IIAEK Targets Intestinal Alkaline Phosphatase (IAP) to Improve Cholesterol Metabolism with a Specific Activation of IAP and Downregulation of ABCA1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemicals
2.3. Cholesterol Absorption Assay for Caco-2 Cells
2.4. RNA Isalation from Caco-2 Cells and Real-Time PCR Using TaqMan Probe
2.5. RNA Isolation from Caco-2 Cells and Real-Time PCR
2.6. Western Blot Analysis of Caco-2 Cells
2.7. Transient Transfections and Luciferase Assay Using Caco-2 Cells
2.8. Alkaline Phosphatase Measurement
2.9. Preparation of Rat Intestinal Mucosal Protein
2.10. Preparation of Intestinal Lipid Rafts from Caco-2 Cells
2.11. Photoaffinity Labeling and Nano LC-MS/MS Analyses
2.12. Statistical Analysis
3. Results
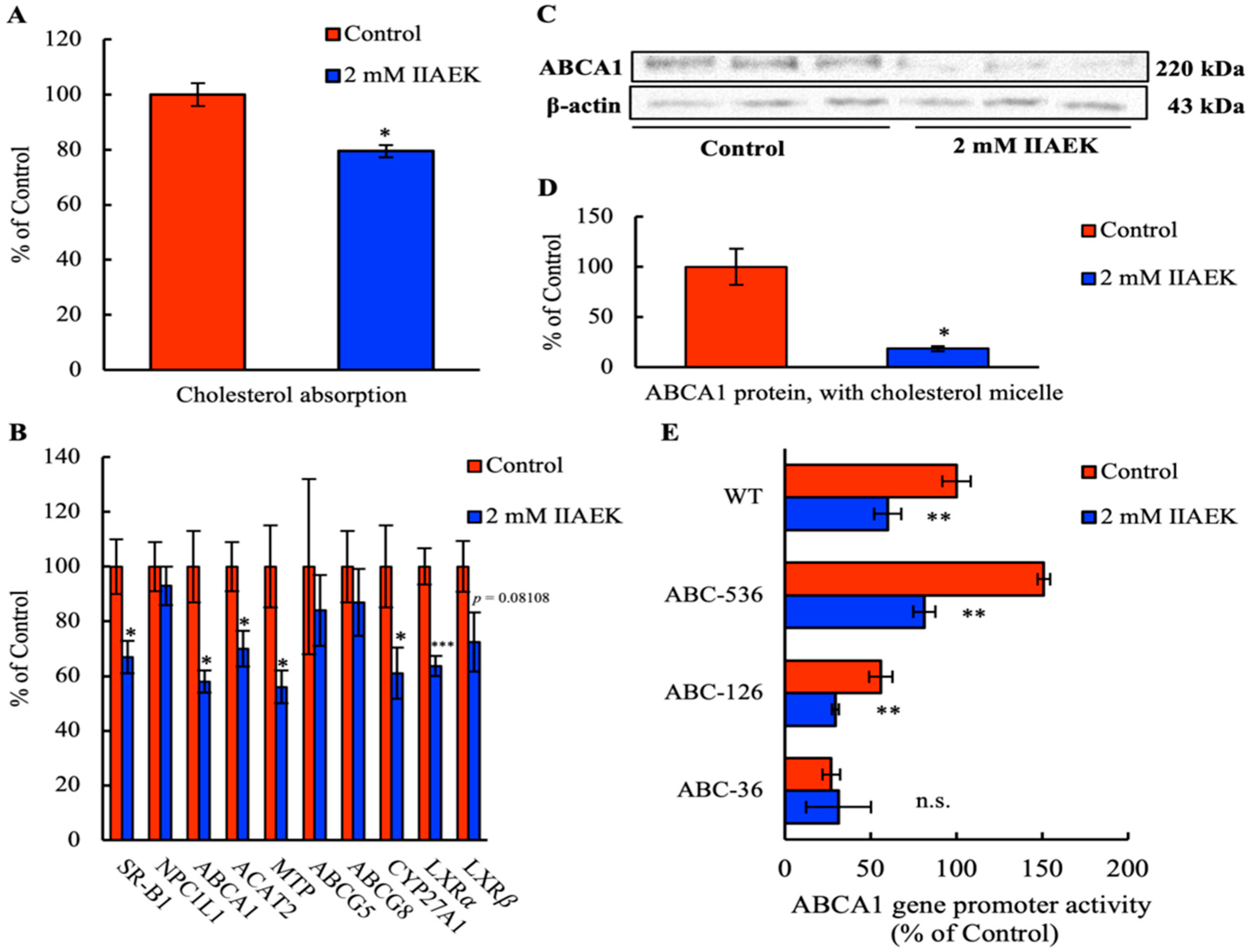
3.1. Effect of IIAEK on Cholesterol Absorption in Caco-2 Cells
3.2. Effect of IIAEK on the mRNA Levels of Cholesterol Metabolism-Associated Genes in Caco-2 Cells
3.3. Effect of IIAEK on ABCA1 Protein Levels in Caco-2 Cells
3.4. Effect of IIAEK on ABCA1 Gene Promoter Activity in Caco-2 Cells
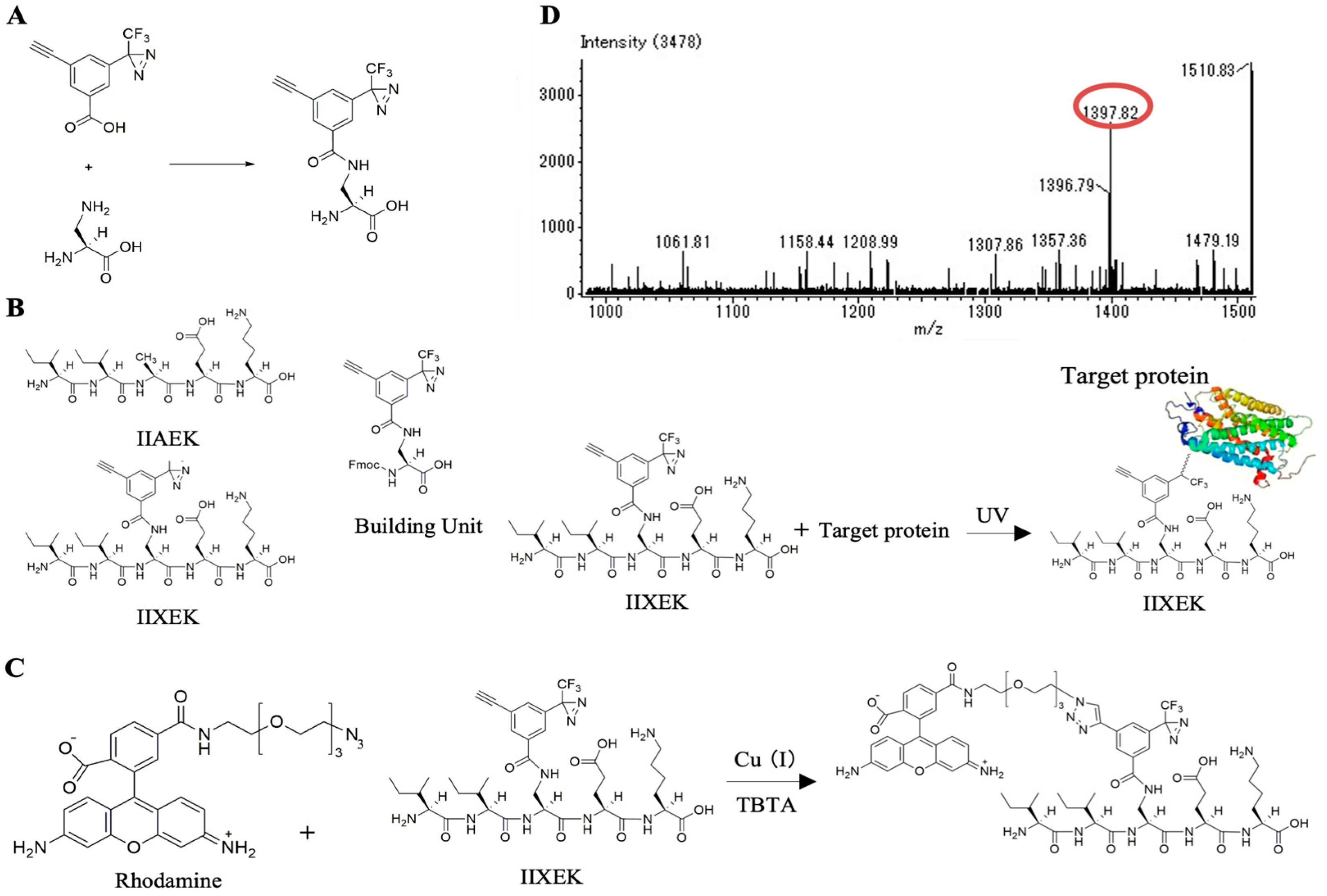
3.5. Chemical Synthesis of IIXEK, a Molecular Probe for Photoaffinity Labeling
3.6. Photoaffinity Labeling and MS Analysis of the Intestinal Lipid Raft Fractions Derived from Caco-2 Cells and Rat Intestinal Mucosal Protein by a Novel Molecular Probe, IIXEK
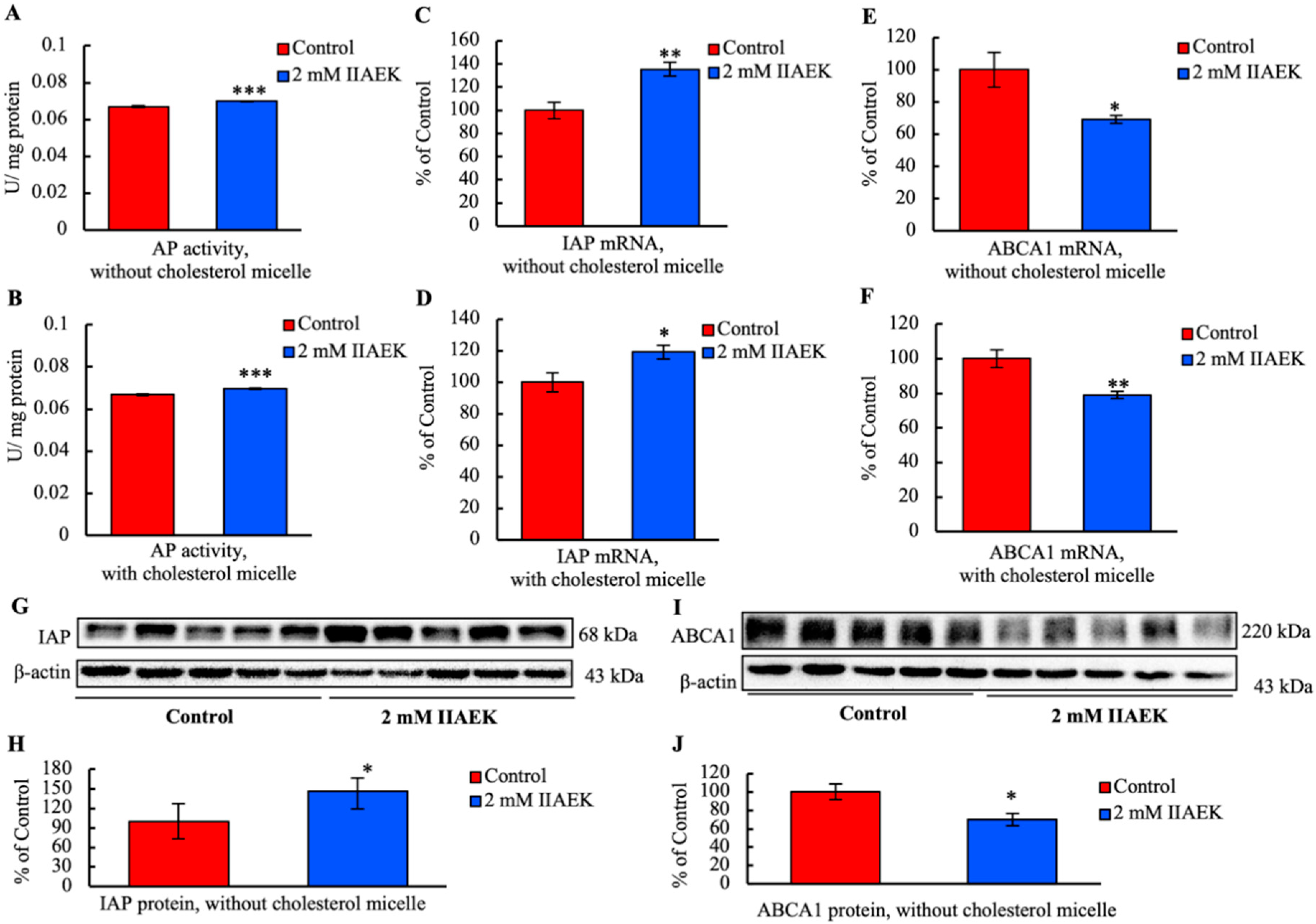
