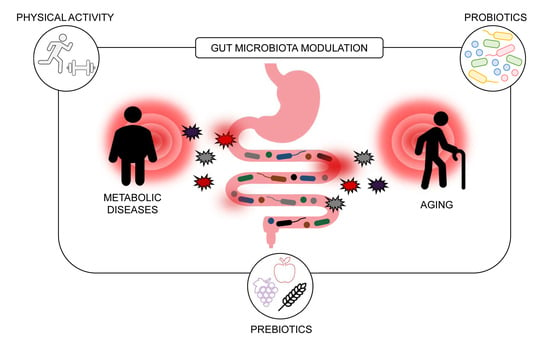
Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions
Abstract
1. Introduction
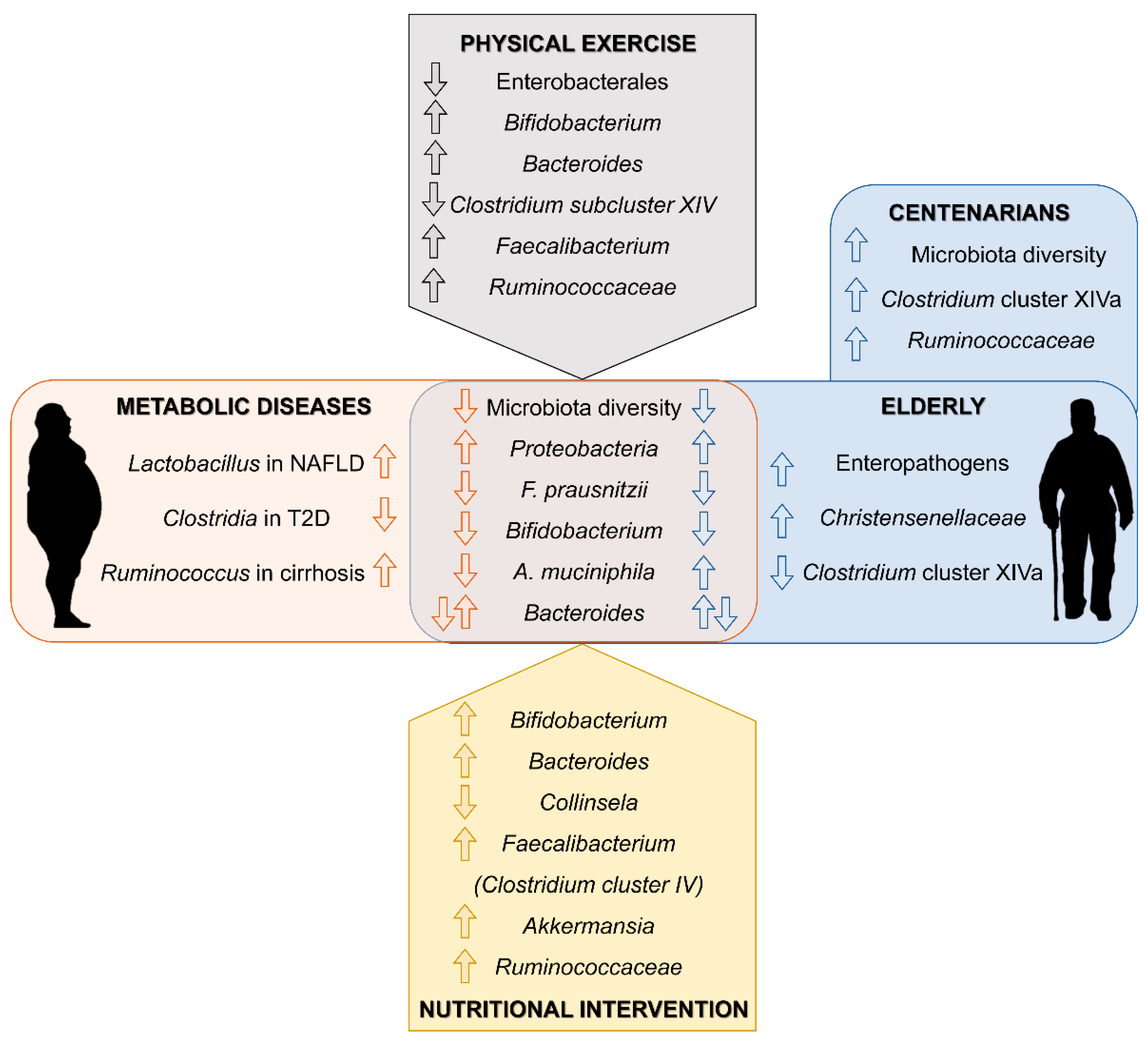
2. Metabolic Diseases in the Elderly
3. The Gut Microbiota as a Key Factor in the Development of Metabolic Diseases
4. Gut Microbiota in the Elderly
5. Gut Microbiota Targeted Interventions in the Elderly
5.1. Dietary Intervention
5.2. Prebiotic and Probiotic Supplementation
5.3. Physical Activity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ho, J.Y.; Hendi, A.S. Recent trends in life expectancy across high income countries: Retrospective observational study. BMJ 2018, 362, k2562. [Google Scholar] [CrossRef]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Kalish, V.B. Obesity in older adults. Prim. Care Clin. Off. Pract. 2016, 43, 137–144. [Google Scholar] [CrossRef]
- Cooper, J.A.; Cadogan, C.A.; Patterson, S.M.; Kerse, N.; Bradley, M.C.; Ryan, C.; Hughes, C.M. Interventions to improve the appropriate use of polypharmacy in older people: A Cochrane systematic review. BMJ Open 2015, 5, e009235. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef]
- Chekroud, S.R.; Gueorguieva, R.; Zheutlin, A.B.; Paulus, M.; Krumholz, H.M.; Krystal, J.H.; Chekroud, A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 2018, 5, 739–746. [Google Scholar] [CrossRef]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of exercise in the older population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Muñoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8263–8276. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Oreopoulos, A.; Kalantar-Zadeh, K.; Sharma, A.M.; Fonarow, G.C. The obesity paradox in the elderly: Potential mechanisms and clinical implications. Clin. Geriatr. Med. 2009, 25, 643–659. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Hou, Q.; Guan, Y.; Yu, W.; Liu, X.; Wu, L.; Xiao, M.; Lü, Y. Associations between obesity and cognitive impairment in the Chinese elderly: An observational study. Clin. Interv. Aging 2019, 14, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Knudsen, C.; Beaumont, M.; Rodriguez, J.; Neyrinck, A.M.; Bindels, L.B. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: A focus on the gut-liver axis. Proc. Nutr. Soc. 2019, 78, 319–328. [Google Scholar] [CrossRef]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.F.J.; De Vos, P.; Dekker, J.; et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 2019, 9, 1437. [Google Scholar] [CrossRef]
- Fava, F.; Rizzetto, L.; Tuohy, K.M. Gut microbiota and health: Connecting actors across the metabolic system. Proc. Nutr. Soc. 2019, 78, 177–188. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Q.; Li, H. Gut microbiota and nonalcoholic fatty liver disease: Insights on mechanisms and therapy. Nutrients 2017, 9, 1124. [Google Scholar] [CrossRef]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 2016, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Foster, B.A.; Dahdouli, M.; Ramirez, J.; Lai, Z. Characterization of the stool microbiome in hispanic preschool children by weight status and time. Child. Obes. 2018, 14, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Ohno, H. Gut microbiome and metabolic diseases. Semin. Immunopathol. 