Title Changes in the Mineral Composition of Rat Femoral Bones Induced by Implantation of LNCaP Prostate Cancer Cells and Dietary Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Dietary Ingredients
2.3. Animal Experiment
- -
- Zinc 4.6 mg/mL (1.85 mg Zn (II)/day/rat) (as ZnSO4 × 7H2O in aqueous suspension);
- -
- Iron 7.5 mg/mL (3 mg Fe (II)/day/rat) (as FeSO4 × 7H2O in aqueous suspension);
- -
- Calcium 75 mg/mL (30 mg Ca/day/rat) (as CaCl2 × 6H2O in aqueous suspension).
- (1)
- Five-fold for determination of Co, Cu, Mn, Mo, Ni, Se and Zn;
- (2)
- 500-fold for determination of Ca, K, Sr and Fe.
2.3.1. Chemicals and Reagents
2.3.2. Instrumentation
2.3.3. Analytical Procedure
2.4. Histopathology
2.5. Statistics
3. Results
3.1. Characteristics of the Animals’ Body Weight Gain
3.2. Mean Values with Standard Deviations for the Content of Ten Elements in Bone of Rats
3.3. Experimental to Control Group Comparison
3.4. Comparison of Diet Groups
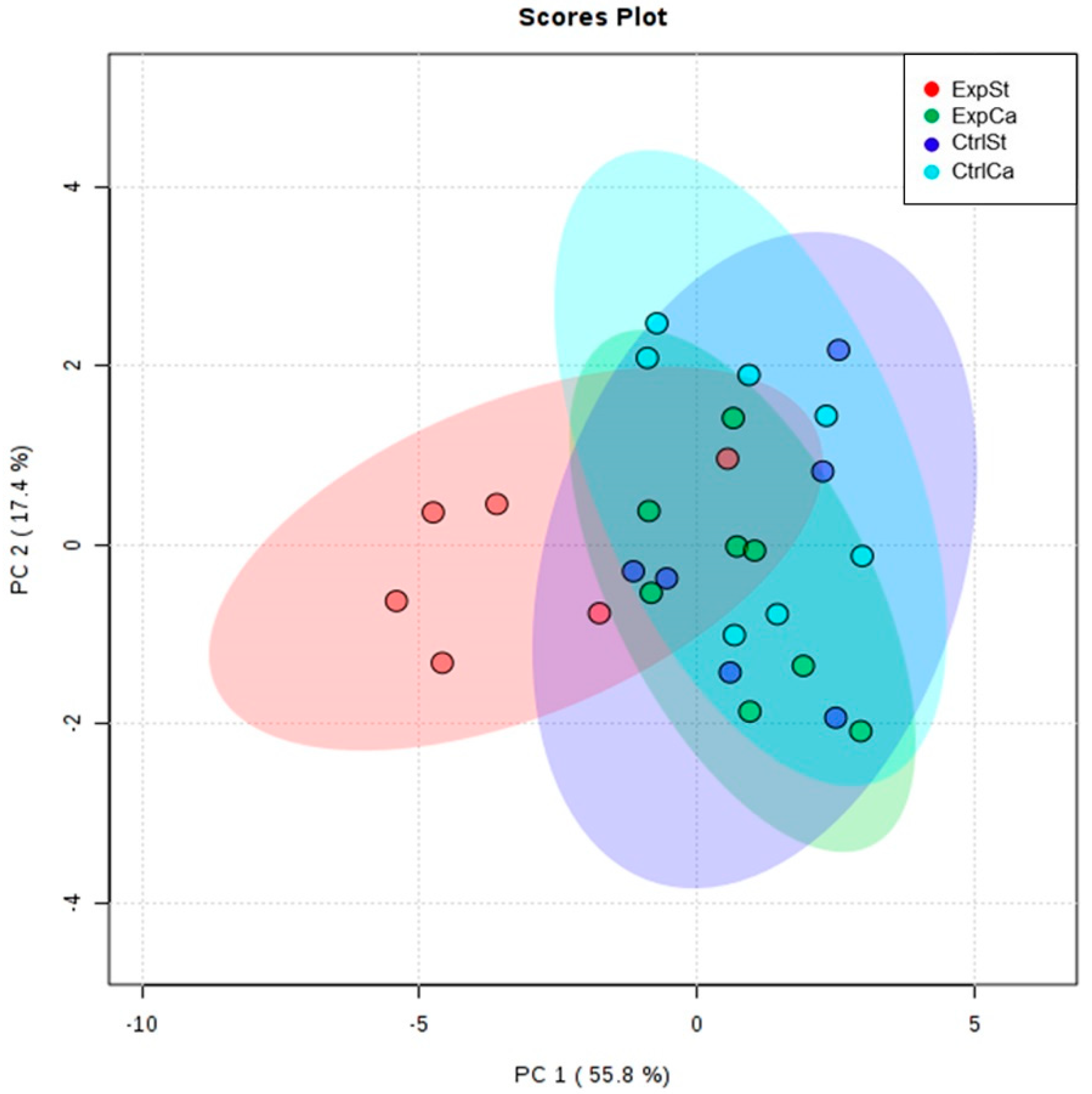
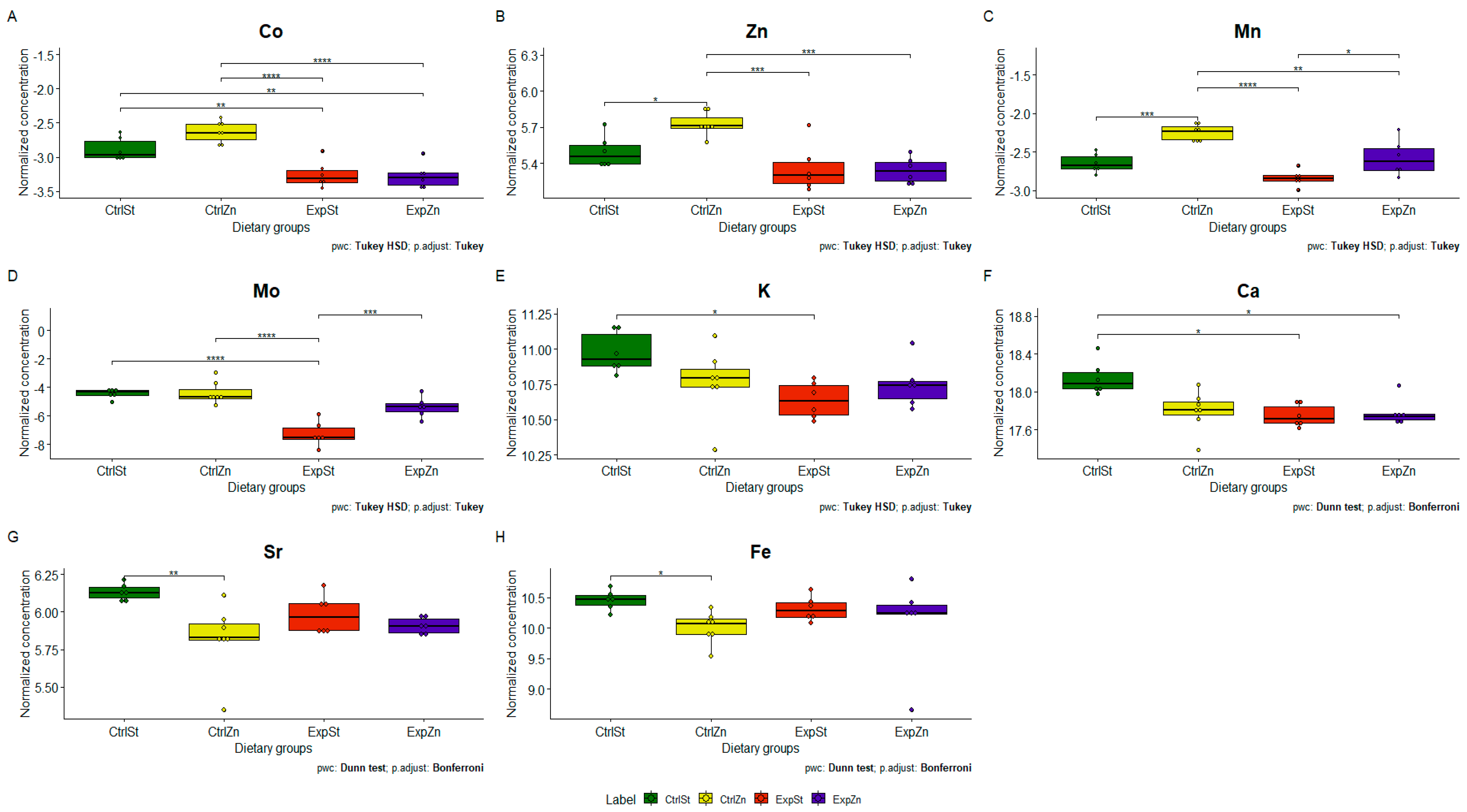
3.4.1. Dietary Supplementation with Calcium Relative to Other Experimental Groups
- -
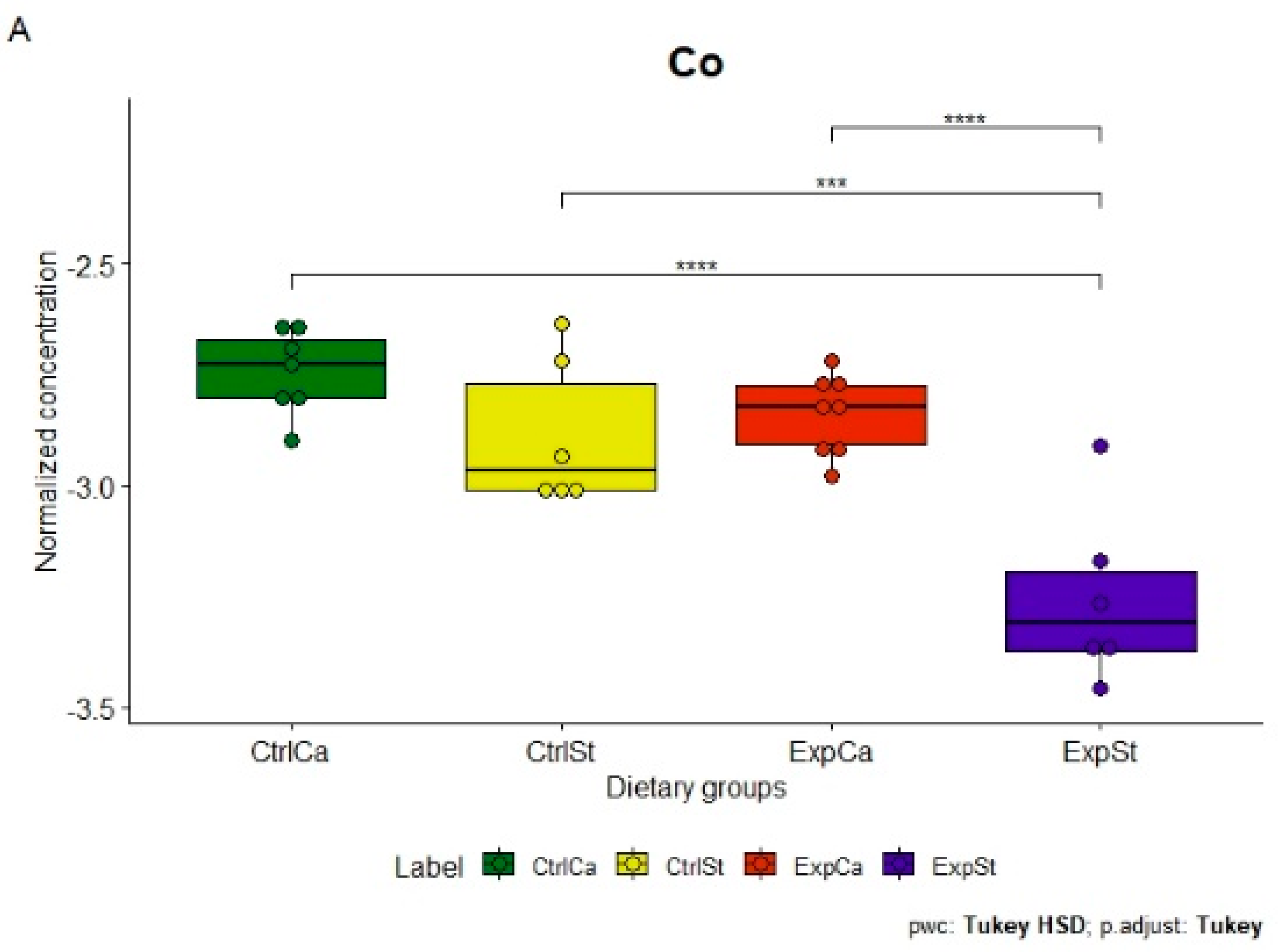
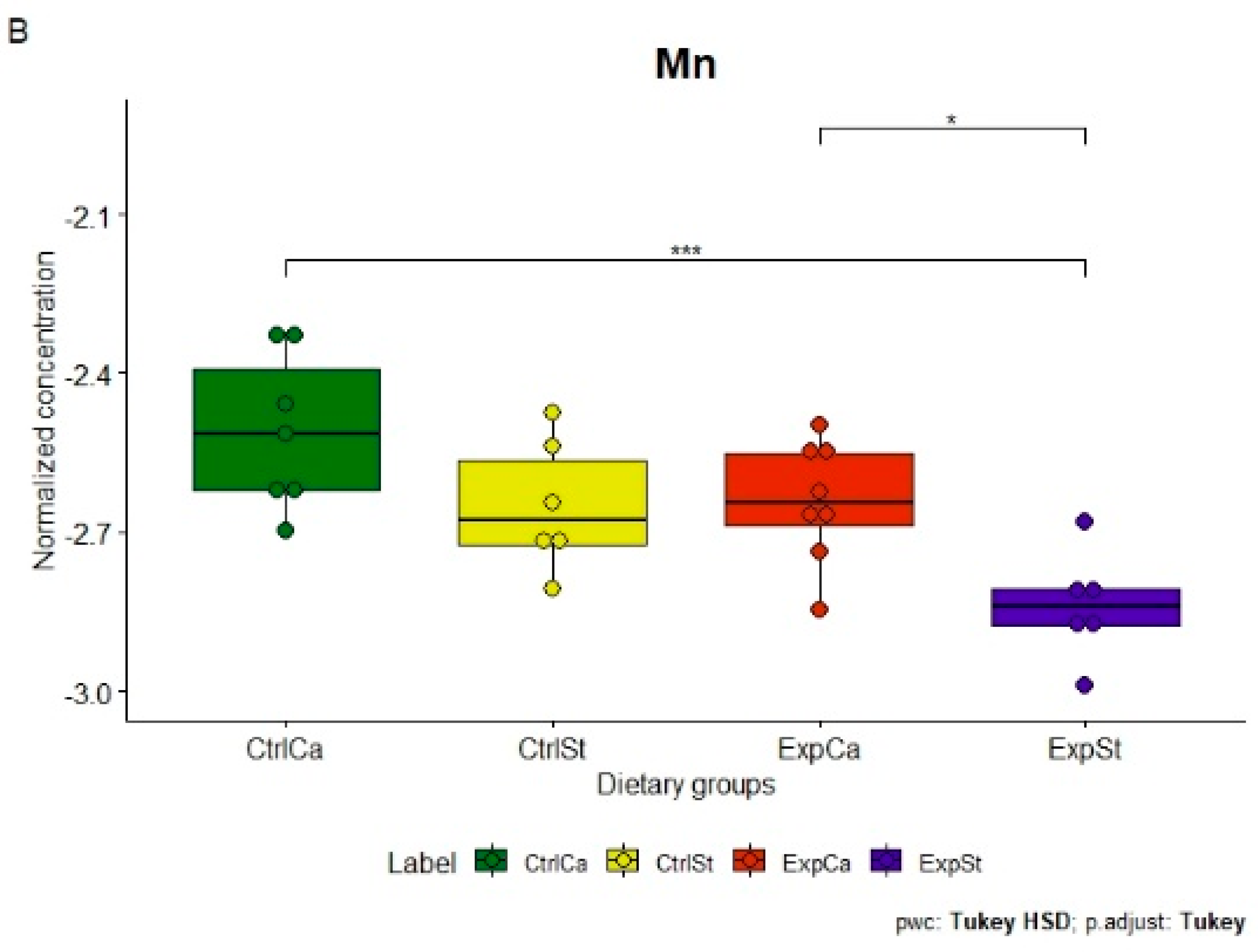
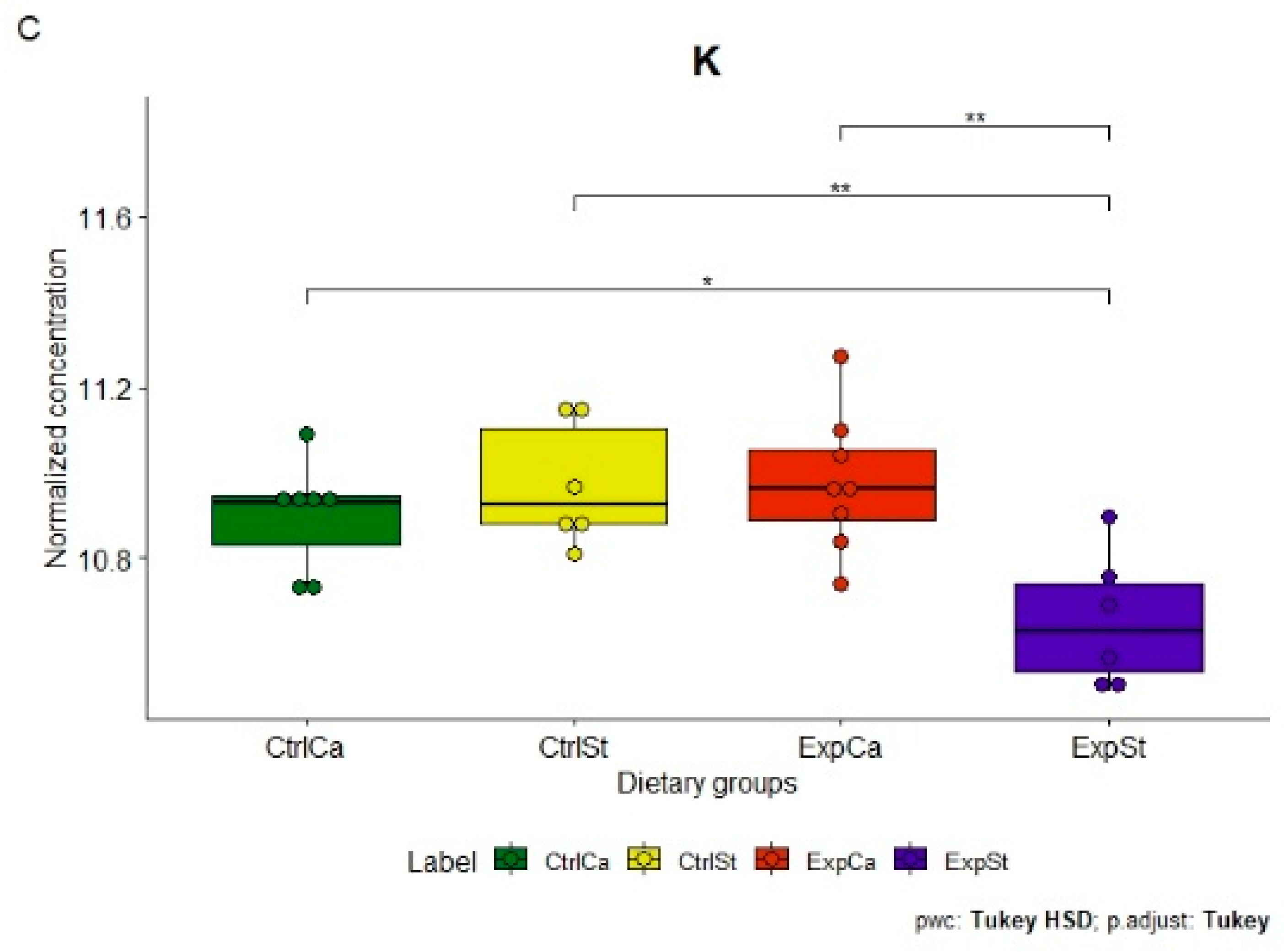
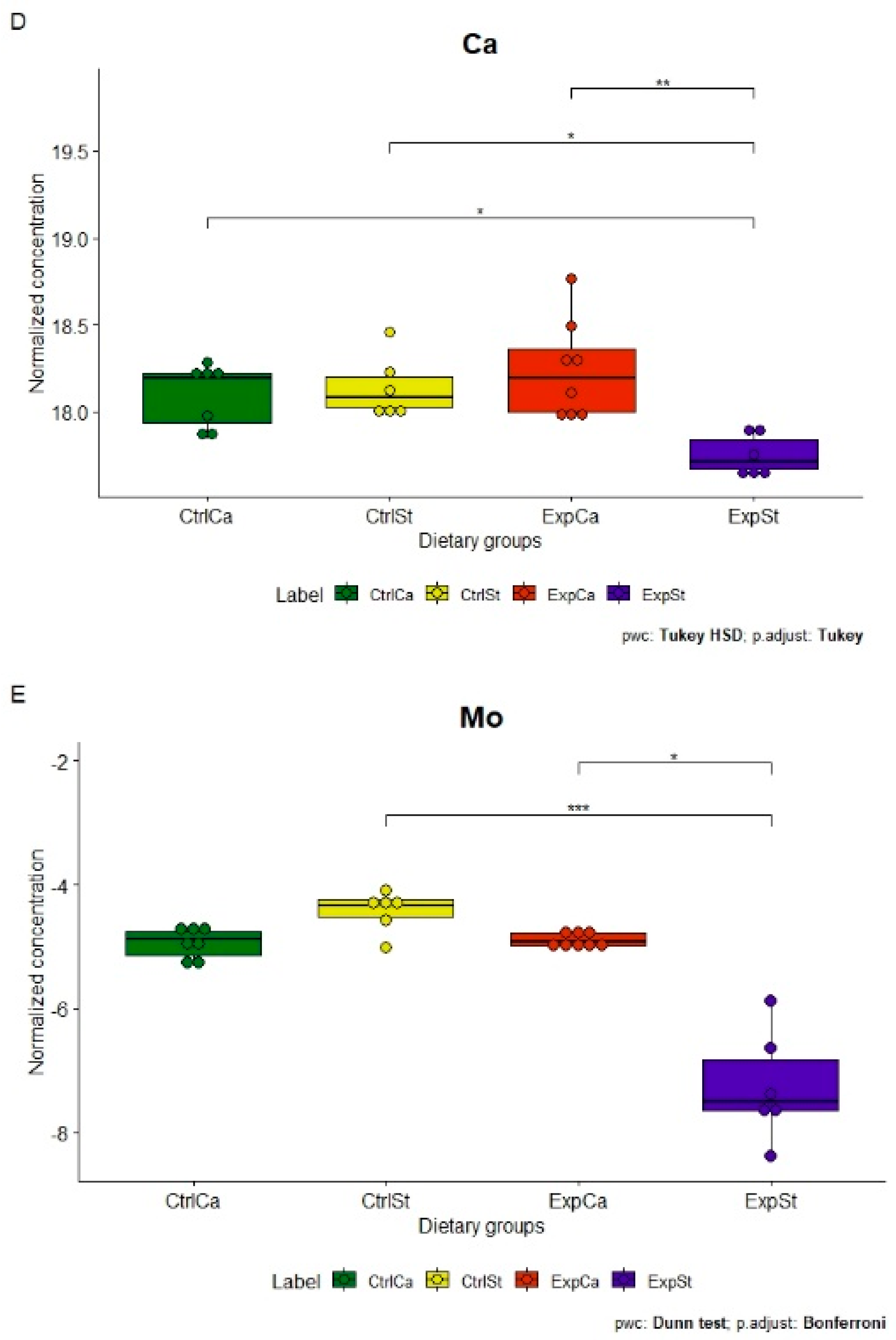
- Supplementation of the diet of rats with calcium ions, irrespective of LNCaP implantation (CtrlCa and ExpCa), caused a significant increase in the content of Co, Mn, K and Ca in comparison to the group with implanted LNCaP on the standard diet (ExpSt) (Figure 2A–D). In the case of Mo, such a relationship was noted only for the group with implanted LNCaP cells (ExpCa) (Figure 2E);
- -
- Supplementation of the diet of rats with calcium ions in the groups with implanted LNCaP (ExpCa) caused no differences in the level of elements in relation to the other groups (CtrlCa and CtrlSt) (Figure 2A–E);
- -
- Implantation of LNCaP cancer cells in the group on the standard diet (ExpSt) significantly decreased the level of Co, K, Ca, Mo and Mn in the bones (Figure 2A–E);
- -
- No significant differences were noted in the case of the other elements tested (Fe, Zn, Sr, Cu and Ni; data not shown);
- -
- The PCA (principal component analysis) score plot confirms the pronounced effect of implanted LNCaP cells and the standard diet on bone composition (ExpSt is separated from the other groups) (Figure 3). At the same time, supplementation with calcium seems to improve bone composition (similarity of ExpCa to CtrlSt and CtrlCa).