3.7. IIAEK Induces IAP Activation and Down-Regulation of ABCA1 in Caco-2 Cells
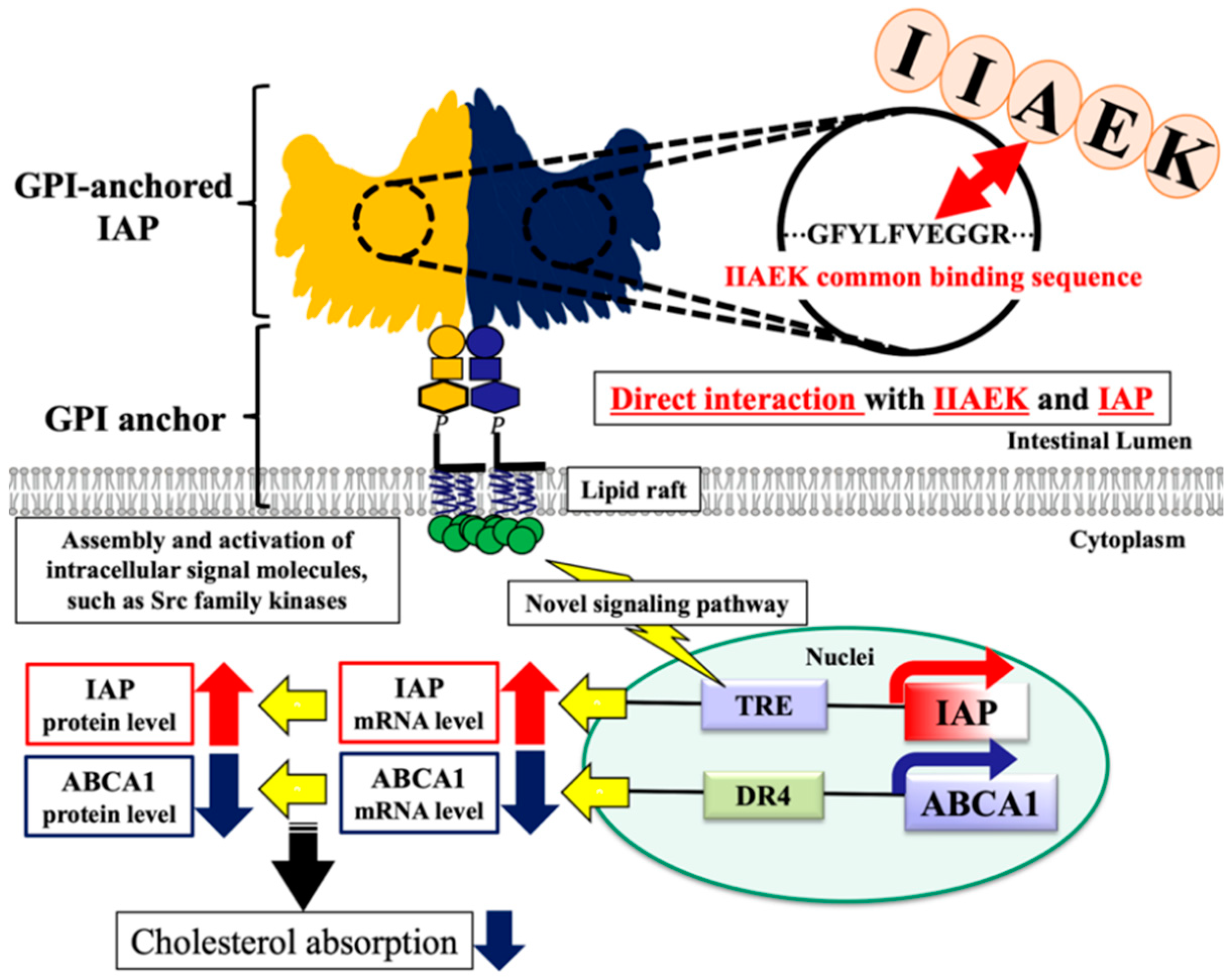
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ginsberg, H.N.; Barr, S.L.; Gilbert, A.; Karmally, W.; Deckelbaum, R.; Kaplan, K.; Ramakrishnan, R.; Holleran, S.; Dell, R.B. Reduction of plasma cholesterol levels in normal men on an American Heart Association step 1 diet or a step 1 diet with added monounsaturated fat. N. Engl. J. Med. 1990, 322, 574–579. [Google Scholar] [CrossRef]
- Hori, G.; Wang, M.F.; Chan, Y.C.; Komatsu, T.; Wong, Y.; Chen, T.H.; Yamamoto, K.; Nagaoka, S.; Yamamoto, S. Soy protein hydrolysate with bound phospholipids reduces serum cholesterol levels in hypercholesterolemic adult male volunteers. Biosci. Biotechnol. Biochem. 2001, 65, 72–78. [Google Scholar] [CrossRef]
- Nagaoka, S.; Futamura, Y.; Miwa, K.; Awano, T.; Yamauchi, K.; Kanamaru, Y.; Kojima, T.; Kuwata, T. Identification of novel hypocholesterolemic peptides derived from bovine milk β-lactoglobulin. Biochem. Biophys. Res. Commun. 2001, 281, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K.; Cuthbert, J.A.; Willard, M.N.; Meidell, R.S. Adenovirus-mediated transfer of a gene encoding cholesterol 7α-hydroxylase into hamsters increases hepatic enzyme activity and reduces plasma total and low density lipoprotein cholesterol. J. Clin. Investig. 