2014, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Sáenz de Miera, L.E.; Ballesteros Pomar, M.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Álvarez-Cuenllas, B.; Linares, P.; Olcoz, J.L.; Arias-Loste, M.T.; García-Lobo, J.M.; et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev. Esp. Enfermedades Dig. 2019, 111, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, M.; Xue, J.; Huang, J.; Zhuang, R.; Zhou, X.; Zhang, H.; Fu, Q.; Hao, Y. Body mass index differences in the gut microbiota are gender specific. Front. Microbiol. 2018, 9, 1250. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Iebba, V.; Guerrieri, F.; Di Gregorio, V.; Levrero, M.; Gagliardi, A.; Santangelo, F.; Sobolev, A.P.; Circi, S.; Giannelli, V.; Mannina, L.; et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Sci. Rep. 2018, 8, 8210. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut microbiome dysbiosis and immunometabolism: New frontiers for treatment of metabolic diseases. Mediators Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The gut microbiota and healthy aging: A mini-review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- García-Peña, C.; Álvarez-Cisneros, T.; Quiroz-Baez, R.; Friedland, R.P. Microbiota and aging. A review and commentary. Arch. Med. Res. 2017, 48, 681–689. [Google Scholar] [CrossRef]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Lynch, D.B.; O’Toole, P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Jeffery, I.B. Microbiome–health interactions in older people. Cell. Mol. Life Sci. 2018, 75, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, E.S.; Utami, T.; Mariyatun, M.; Hasan, P.N.; Kamil, R.Z.; Setyawan, R.H.; Pamungkaningtyas, F.H.; Harahap, I.A.; Wiryohanjoyo, D.V.; Pramesi, P.C.; et al. Gut microbiota profile in healthy Indonesians. World J. Gastroenterol. 2019, 25, 1478–1491. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Bian, G.; Gloor, G.B.; Gong, A.; Jia, C.; Zhang, W.; Hu, J.; Zhang, H.; Zhang, Y.; Zhou, Z.; Zhang, J.; et al. The gut microbiota of healthy aged chinese is similar to that of the healthy young. mSphere 2017, 2, e00327-17. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Jackson, M.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef]
- Mangiola, F.; Nicoletti, A.; Gasbarrini, A.; Ponziani, F.R. Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7404–7413. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, T.; Huang, G.; Cai, D.; Liang, X.; Su, H.; Zhu, Z.; Li, D.; Yang, Y.; Shen, P.; et al. Gut Microbiota community and its assembly associated with age and diet in Chinese centenarians. J. Microbiol. Biotechnol. 2015, 25, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, R.; Lin, H.; Fu, C.; Wang, X.; Zhang, Y.; Su, M.; Huang, P.; Qian, J.; Jiang, F.; et al. Enriched taxa were found among the gut microbiota of centenarians in East China. PLoS ONE 2019, 14, e0222763. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, C.W.; Shin, H.; Jin, S.P.; Bae, J.S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the gut microbiota of centenarians in longevity villages of South Korea with those of other age groups. J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut microbiota and extreme longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Collino, S.; Montoliu, I.; Martin, F.P.J.