3.4.2. Dietary Supplementation with Zinc Relative to Other Experimental Groups
- -
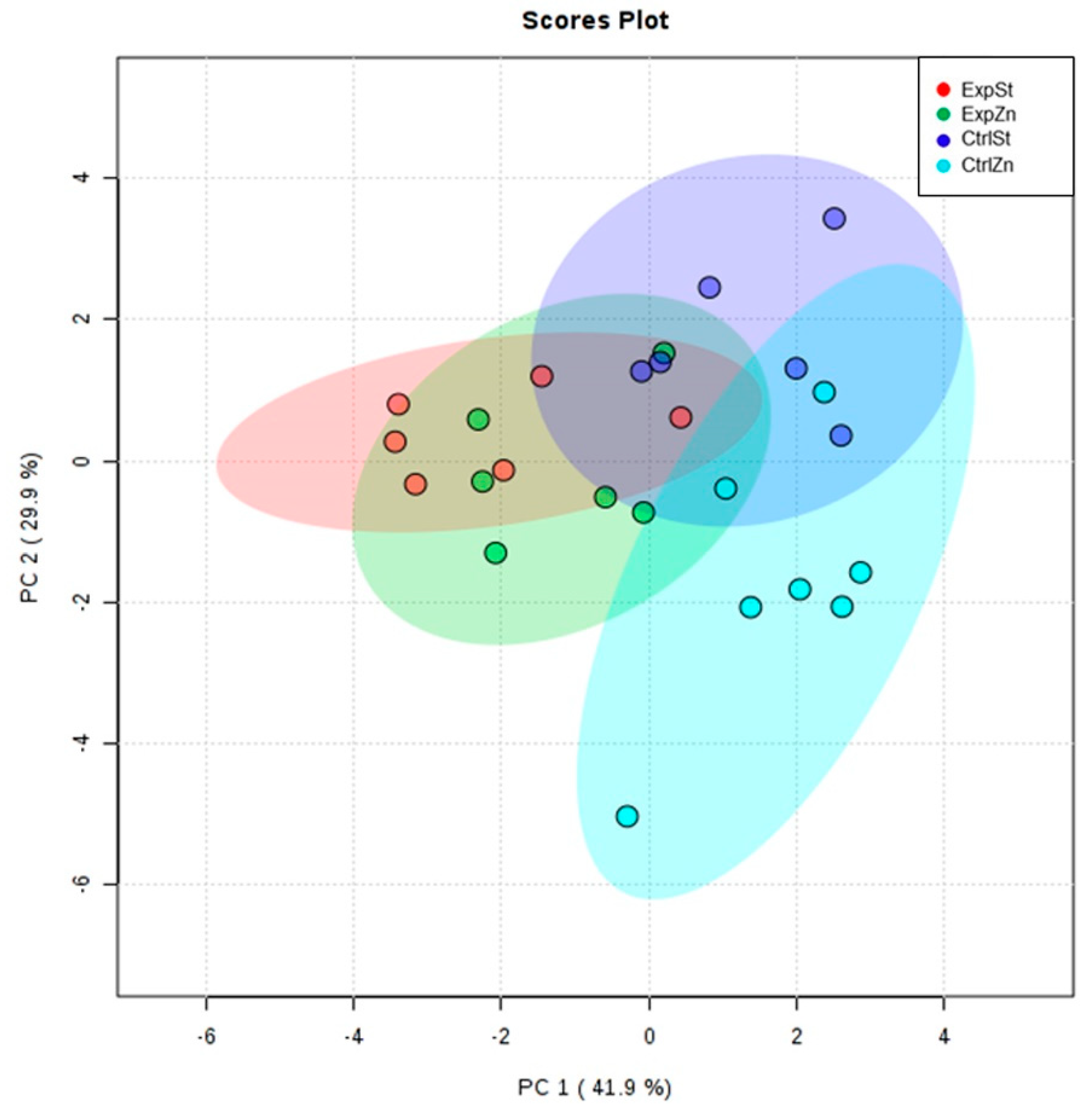
- Dietary supplementation with zinc generated the most differences between groups in the case of Co, Mn, Mo and Zn (Figure 4A–D);
- -
- -
- The content of Sr and Fe was reduced in the control groups receiving a zinc supplement (CtrlZn) relative to the standard control group (CtrlSt) (Figure 4G–H);
- -
- Implantation of LNCaP cells in groups ExpSt and ExpZn reduced the level of Ca relative to the standard control group (CtrlSt) (Figure 4F);
- -
- No significant differences were noted in the case of Cu or Ni (data not shown);
- -
- Principal component analysis shows a clear influence of dietary supplementation with zinc in rats without LNCaP implantation on bone composition (CtrlZn is separated from the other groups). At the same time, LNCaP implantation causes changes in bone composition (similarity of ExpZn to ExpSt) (Figure 5).
3.4.3. Dietary Supplementation with Iron Relative to Other Experimental Groups
- -
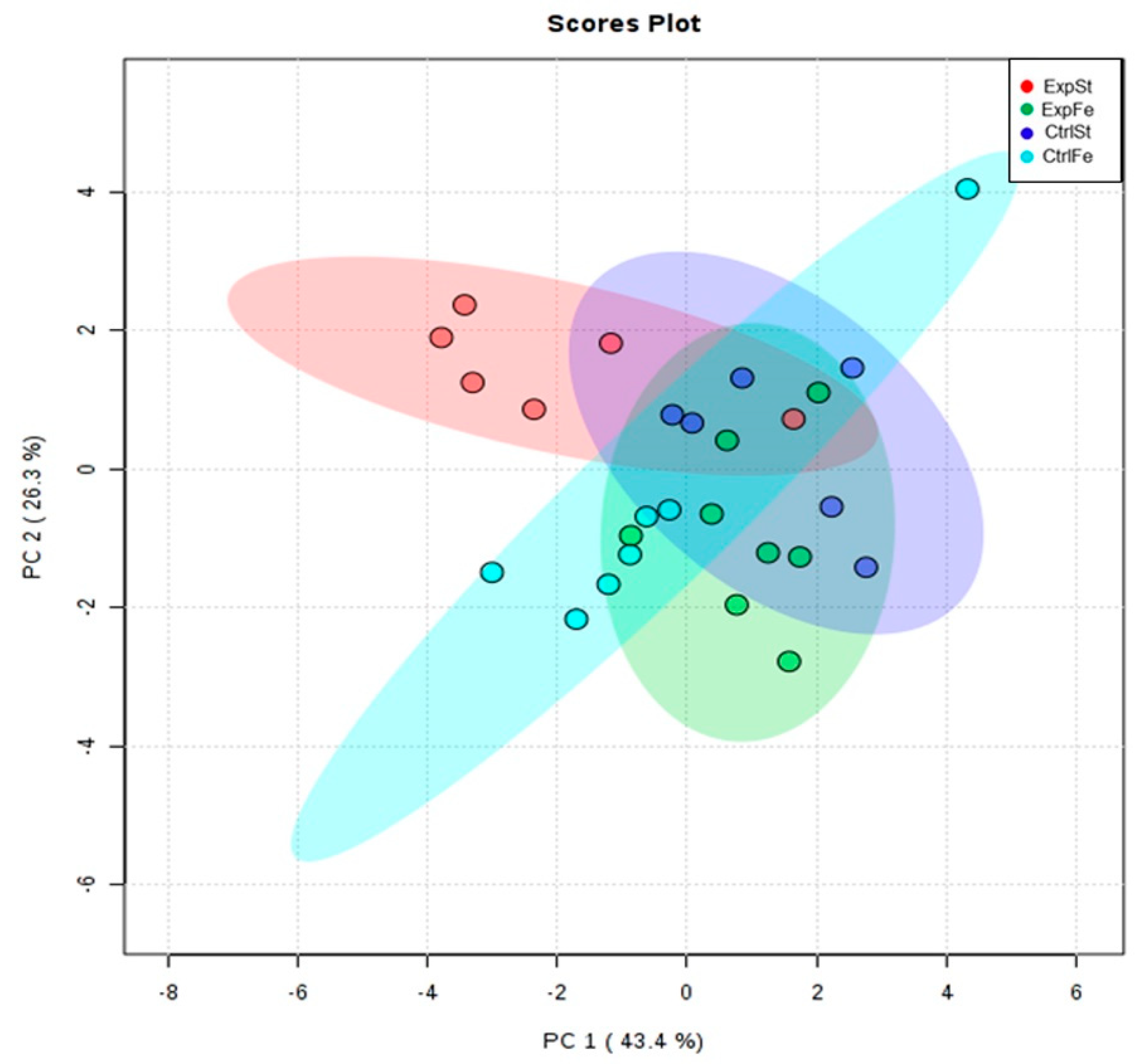
- The Mo level in the groups whose diet was supplemented with iron, irrespective of the presence or absence of LNCaP cells (CtrlFe and ExpFe), as well as in group CtrlSt, was significantly higher than in the standard group with implanted LNCaP (ExpSt) (Figure 6A);
- -
- Implantation of cells of the LNCaP line in the group on an iron diet (ExpFe) significantly increased the content of Co and Mn in the bones relative to the experimental group on the standard diet (ExpSt) (Figure 6B–C);
- -
- -
- The calcium concentration in the femoral bone of rats in the implantation groups (ExpSt) was lower than in the groups without implanted cells on the standard diet (CtrlSt) (Figure 6F);
- -
- There were no changes in the case of Cu, Zn, Sr or K (data not shown);
- -
- Principal component analysis indicates that the presence of cancer cells had a marked effect on the bone composition of rats on the standard diet (ExplSt is separated from the other groups). At the same time, iron supplementation improved bone composition (similarity of ExpFe to CtrlSt and CtrlFe) (Figure 7).