1995, 96, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K.; Cuthbert, J.A.; Willard, M.N.; Meidell, R.S. Overexpression of cholesterol 7α-hydroxylase (CYP7A) in mice lacking the low density lipoprotein (LDL) receptor gene. LDL transport and plasma LDL concentrations are reduced. J. Biol. Chem. 1998, 273, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Kondo, I.; Kanamaru, Y.; Nagaoka, S. A novel regulatory pathway for cholesterol degradation via lactostatin. Biochem. Biophys. Res. Commun. 2007, 352, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Thornton, E.R.; Westheimer, F.H. The photolysis of diazoacetylchymotrypsin. J. Biol. Chem. 1962, 237, 3006–3008. [Google Scholar]
- Tomohiro, T.; Hatanaka, Y. Diazirine-based multifunctional photo-probes for affinity-based elucidation of protein-ligand interaction. Heterocycles 2014, 89, 2697–2727. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper (I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper (I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Banno, A.; Wang, J.; Okada, K.; Mori, R.; Maihemuti, M.; Nagaoka, S. Identification of a novel cholesterol-lowering dipeptide, phenylalanine-proline (FP), and its down-regulation of intestinal ABCA1 in hypercholesterolemic rats and Caco-2 cells. Sci. Rep. 2019, 9, 19416. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Watt, K.; Mathur, S.N. TNF-α decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J. Lipid Res. 2010, 51, 1407–1415. [Google Scholar] [CrossRef]