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; Biagi, E.; et al. Metabolic signatures of extreme longevity in Northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Johnson, L.C.; Martens, C.R.; Santos-Parker, J.R.; Bassett, C.J.; Strahler, T.R.; Cruickshank-Quinn, C.; Reisdorph, N.; McQueen, M.B.; Seals, D.R. Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with ageing. Clin. Sci. 2018, 132, 1765–1777. [Google Scholar] [CrossRef]
- Cai, D.; Zhao, S.; Li, D.; Chang, F.; Tian, X.; Huang, G.; Zhu, Z.; Liu, D.; Dou, X.; Li, S.; et al. Nutrient intake is associated with longevity characterization by metabolites and element profiles of healthy centenarians. Nutrients 2016, 8, 564. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Intestinal microbiota modulation in obesity-related non-alcoholic fatty liver disease. Front. Physiol. 2018, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.M.; McKenzie, E.J.; Mitchell, C.J.; Milan, A.M.; Zeng, N.; D’Souza, R.F.; Ramzan, F.; Sharma, P.; Rettedal, E.; Knowles, S.O.; et al. A period of 10 weeks of increased protein consumption does not alter faecal microbiota or volatile metabolites in healthy older men: A randomised controlled trial. J. Nutr. Sci. 2020, 9, e25. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Cousin, F.J.; Lynch, D.B.; Menon, R.; Brulc, J.; Brown, J.R.M.; O’Herlihy, E.; Butto, L.F.; Power, K.; Jeffery, I.B.; et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 2019, 7, 39. [Google Scholar] [CrossRef]
- Kim, C.-S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.-M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. Ser. A 2020, glaa090. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Amamoto, R.; Park, S.; Honda, Y.; Shimamoto, K.; Kushiro, A.; Tsuji, H.; Matsumoto, H.; Shimizu, K.; Miyazaki, K.; et al. Independent and interactive effects of habitually ingesting fermented milk products containing lactobacillus casei strain shirota and of engaging in moderate habitual daily physical activity on the intestinal health of older people. Front. Microbiol. 2019, 10, 1477. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; Khakimov, B.; Krych, Ł.; Bülow, J.; Bechshøft, R.L.; Højfeldt, G.; Mertz, K.H.; Garne, E.S.; Schacht, S.R.; Ahmad, H.F.; et al. Physical fitness in community-dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell 2020, 19, e13105. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef]
- Yu, Y.; Mao, G.; Wang, J.; Zhu, L.; Lv, X.; Tong, Q.; Fang, Y.; Lv, Y.; Wang, G. Gut dysbiosis is associated with the reduced exercise capacity of elderly patients with hypertension. Hypertens. Res. 2018, 41, 1036–1044. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, S.; Du, G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiologyopen 2020, 9, e1053. [Google Scholar] [CrossRef]