3.5. The Effect of Supplementation with Calcium, Iron and Zinc on the Occurrence of Tumour Hyperplasia in Rats
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.-V.; Giaze, T.R.; Chin, K.-Y.; Mohamed, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Sterling, J.A.; Edwards, J.R.; Martin, T.J.; Mundy, G.R. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone 2011, 48, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Coenegrachts, L.; Maes, C.; Torrekens, S.; Van Looveren, R.; Mazzone, M.; Guise, T.A.; Bouillon, R.; Stassen, J.-M.; Carmeliet, P.; Carmeliet, G. Anti-placental growth factor reduces bone metastasis by blocking tumor cell engraftment and osteoclast differentiation. Cancer Res. 2010, 70, 6537–6547. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Wong, M.H.; Pavlakis, N. Treatment and Prevention of Bone Metastases from Breast Cancer: A Comprehensive Review of Evidence for Clinical Practice. J. Clin. Med. 2014, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. Bone cancer pain: From mechanism to therapy. Curr. Opin. Support. Palliat. Care 2014, 8, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hiasa, M.; Nagata, Y.; Okui, T.; White, F.A. Acidic microenvironment and bone pain in cancer-colonized bone. BoneKEy Rep. 2015, 4, 690. [Google Scholar] [CrossRef] [PubMed]
- Dushyanthen, S.; Cossigny, D.A.F.; Quan, G.M.Y. The Osteoblastic and Osteoclastic Interactions in Spinal Metastases Secondary to Prostate Cancer. Cancer Growth Metastasis 2013, 6, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sircar, K.; Aprikian, A.; Potti, A.; Goltzman, D.; Rabbani, S.A. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 2006, 107, 289–298. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of Iron on Bone Homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Wysoczańska, A.; Ślęzak, A.; Barg, E. Effects of oncological therapy on bone disorders. Pediatr. Endocrinol. Diabetes Metab. 2017, 23, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zofková, I.; Nemcikova, P.; Matucha, P. Trace elements and bone health. Clin. Chem. Lab. Med. 2013, 51, 1555–1561. [Google Scholar] [CrossRef]
- Amin, N.; Clark, C.C.T.; Taghizadeh, M.; Djafarnejad, S. Zinc supplements and bone health: The role of the RANKL-RANK axis as a therapeutic target. J. Trace Elem. Med. Biol. 2020, 57, 126417. [Google Scholar] [CrossRef]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Rai, D. Zinc Improves the Bone Mechanical Strength in Ovariectomized Rat Model by Restoring Bone Composition and Hydroxyapatite Crystallite Dimension. Vitam. Miner. 2016, 5. [Google Scholar] [CrossRef]
- Razmandeh, R.; Nasli-Esfahani, E.; Heydarpour, R.; Faridbod, F.; Ganjali, M.R.; Norouzi, P.; Larijani, B.; Khoda-amorzideh, D. Association of Zinc, Copper and Magnesium with bone mineral density in Iranian postmenopausal women—A case control study. J. Diabetes Metab. Disord. 2014, 13, 43. [Google Scholar] [CrossRef]
- Pepa, G.D.; Brandi, M.L. Microelements for bone boost: The last but not the least. Clin. Cases Miner. Bone Metab. 2016, 13, 181–185. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Ebrahimi, M.; Ebrahimi, A. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2015, 12, 18–21. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of Zinc Signaling Studies in Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 667. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, Q.; Hu, X.; Dong, X.; Wang, L.; Liu, Q.; Long, Z.; Li, L. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 25778. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef]
- Xue, Y.-N.; Liu, Y.-N.; Su, J.; Li, J.-L.; Wu, Y.; Guo, R.; Yu, B.-B.; Yan, X.-Y.; Zhang, L.-C.; Sun, L.-K.; et al. Zinc cooperates with p53 to inhibit the activity of mitochondrial aconitase through reactive oxygen species accumulation. Cancer Med. 2019, 8, 2462–2473. [Google Scholar] [CrossRef]
- Çınar, V.; Talaghir, L.G.; Akbulut, T.; Turgut, M.; Sarıkaya, M. The effects of the zinc supplementation and weight trainings on the testosterone levels. Чeлoвeк Cпopт Meдицинa 2017, 17, 58–63. [Google Scholar]
- Chu, Q.; Chi, Z.-H.; Zhang, X.; Liang, D.; Wang, X.; Zhao, Y.; Zhang, L.; Zhang, P. A potential role for zinc transporter 7 in testosterone synthesis in mouse Leydig tumor cells. Int. J. Mol. Med. 2016, 37, 1619–1626. [Google Scholar] [CrossRef]
- Koehler, K.; Parr, M.K.; Geyer, H.; Mester, J.; Schänzer, W. Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement. Eur. J. Clin. Nutr. 2009, 63, 65–70. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; Castelló, A.; Fernández-Navarro, P.; Castaño-Vinyals, G.; Llorca, J.; Salas-Trejo, D.; Salcedo-Bellido, I.; Aragonés, N.; Fernández-Tardón, G.; Alguacil, J.; et al. Dietary Zinc and Risk of Prostate Cancer in Spain: MCC-Spain Study. Nutrients 2019, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Farahzadi, R.; Fathi, E.; Mesbah-Namin, S.A.; Zarghami, N. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0188052. [Google Scholar] [CrossRef]
- Boissier, S.; Ferreras, M.; Peyruchaud, O.; Magnetto, S.; Ebetino, F.H.; Colombel, M.; Delmas, P.; Delaissé, J.M.; Clézardin, P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000, 60, 2949–2954. [Google Scholar] [PubMed]
- Holmes, M.D.; Pollak, M.N.; Willett, W.C.; Hankinson, S.E. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol. Biomark. 2002, 11, 852–861. [Google Scholar]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F. Iron and Its Role in Cancer Defense: A Double-Edged Sword. Met. Ions Life Sci. 2018, 18. [Google Scholar] [CrossRef]
- Banas, A.; Kwiatek, W.M.; Banas, K.; Gajda, M.; Pawlicki, B.; Cichocki, T. Correlation of concentrations of selected trace elements with Gleason grade of prostate tissues. J. Biol. Inorg. Chem. 2010, 15, 1147–1155. [Google Scholar] [CrossRef]
- Jian, J.; Yang, Q.; Dai, J.; Eckard, J.; Axelrod, D.; Smith, J.; Huang, X. Effects of Iron Deficiency and Overload on Angiogenesis and Oxidative Stress—A Potential Dual Role for Iron in Breast Cancer. Free Radic. Biol. Med. 2011, 50, 841–847. [Google Scholar] [CrossRef]
- Brown, R.A.M.; Richardson, K.L.; Kabir, T.D.; Trinder, D.; Ganss, R.; Leedman, P.J. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Neuhouser, M.L.; Barnett, M.J.; Hong, C.-C.; Kristal, A.R.; Thornquist, M.D.; King, I.B.; Goodman, G.E.; Ambrosone, C.B. Iron intake, oxidative stress-related genes (MnSOD and MPO) and prostate cancer risk in CARET cohort. Carcinogenesis 2008, 29, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Cross, A.J.; Abnet, C.C.; Sinha, R.; Markin, R.S.; Weisenburger, D.D. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur. J. Cancer Prev. 2012, 21, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Varenna, M.; Fracanzani, A.L.; Rossi, V.; Fargion, S.; Sinigaglia, L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos. Int. 2009, 20, 549–555. [Google Scholar] [CrossRef]
- Doyard, M.; Chappard, D.; Leroyer, P.; Roth, M.-P.; Loréal, O.; Guggenbuhl, P. Decreased Bone Formation Explains Osteoporosis in a Genetic Mouse Model of Hemochromatosiss. PLoS ONE 2016, 11, e0148292. [Google Scholar] [CrossRef]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Zhu, K.; Prince, R.L. Calcium and bone. Clin. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef]
- Seccareccia, D. Cancer-related hypercalcemia. Can. Fam. Physician 2010, 56, 244–246. [Google Scholar]
- Skrajnowska, D.; Bobrowska-Korczak, B.; Tokarz, A. Disorders of Mechanisms of Calcium Metabolism Control as Potential Risk Factors of Prostate Cancer. Curr. Med. Chem. 2017, 24, 4229–4244. [Google Scholar] [CrossRef]
- Wolf, A.; Wennemuth, G. Ca2+ clearance mechanisms in cancer cell lines and stromal cells of the prostate. Prostate 2014, 74, 29–40. [Google Scholar] [CrossRef]