- Kitamura, K.; Okada, Y.; Okada, K.; Kawaguchi, Y.; Nagaoka, S. Epigallocatechin gallate induces an up-regulation of LDL receptor accompanied by a reduction of PCSK9 via the annexin A2-independent pathway in HepG2 cells. Mol. Nutr. Food. Res. 2017, 61, 1600836. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Yamada, A.; Nakaoka, K.; Goseki-sone, M. 1-alpha, 25-Dihydroxyvitamin D3 up-regulates the expression of 2 types of human intestinal alkaline phosphatase alternative splicing variants in Caco-2 cells and may be an important regulator of their expression in gut homeostasis. Nutr. Res. 2017, 46, 59–67. [Google Scholar] [CrossRef]
- Ilboudo, S.; Fouche, E.; Rizzati, V.; Toè, A.M.; Gamet-Payrastre, L.; Guissou, P.I. In vitro impact of five pesticides alone or in combination on human intestinal cell line Caco-2. Toxicol. Rep. 2014, 1, 474–489. [Google Scholar] [CrossRef]
- Yuyama, K.; Sekino-Suzuki, N.; Sanai, Y.; Kasahara, K. Translocation of activated heterotrimeric G protein GαO to ganglioside-enriched detergent-resistant membrane rafts in developing cerebellum. J. Biol. Chem. 2007, 282, 26392–26400. [Google Scholar] [CrossRef]
- Nakamoto, K.; Akao, Y.; Ueno, Y. Diazirine-containing tag-free RNA probes for efficient RISC-loading and photoaffinity labeling of microRNA targets. Bioorg. Med. Chem. Lett. 2018, 28, 2906–2909. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; The Iowa State University Press: Ames, IA, USA, 1967. [Google Scholar]