- Salazar, N.; González, S.; Nogacka, A.M.; Rios-Covián, D.; Arboleya, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Microbiome: Effects of ageing and diet. Curr. Issues Mol. Biol. 2019, 36, 33–62. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Bellanti, F.; Lo Buglio, A.; Vendemiale, G. The mediterranean diet slows down the progression of aging and helps to prevent the onset of frailty: A narrative review. Nutrients 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein intake and frailty: A matter of quantity, quality, and timing. Nutrients 2020, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Tammela, L.; Korpela, J.; Parhiala, R.; Ahokoski, H.; Mykkänen, H.; Salminen, S.J. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Omaha) 2009, 31, 59–66. [Google Scholar] [CrossRef]
- Rampelli, S.; Candela, M.; Severgnini, M.; Biagi, E.; Turroni, S.; Roselli, M.; Carnevali, P.; Donini, L.; Brigidi, P. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J. Nutr. Heal. Aging 2013, 17, 166–172. [Google Scholar] [CrossRef]
- Macfarlane, S.; Cleary, S.; Bahrami, B.; Reynolds, N.; Macfarlane, G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013, 38, 804–816. [Google Scholar] [CrossRef]
- Ahmed, M.; Prasad, J.; Gill, H.; Stevenson, L.; Gopal, P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J. Nutr. Heal. Aging 2007, 11, 26–31. [Google Scholar]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Häggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L.J. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.W.; Park, Y.M.; Holscher, H.D.; Padilla, J.; Scroggins, R.J.; Welly, R.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J.; Swanson, K.S. Physical activity differentially affects the cecal microbiota of ovariectomized female rats selectively bred for high and low aerobic capacity. PLoS ONE 2015, 10, e0136150. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, S.; Lensu, S.; Nokia, M.; Vanhatalo, S.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Intrinsic aerobic capacity governs the associations between gut microbiota composition and fat metabolism age-dependently in rat siblings. Physiol. Genom. 2017, 49, 733–746. [Google Scholar] [CrossRef]
- Houghton, D.; Stewart, C.J.; Stamp, C.; Nelson, A.; Aj Ami, N.J.; Petrosino, J.F.; Wipat, A.; Trenell, M.I.; Turnbull, D.M.; Greaves, L.C. Impact of age-related mitochondrial dysfunction and exercise on intestinal microbiota composition. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; Garcia-Mediavilla, M.V.V.; Martinez-Florez, S.; Juarez-Fernandez, M.; Cuevas, M.J.J.; Mauriz, J.L.L.; Gonzalez-Gallego, J.; Nistal, E.; Sanchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. DMM Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
| Reference | Subjects | Methodological Approach | Main Findings in Gut Microbiota Composition | |
|---|---|---|---|---|
| [67] Biagi et al. 2010 | 84 subjects from Northern Italy (50F, 34M). Young adults (20–40 years old) (Y), elderly (60–80 years old) (E), centenarians group (99–104 years old) (C) and offspring of the centenarians (59–78 years old) (F) | HITChip analysis and qPCR (16S rRNA) | Elderly | Centenarians |
|
| |||
| [58] Claesson et al. 2011 | 161 elderly Irish subjects (82F, 79M) (>65 years old) and a control group (5F, 4M) (28–46 years old) | Pyrosequencing with 454 system (16S rRNA V4 region) |
| |
| [72] Wang et al. 2015 | 24 volunteers from China (14F, 10M) classified in Group RC (100–108 years old), Group RE (85–99 years old) and Group CE (80–92 years old) | Illumina MiSeq and qPCR (16S rRNA V4 region) |