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and phosphorus intake and prostate cancer risk: A 24-y follow-up study. Am. J. Clin. Nutr. 2015, 101, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, S.; Colditz, G.A. Prostate cancer: Is it time to expand the research focus to early-life exposures? Nat. Rev. Cancer 2013, 13, 208–518. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Whitley, B.M.; Hoyo, C.; Grant, D.J.; Schwartz, G.G.; Presti, J.C.; Iraggi, J.D.; Newman, K.A.; Gerber, L.; Taylor, L.A.; et al. Dietary calcium and risk for prostate cancer: A case-control study among US veterans. Prev. Chronic. Dis. 2012, 9, E39. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Wong, A.S.; Koh, W.-P.; Wang, R.; Yuan, J.-M.; Yu, M.C. Calcium intake increases risk of prostate cancer among Singapore Chinese. Cancer Res. 2010, 70, 4941–4948. [Google Scholar] [CrossRef]
- Aune, D.; Navarro Rosenblatt, D.A.; Chan, D.S.M.; Vieira, A.R.; Vieira, R.; Greenwood, D.C.; Vatten, L.J.; Norat, T. Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr. 2015, 101, 87–117. [Google Scholar] [CrossRef]
- Binder, M.; Shui, I.M.; Wilson, K.M.; Penney, K.L.; Mucci, L.A.; Kibel, A.S. Calcium intake, polymorphisms of the calcium-sensing receptor, and recurrent/aggressive prostate cancer. Cancer Causes Control CCC 2015, 26, 1751–1759. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Berryman, K.; Langdon, R.; Bonilla, C.; Davey Smith, G.; Martin, R.M.; Lewis, S.J.; Practical Consortium. Mendelian randomization does not support serum calcium in prostate cancer risk. Cancer Causes Control 2018, 29, 1073–1080. [Google Scholar] [CrossRef]
- Bonithon-Kopp, C.; Kronborg, O.; Giacosa, A.; Räth, U.; Faivre, J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: A randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet Lond. Engl. 2000, 356, 1300–1306. [Google Scholar] [CrossRef]
- Grau, M.V.; Baron, J.A.; Sandler, R.S.; Wallace, K.; Haile, R.W.; Church, T.R.; Beck, G.J.; Summers, R.W.; Barry, E.L.; Cole, B.F.; et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J. Natl. Cancer Inst. 2007, 99, 129–136. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Lytras, T.; Malesci, A.; Danese, S. Calcium supplementation for the prevention of colorectal adenomas: A systematic review and meta-analysis of randomized controlled trials. World J. Gastroenterol. 2016, 22, 4594–4603. [Google Scholar] [CrossRef]
- Pasha, Q.; Malik, S.A.; Iqbal, J.; Shaheen, N.; Shah, M.H. Comparative evaluation of trace metal distribution and correlation in human malignant and benign breast tissues. Biol. Trace Elem. Res. 2008, 125, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.P.; Valentine, R.J.; Moulton, C.J.; Wagoner Johnson, A.J.; Evans, E.M.; Layman, D.K. Breast tumors induced by N-methyl-N-nitrosourea are damaging to bone strength, structure, and mineralization in the absence of metastasis in rats. J. Bone Miner. Res. 2011, 26, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Scorei, I.D.; Scorei, R.I. Calcium Fructoborate Helps Control Inflammation Associated with Diminished Bone Health. Biol. Trace Elem. Res. 2013, 155, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Baron, J.A.; Burckhardt, P.; Li, R.; Spiegelman, D.; Specker, B.; Orav, J.E.; Wong, J.B.; Staehelin, H.B.; et al. Calcium intake and hip fracture risk in men and women: A meta-analysis of prospective cohort studies and randomized controlled trials. Am. J. Clin. Nutr. 2007, 86, 1780–1790. [Google Scholar] [CrossRef]
- Warensjö, E.; Byberg, L.; Melhus, H.; Gedeborg, R.; Mallmin, H.; Wolk, A.; Michaëlsson, K. Dietary calcium intake and risk of fracture and osteoporosis: Prospective longitudinal cohort study. BMJ 2011, 342. [Google Scholar] [CrossRef]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351. [Google Scholar] [CrossRef]
- Bortolin, R.H.; Abreu, B.J.d.G.A.; Ururahy, M.A.G.; de Souza, K.S.C.; Bezerra, J.F.; Loureiro, M.B.; da Silva, F.S.; Marques, D.E.d.S.; Batista, A.A.d.S.; Oliveira, G.; et al. Protection against T1DM-Induced Bone Loss by Zinc Supplementation: Biomechanical, Histomorphometric, and Molecular Analyses in STZ-Induced Diabetic Rats. PLoS ONE 2015, 10, e0125349. [Google Scholar] [CrossRef]
- Suzuki, T.; Kajita, Y.; Katsumata, S.; Matsuzaki, H.; Suzuki, K. Zinc Deficiency Increases Serum Concentrations of Parathyroid Hormone through a Decrease in Serum Calcium and Induces Bone Fragility in Rats. J. Nutr. Sci. Vitaminol. 2015, 61, 382–390. [Google Scholar] [CrossRef]
- Berger, P.K.; Pollock, N.K.; Laing, E.M.; Chertin, V.; Bernard, P.J.; Grider, A.; Shapses, S.A.; Ding, K.-H.; Isales, C.M.; Lewis, R.D. Zinc Supplementation Increases Procollagen Type 1 Amino-Terminal Propeptide in Premenarcheal Girls: A Randomized Controlled Trial. J. Nutr. 2015, 145, 2699–2704. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, Y.; Yoon, D.S.; Lee, K.-M.; Kim, D.; Lee, J.W. Zinc Promotes Osteoblast Differentiation in Human Mesenchymal Stem Cells Via Activation of the cAMP-PKA-CREB Signaling Pathway. Stem Cells Dev. 2018, 27, 1125–1135. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; López-Frías, M.R.; Campos, M.S.; López-Frías, M.; Alférez, M.J.M.; Nestares, T.; Ojeda, M.L.; López-Aliaga, I. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur. J. Nutr. 2012, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Liu, Z.; McQueen, A.K.; Acton, D.; Acton, A.J.; Padgett, L.R.; Peacock, M.; Econs, M.J. Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone 2016. [Google Scholar] [CrossRef] [PubMed]
- Kubaszewski, Ł.; Zioła-Frankowska, A.; Frankowski, M.; Rogala, P.; Gasik, Z.; Kaczmarczyk, J.; Nowakowski, A.; Dabrowski, M.; Labedz, W.; Miękisiak, G.; et al. Comparison of trace element concentration in bone and intervertebral disc tissue by atomic absorption spectrometry techniques. J. Orthop. Surg. 2014, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska-Korczak, B.; Skrajnowska, D.; Kiss, A.K.; Wrzesień, R.; Bielecki, W.; Orzoł, A.; Żebrowski, P.; Białek, S. Lipid peroxidation as a predictive biomarker of the early stage of cancer. J. Biol. Regul. Homeost. Agents 2019, 33, 799–810. [Google Scholar] [PubMed]
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. CA. Cancer J. Clin. 2018, 68, 377–386. [Google Scholar] [CrossRef]
- Chung, S.-H.; Thomas, S.A. Management of Hypercalcemia of Malignancy. JHOP 2016, 6. [Google Scholar] [CrossRef][Green Version]
- Jagielska, A.; Ruszczyńska, A.; Wagner, B.; Bulska, E.; Skrajnowska, D.; Bobrowska-Korczak, B. ICP-MS analysis of diet supplementation influence on the elemental content of rat prostate gland. Mon. Chem. Chem. Mon. 2019, 150, 1681–1690. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Grajeta, H. The role of manganese in etiopathogenesis and prevention of selected diseases. Postępy Hig. Med. Dośw. 2012, 66, 549–553. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Pang, X.; Tang, B.; Lin, L. Synthesis of chondroitin sulfate magnesium for osteoarthritis treatment. Carbohydr. Polym. 2019, 212, 387–394. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Gabr, S.A.; Al-Eisa, E.S.; Alghadir, M.H. Correlation between bone mineral density and serum trace elements in response to supervised aerobic training in older adults. Clin. Interv. Aging 2016, 265. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Hong, L.; Fu, S.; Jiang, W.; Jones, A.; Nie, L.H.; Zheng, W. Manganese accumulation in bone following chronic exposure in rats: Steady-state concentration and half-life in bone. Toxicol. Lett. 2014, 229, 93–100. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Liu, Y.; Koltick, D.; Byrne, P.; Wang, H.; Zheng, W.; Nie, L.H. Development of a transportable neutron activation analysis system to quantify manganese in bonein vivo: Feasibility and methodology. Physiol. Meas. 2013, 34, 1593–1609. [Google Scholar] [CrossRef]
- Sengupta, P. A Scientific Review of Age Determination for a Laboratory Rat: How Old Is It in Comparison with Human Age? Available online: https://www.semanticscholar.org/paper/A-Scientific-Review-of-Age-Determination-for-a-Rat%3A-Sengupta/0127c296097a86c47bea00957812b4fb4f2de943 (accessed on 19 June 2020).