- Hosoya, T.; Hiramatsu, T.; Ikemoto, T.; Aoyama, H.; Ohmae, T.; Endo, M.; Suzuki, M. Design of dantrolene-derived probes for radioisotope-free photoaffinity labeling of proteins involved in the physiological Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Bioorg. Med. Chem. Lett. 2005, 15, 1289–1294. [Google Scholar] [CrossRef]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta. 2005, 1735, 1–19. [Google Scholar] [CrossRef]
- Iqbal, J.; Parks, J.S.; Hussain, M.M. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J. Biol. Chem. 2013, 288, 30432–30444. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Rogers, C.E.; Harris, H. Expression of alkaline phosphatase loci in mammalian tissues. Proc. Natl. Acad. Sci. USA 1980, 77, 2857–2860. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019, 77, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Yedlin, S.T.; Young, G.P.; Seetharam, B.; Seetharam, S.; Alpers, D.H. Characterization and comparison of soluble and membranous forms of intestinal alkaline phosphatase from the suckling rat. J. Biol. Chem. 1981, 256, 5620–5626. [Google Scholar] [PubMed]
- Sogabe, N.; Mizoi, L.; Asahi, K.; Ezawa, I.; Goseki-Sone, M. Enhancement by lactose of intestinal alkaline phosphatase expression in rats. Bone 2004, 35, 249–255. [Google Scholar] [CrossRef]