| |
| [76] Biagi et al. 2016 | 24 semi-supercentenarians (>105 years old, group S) (18F, 6M) vs. 15 young adults (22–48 years old, group Y) (8F, 7M) from Northern Italy. Results of C and E groups from Biagi et al. 2010 study were incorporated. | Illumina MiSeq and qPCR (16S rRNA V3-V4 region) | Elderly | Centenarians |
|
| |||
| ||||
| [75] Kong et al. 2016 | 168 Chinese individuals (85F, 83M) grouped into long-living group (≥90 years old), elderly group (65–83 years old) and a younger age group (24–64 years old) | Illumina MiSeq (16S rRNA V3-V4 region) |
| |
| [64] Odamaki et al. 2016 | 367 Japanese volunteers between 0 and 104 years old (210F, 157M) | Illumina MiSeq and qPCR (16S rRNA V3-V4 region) | Elderly | Centenarians |
| - |
| |||
| ||||
| [68] Bian et al. 2017 | A total of 1095 healthy Chinese volunteers (533F, 562M) classified into eight groups according to their age (children, adults, elderly, centenarians) | Illumina MiSeq (16S rRNA V4 region) | Elderly | Centenarians |
|
| |||
| ||||
| [66] Rahayu et al. 2019 | 80 Indonesian subjects (50F, 30M): young group (25–45 years old) and elderly group (≥70 years old) | Yakult intestinal flora-scan (YIF-SCAN) (qPCR method) |
| |
| [73] Wang et al. 2019 | 187 elderly subjects from three groups of age (65–70 years old), (90–99 years old) and (100+ years old) from East China (120F, 67M) | Illumina MiSeq (16S rRNA V3, V4 and V5 regions) | Elderly | Centenarians |
|
| |||
| [74] Kim et al. 2019 | 56 South Korea subjects classified in centenarians (95–108 years old) (27F, 3M), elderly (67–79 years old) (7F, 10M) and adults (26–43 years old) (3F, 6M) | Pyrosequencing with 454 system (16S rRNA V1–V3 regions) | Elderly | Centenarians |
|
| |||
| Reference | Subjects | Methodological Approach/Intervention | Main Findings in Gut Microbiota Composition | |
|---|---|---|---|---|
| Dietary Interventions, Probiotics and Prebiotics | ||||
| [82] Cancello et al. 2019 | ≥65 years old obese women (20) | Longitudinal study. Hypocaloric mediterranean diet (15 days, DIET), hypocaloric mediterranean diet + VSL#3 probiotic mix (15 days, PRO) | DIET | PRO |
| ↑ Parabacteroides ↑ Bacteroides ↑ Christensenellaceae ↑ Methanobrebrevibacter ↓ Collinsela | ↑ Akkermansia | |||
| [83] Ghosh et al. 2020 | 65–80 years old (286 M and 326 F) | Randomized multicenter single-blind controlled trial. Mediterranean diet (DIET) and control group (CON) (1 year) | DIET ↑ Faecalibacteriumn prausnitzii ↑ Roseburia ↑ Eubacterium ↑ Bacteroides thetaiotaomicron ↓ Ruminococcus torques ↓ Collinsela aerofaciens | |
| [84] Mitchell et al. 2020 | ≥70 years old men (30) | Randomized single-blind controlled trial. Diet with the recommended protein intake (RDA) or twice the recommended protein intake (2RDA) (10 weeks) | No significant differences reported | |
| [85] Tran et al. 2019 | Young healthy (13 M and 16 F). Community dwelling (>65 years old; 10 M and 18 F) and institutionalized elderly (>65 years old; 9 M and 13 F) | Randomized single-blind controlled trial. Prebiotic mix (PRE) vs. placebo (maltodextrin). Single daily dose over 26 weeks (Double dose for frail subjects). | PRE ↑ Ruminoccocaceae ↑ Parabacteroides ↑ Clostridium cluster IV ↑ Phascolarctobacterium (Significance reached for institutionalized subjects at 13 week) | |