- Smith, D.; Gwiazda, R.; Bowler, R.; Roels, H.; Park, R.; Taicher, C.; Lucchini, R. Biomarkers of Mn exposure in humans. Am. J. Ind. Med. 2007, 50, 801–811. [Google Scholar] [CrossRef]
- Zheng, W.; Kim, H.; Zhao, Q. Comparative Toxicokinetics of Manganese Chloride and Methylcyclopentadienyl Manganese Tricarbonyl (MMT) in Sprague-Dawley Rats. Toxicol. Sci. 2000, 54, 295–301. [Google Scholar] [CrossRef]
- Wang, D.; Du, X.; Zheng, W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol. Lett. 2008. [Google Scholar] [CrossRef]
- Rolle-McFarland, D.; Liu, Y.; Zhou, J.; Mostafaei, F.; Zhou, Y.; Li, Y.; Fan, Q.; Zheng, W.; Nie, L.; Wells, E. Development of a Cumulative Exposure Index (CEI) for Manganese and Comparison with Bone Manganese and Other Biomarkers of Manganese Exposure. Int. J. Environ. Res. Public. Health 2018, 15, 1341. [Google Scholar] [CrossRef]
- Strause, L.; Saltman, P. Role of Manganese in Bone Metabolism. Nutr. Bioavailab. Manganese 1987, 354, 46–55. [Google Scholar]
- Reginster, J.-Y.; Strause, L.G.; Saltman, P.; Franchimont, P. Trace elements and postmenopausal osteoporosis: A preliminary report of decreased serum manganese. Med. Sci. Res. 1988, 16, 337–338. [Google Scholar]
- Kara, E. Effect of a three-month football training program on trace element metabolism of boys in the eight to twelve age group. Afr. J. Biotechnol. 2012, 11, 169–172. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Lamari, F.N.; Theocharis, A.D.; Schuschke, D.A.; Karamanos, N.K.; Klimis-Zacas, D.J. Dietary manganese affects the concentration, composition and sulfation pattern of heparan sulfate glycosaminoglycans in Sprague-Dawley rat aorta. Biometals 2006, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, J.M.; González-Reimers, E.; DeLaVega-Prieto, M.J.; del Carmen Durán-Castellón, M.; Viña-Rodríguez, J.; Galindo-Martín, L.; Alvisa-Negrín, J.; Santolaria-Fernández, F. Relative and Combined Effects of Ethanol and Protein Deficiency on Bone Manganese and Copper. Biol. Trace Elem. Res. 2012, 147, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Budis, H.; Kalisinska, E.; Lanocha, N.; Kosik-Bogacka, D.I. The Concentration of Manganese, Iron and Strontium in Bone of Red Fox Vulpes vulpes (L. 1758). Biol. Trace Elem. Res. 2013, 155, 361–369. [Google Scholar] [CrossRef]
- Zhao-jun, W.; Lin, W.; Zhen-yong, W.; Jian, W.; Ran, L. Effects of manganese deficiency on serum hormones and biochemical markers of bone metabolism in chicks. J. Bone Miner. Metab. 2012. [Google Scholar] [CrossRef]
- Aljawadi, Z.A.M. The Relationship between Zinc, Copper and Manganese Levels on Bone Formation, Which Increases the Risk of Uterine Cancer. Eurasia Proc. Sci. Technol. Eng. Math. 2018, 4, 108–112. [Google Scholar]
- Schwarz, G.; Belaidi, A.A. Molybdenum in Human Health and Disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; pp. 415–450. ISBN 978-94-007-7500-8. [Google Scholar]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Exploratory analysis of the potential relationship between urinary molybdenum and bone mineral density among adult men and women from NHANES 2007–2010. Chemosphere 2016, 164, 677–682. [Google Scholar] [CrossRef]
- Maciejewski, R.; Glickman, N.; Moore, G.; Zheng, C.; Tyner, B.; Cleveland, W.; Ebert, D.; Glickman, L. Companion Animals as Sentinels for Community Exposure to Industrial Chemicals: The Fairburn, GA, Propyl Mercaptan Case Study. Public Health Rep. 2008, 123, 333–342. [Google Scholar] [CrossRef]
- Lanocha, N.; Kalisinska, E.; Kosik-Bogacka, D.I.; Budis, H. Evaluation of Dog Bones in the Indirect Assessment of Environmental Contamination with Trace Elements. Biol. Trace Elem. Res. 2012, 147, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.; Havemeyer, A.; Clement, B. The mammalian molybdenum enzymes of mARC. J. Biol. Inorg. Chem. JBIC 2015, 20, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. Metabolism of Molybdenum. In Metallomics and the Cell; Banci, L., Ed.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; pp. 503–528. ISBN 978-94-007-5561-1. [Google Scholar]
- Mendel, R.R. Cell biology of molybdenum. BioFactors 2009, 35, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Serpe, F.P.; Russo, R.; Simone, A.D.; Florio, S.; Esposito, M.; Severino, L. Levels of heavy metals in liver and kidney of dogs from urban environment. Open Vet. J. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Schmidt, P.L. Companion Animals as Sentinels for Public Health. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 241–250. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Detvanova, L.; Sirotkin, A.V.; Toman, R.; Kolesarova, A. In vitro changes in secretion activity of rat ovarian fragments induced by molybdenum. Physiol. Res. 2014, 63, 807–809. [Google Scholar] [CrossRef]
- Mendel, R.R. Biology of the molybdenum cofactor. J. Exp. Bot. 2007, 58, 2289–2296. [Google Scholar] [CrossRef]
- Small, R.E. Uses and limitations of bone mineral density measurements in the management of osteoporosis. MedGenMed Medscape Gen. Med. 2005, 7, 3. [Google Scholar]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Nelson, H.D.; Haney, E.M.; Dana, T.; Bougatsos, C.; Chou, R. Screening for Osteoporosis: An Update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2010, 153, 99–111. [Google Scholar] [CrossRef]
- Manolagas, S.C. Steroids and osteoporosis: The quest for mechanisms. J. Clin. Investig. 2013, 123, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. The mutual dependence between bone and gonads. J. Endocrinol. 2012, 213, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Lee, H.; Leder, B.Z.; Burnett-Bowie, S.-A.M.; Goldstein, D.W.; Hahn, C.W.; Hirsch, S.C.; Linker, A.; Perros, N.; Servais, A.B.; et al. Gonadal steroid–dependent effects on bone turnover and bone mineral density in men. J. Clin. Investig. 2016, 126, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J. Sex Steroids and the Construction and Conservation of the Adult Skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.S.; Hwang, T.I.S.; Hsui, Y.S.; Lin, Y.C.; Chen, H.E.; Chen, G.C.; Liao, C.H. Transdermal testosterone gel increases serum testosterone levels in hypogonadal men in Taiwan with improvements in sexual function. Int. J. Impot. Res. 2007, 19, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Meeker, J.D. Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the United States National Health and Nutrition Examination Survey 2011-2012. Fertil. Steril. 2015. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Bouillon, R. Androgens and bone. Calcif. Tissue Int. 1995, 56, 341–346. [Google Scholar] [CrossRef]
