.96, 0.84) | 1.73 (−0.85, 3.77) |
| Vitamin D, nmol/L | 92 | 6.25 −1.43, 16.02) | 88 | 4.35 (0.08, 10.82) | 1.90 (−8.88, 13.48) |
| Zinc, µg/dL | 77 | −2.42 (−6.36, 3.57) | 71 | −4.22 (−9.36, 1.44) | 1.80 (−5.70, 10.17) |
| Ferritin, µg/L | 78 | −0.84 (−3.56, 3.27) | 72 | −4.84 (−8.32, 0.62) | 4.00 (−3.11, 8.73) |
| Folate, ng/mL | 90 | −0.87 (−1.38, -0.44) | 89 | −3.83 (−4.65, −3.03) | 2.96 (2.02, 3.84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Pasquel, M.; Flores-Aldana, M.; Parra-Cabrera, M.-S.; Quezada-Sánchez, A.D.; García-Guerra, A.; Maldonado-Hernández, J. Effect of Milk-Based Infant Formula Fortified with PUFAs on Lipid Profile, Growth and Micronutrient Status of Young Children: A Randomized Double-Blind Clinical Trial. Nutrients 2021, 13, 4. https://doi.org/10.3390/nu13010004
Rivera-Pasquel M, Flores-Aldana M, Parra-Cabrera M-S, Quezada-Sánchez AD, García-Guerra A, Maldonado-Hernández J. Effect of Milk-Based Infant Formula Fortified with PUFAs on Lipid Profile, Growth and Micronutrient Status of Young Children: A Randomized Double-Blind Clinical Trial. Nutrients. 2021; 13(1):4. https://doi.org/10.3390/nu13010004
Chicago/Turabian StyleRivera-Pasquel, Marta, Mario Flores-Aldana, María-Socorro Parra-Cabrera, Amado David Quezada-Sánchez, Armando García-Guerra, and Jorge Maldonado-Hernández. 2021. "Effect of Milk-Based Infant Formula Fortified with PUFAs on Lipid Profile, Growth and Micronutrient Status of Young Children: A Randomized Double-Blind Clinical Trial" Nutrients 13, no. 1: 4. https://doi.org/10.3390/nu13010004
APA StyleRivera-Pasquel, M., Flores-Aldana, M., Parra-Cabrera, M.-S., Quezada-Sánchez, A. D., García-Guerra, A., & Maldonado-Hernández, J. (2021). Effect of Milk-Based Infant Formula Fortified with PUFAs on Lipid Profile, Growth and Micronutrient Status of Young Children: A Randomized Double-Blind Clinical Trial. Nutrients, 13(1), 4. https://doi.org/10.3390/nu13010004