| [86] Kim et al. 2020 | ≥65 years old (53) | Randomized double-blind, multicenter controlled trial. Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI (1 × 109 CFU/dose) vs. placebo (two doses/day, 12 weeks) | PRO ↓ Clostridiales ↓ Prevotellaceae ↓ Allisonela ↓ Eubacterium | |
| Physical activity | ||||
| [87] Aoyagi et al. 2019 | 65–92 years old (140 M and 198 F) | Monitoring of daily physical activity by electronic device over 1 month. Comparative study between more active (>15 min/day at >3 METS;MA) and less active (<15 min min/day at >3 METS; LA) | MA ↑ Bacillaceae ↓ Fusobacteriaceae | |
| [88] Castro-Mejía et al. 2020 | >65 years old (109 M and 98 F) | Comparative study of patients stratified into high (HF) and low-physical fitness (LF) | HF | LF |
| Bifidobacterium adolescentis Christensenella | Enterobacterales | |||
| [89] Taniguchi et al. 2018 | 62–76 years old men (33) | Randomised crossover trial. 3 sessions/week exercise in a cyclo-ergometer (TRA) and control sedentary period (CON; 5 weeks each) | TRA ↑ Oscillospira (CON first only) ↓ C. difficile | |
| [90] Morita et al. 2019 | >65 years old women (32) | Non-randomized comparative trial. 12 weeks of 1 h/daily brisk walking (TRA) vs. 1 h/weekly session of trunk muscle training (CON) | TRA | CON |
| ↑ Bacteroides ↓ Clostridium subcluster XIV | ↓ Clostridium subcluster IX | |||
| [91] Yu et al. 2018 | 65–80 years old patients with hypertension (32 M and 24 F) | Comparative study of patients classified according to Weber’ system for functional capacity. Normal exercise capacity (NE) vs. reduced exercise capacity (RE) | NE | RE |
| ↑ Betaproteobacteria ↑ Ruminococcaceae ↑ Faecalibacterium | ↑ Escherichia_Sigella ↑ Lactobacillales ↑ Lachnospiraceae ↑ Blautia | |||
| [92] Zhu et al. 2020 | Healthy elderly and overweight elderly (>61 years old) (897) | Comparative study. Overweight elderly daily exercise (DROE, 74) group vs. overweight elderly never or rare exercise group (NROE, 222) | DROE ↑ Turicibacteraceae ↓ Pseudomonadaceae ↓ Oxalobacteraceae ↓Odoribacteraceae ↓Barnesiellaceae | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez-Fernández, M.; Porras, D.; García-Mediavilla, M.V.; Román-Sagüillo, S.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions. Nutrients 2021, 13, 16. https://doi.org/10.3390/nu13010016
Juárez-Fernández M, Porras D, García-Mediavilla MV, Román-Sagüillo S, González-Gallego J, Nistal E, Sánchez-Campos S. Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions. Nutrients. 2021; 13(1):16. https://doi.org/10.3390/nu13010016
Chicago/Turabian StyleJuárez-Fernández, María, David Porras, María Victoria García-Mediavilla, Sara Román-Sagüillo, Javier González-Gallego, Esther Nistal, and Sonia Sánchez-Campos. 2021. "Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions" Nutrients 13, no. 1: 16. https://doi.org/10.3390/nu13010016
APA StyleJuárez-Fernández, M., Porras, D., García-Mediavilla, M. V., Román-Sagüillo, S., González-Gallego, J., Nistal, E., & Sánchez-Campos, S. (2021). Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions. Nutrients, 13(1), 16. https://doi.org/10.3390/nu13010016