- Tang, Q.; Fu, W.; Zhang, M.; Wang, E.; Shan, L.; Chai, X.; Pang, J.; Wang, X.; Xu, X.; Xu, L.; et al. Novel androgen receptor antagonist identified by structure-based virtual screening, structural optimization, and biological evaluation. Eur. J. Med. Chem. 2020, 192, 112156. [Google Scholar] [CrossRef]
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 1568–1579. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpilowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Unice, K.M.; Monnot, A.D.; Gaffney, S.H.; Tvermoes, B.E.; Thuett, K.A.; Paustenbach, D.J.; Finley, B.L. Inorganic cobalt supplementation: Prediction of cobalt levels in whole blood and urine using a biokinetic model. Food Chem. Toxicol. 2012, 50, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Münch, H.; Jacobsen, S.; Olesen, J.; Menné, T.; Søballe, K.; Johansen, J.; Thyssen, J. The association between metal allergy, total knee arthroplasty, and revision: Study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015, 86, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Von Schewelov, T.; Sanzén, L. Catastrophic failure due to aggressive metallosis 4 years after hip resurfacing in a woman in her forties—A case report. Acta Orthop. 2010, 81, 402–404. [Google Scholar] [CrossRef]
- Langton, D.J.; Sidaginamale, R.P.; Joyce, T.; Bowsher, J.G.; Holland, J.P.; Deehan, D.J.; Nargol, A.V.F.; Natu, S. Aseptic lymphocyte-dominated vasculitis-associated lesions are related to changes in metal ion handling in the joint capsules of metal-on-metal hip arthroplasties. Bone Jt. Res. 2018. [Google Scholar] [CrossRef]
- Brandão, M.H.T.; Gontijo, B. Contact sensitivity to metals (chromium, cobalt and nickel) in childhood. An. Bras. Dermatol. 2012. [Google Scholar] [CrossRef]
- Andrews, R.E.; Shah, K.M.; Wilkinson, J.M.; Gartland, A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: Implications for skeletal health. Bone 2011, 49, 717–723. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Anissian, L.; Stark, A.; Dahlstrand, H.; Granberg, B.; Good, V.; Bucht, E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop. Scand. 2002, 73, 369–374. [Google Scholar] [CrossRef]
- Zofková, I.; Davis, M.A.; Blahoš, J. Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiol. Res. 2017. [Google Scholar] [CrossRef]




| Element | Element Concentration in Feed (Per 1 kg) |
| Ca | 10.0 g |
| Zn | 76.9 mg |
| Fe | 250.0 mg |
| Step | Time (min) | Power (%) | Minimum Pressure (atm) | Maximum Pressure (atm) |
| 1 | 1 | 70 | 17 | 20 |
| 2 | 5 | 80 | 27 | 30 |
| 3 | 6 | 90 | 36 | 39 |
| ICP-MS Nexion 300D | |
|---|---|
| RF power | 1350 W |
| nebulizer gas flow (Ar) | 0.9 l/min |
| dwell time | 50 ms |
| readings | 5 |
| sweeps | 1 |
| replicates | 3 |
| monitored isotopes | 24Mg, 27Al, 39K, 43Ca, 51V, 55Mn, 57Fe, 59Co, 63Cu, 60Ni, 66Zn, 78Se, 88Sr, 95Mo, 111Cd, 208Pb |
| Diets | The Animals’ Body Weight Gain (g) (Week 10–21) |
|---|---|
| ExpSt | 117.4 ± 31.2 |
| ExpZn (4.6 mg Zn/mL) | 101.8 ± 11.0 |
| ExpCa (75 mg Ca/mL) | 102.4 ± 11.6 |
| ExpFe (7.5 mg Fe/mL) | 120.6 ± 12.2 |
| CtrlSt | 100.2 ± 11.7 * |
| CtrlZn (4.6 mg Zn/mL) | 113.1 ± 14.9 |
| CtrlCa (75 mg Ca/mL) | 94.8 ± 8.5 |
| CtrlFe (7.5 mg Fe/mL) | 124.7 ± 10.6 * |
| Elements ↓ | CtrlSt ExpSt (n = 12) | CtrlZn ExpZn (n = 14) | CtrlFe ExpFe (n = 14) | CtrlCa ExpCa (n = 14) |
|---|---|---|---|---|
| Zn | 45.3 ± 4.3 | 53.0 ± 3.6 | 40.9 ± 2.0 | 45.3 ± 2.7 |
| 41.3 ± 6.0 | 40.6 ± 3.1 | 44.5 ± 2.9 | 43.2 ± 1.4 | |
| Fe | 1411 ± 158 | 1040 ± 176 | 961 ± 482 | 1371 ± 228 |
| 1281 ± 184 | 1197 ± 449 | 1224 ± 169 | 1381 ± 118 | |
| Ca | 291 ± 38 | 230 ± 32 | 293 ± 148 | 280 ± 34 |
| 221 ± 18 | 226 ± 24 | 256 ± 33 | 315 ± 67 | |
| Cu | 0.494 ± 0.261 | 0.386 ± 0.03 | 0.273 ± 0,013 | 0.343 ± 0.026 |
| 0.272 ± 0.084 | 0.241 ± 0.04 | 0.305 ± 0.044 | 0.312 ± 0.013 | |
| Mn | 0.160 ± 0.014 | 0.211 ± 0.015 | 0.158 ± 0.009 | 0.176 ± 0.018 |
| 0.140 ± 0.010 | 0.169 ± 0.031 | 0.208 ± 0.039 | 0.160 ± 0.012 | |
| Co | 0.136 ± 0.016 | 0.162 ± 0.018 | 0.131 ± 0.007 | 0.150 ± 0.01 |
| 0.106 ± 0.015 | 0.104 ±0.014 | 0.142 ± 0.005 | 0.141 ± 0.008 | |
| Mo | 0.047 ± 0.010 | 0.056 ± 0.036 | 0.029 ± 0.008 | 0.033 ± 0.006 |
| 0.008 ± 0.005 | 0.027 ± 0.015 | 0.047 ± 0.024 | 0.035 ± 0.002 | |
| K | 2018 ± 206 | 1758 ± 281 | 1756 ± 631 | 1918 ± 169 |
| 1753 ± 484 | 1732 ± 205 | 2027 ± 255 | 2028 ± 236 | |
| Sr | 70.1 ± 2.7 | 57.2 ± 8.6 | 67.4 ± 25 | 67.8 ± 7.3 |
| 63.5 ± 5.8 | 62.2 ± 6.6 | 64.2 ± 7.01 | 72.1 ± 6.8 | |
| Ni | 3.71 ± 0.69 | 4.19 ± 0.53 | 4.22 ± 0.31 | 3.91 ± 0.28 |
| 3.36 ± 0.55 | 6.60 ± 7.06 | 3.83 ± 0.35 | 3.77 ± 0.25 |
| Elements | ExpSt:CtrlSt | ExpZn:CtrlZn | ExpFe:CtrlFe | ExpCa:CtrlCa | |
|---|---|---|---|---|---|
| Zn | ↓9% | ↓23% | ↑9% | ↓5% | |
| p value | ns | ns | 0.013 | ns | |
| Fe | ↓9% | ↑15% | ↑27% | ↑1% | |
| p value | ns | ns | ns | ns | |
| Ca | ↓24% | ↓2% | ↓13% | ↑13% | |
| p value | 0.002 | ns | ns | ns | |
| Cu | ↓45% | ↓38% | ↑12% | ↓9% | |
| p value | ns | 0.0001 | ns | ns | |
| Mn | ↓13% | ↓20% | ↑32% | ↓9% | |
| p value | 0.017 | 0.011 | 0.005 | ns | |
| Co | ↓22% | ↓36% | ↑8% | ↓6% | |
| p value | 0.007 | 0.0001 | 0.003 | ns | |
| Mo | ↓83% | ↓48% | ↑62% | ↑6% | |
| p value | 0.0001 | ns | ns | ns | |
| K | ↓13% | ↓2% | ↑15% | ↑6% | |
| p value | 0.002 | ns | ns | ns | |
| Sr | ↓9% | ↑9% | ↓5% | ↑6% | |
| p value | 0.029 | ns | ns | ns | |
| Ni | ↓9% | ↑58% | ↓9% | ↓4% | |
| p value | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrajnowska, D.; Jagielska, A.; Ruszczyńska, A.; Wagner, B.; Bielecki, W.; Bobrowska-Korczak, B. Title Changes in the Mineral Composition of Rat Femoral Bones Induced by Implantation of LNCaP Prostate Cancer Cells and Dietary Supplementation. Nutrients 2021, 13, 100. https://doi.org/10.3390/nu13010100
Skrajnowska D, Jagielska A, Ruszczyńska A, Wagner B, Bielecki W, Bobrowska-Korczak B. Title Changes in the Mineral Composition of Rat Femoral Bones Induced by Implantation of LNCaP Prostate Cancer Cells and Dietary Supplementation. Nutrients. 2021; 13(1):100. https://doi.org/10.3390/nu13010100
Chicago/Turabian StyleSkrajnowska, Dorota, Agata Jagielska, Anna Ruszczyńska, Barbara Wagner, Wojciech Bielecki, and Barbara Bobrowska-Korczak. 2021. "Title Changes in the Mineral Composition of Rat Femoral Bones Induced by Implantation of LNCaP Prostate Cancer Cells and Dietary Supplementation" Nutrients 13, no. 1: 100. https://doi.org/10.3390/nu13010100
APA StyleSkrajnowska, D., Jagielska, A., Ruszczyńska, A., Wagner, B., Bielecki, W., & Bobrowska-Korczak, B. (2021). Title Changes in the Mineral Composition of Rat Femoral Bones Induced by Implantation of LNCaP Prostate Cancer Cells and Dietary Supplementation. Nutrients, 13(1), 100. https://doi.org/10.3390/nu13010100