- Xie, Q.M.; Zhang, Y.; Mahmood, S.; Alpers, D.H. Rat intestinal alkaline phosphatase II messenger RNA is present in duodenal crypt and villus cells. Gastroenterology 1997, 112, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Butterworth, P.J. Molecular properties of rat intestinal alkaline phosphatase. Biochim. Biophys. Acta 1976, 446, 105–114. [Google Scholar] [CrossRef]
- Hirano, K.; Sugiura, M.; Miki, K.; Iino, S.; Suzuki, H.; Oda, T. Characterization of Tissue-specific Isozyme of Alkaline Phosphatase from Human Placenta and Intestine. Chem. Pharm. Bull. 1977, 25, 2524–2529. [Google Scholar] [CrossRef]
- Ghosh, K.; Tagore, D.M.; Anumula, R.; Lakshmaiah, B.; Kumar, P.P.B.S.; Singaram, S.; Matan, T.; Kallipatti, S.; Selvam, S.; Krishnamurthy, P.; et al. Crystal structure of rat intestinal alkaline phosphatase—Role of crown domain in mammalian alkaline phosphatase. J. Struct. Biol. 2013, 184, 182–192. [Google Scholar] [CrossRef]
- Nakano, T.; Inoue, I.; Alpers, D.H.; Akiba, Y.; Katayama, S.; Shinozaki, R.; Kaunitz, J.D.; Ohshima, S.; Akita, M.; Takahashi, S.; et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 207–214. [Google Scholar] [CrossRef]
- Harada, T.; Koyama, I.; Sato, K.; Komoda, T. Induction of rat alkaline phosphatase isozymes bearing a glycan-phosphatidylinositol anchor modified by in vivo treatment with a benzimidazole derivative linked to ethylbenzene. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 127, 193–202. [Google Scholar] [CrossRef]
- Suzuki, K.; Kasai, R.; Hirosawa, K.; Nemoto, Y.; Ishibashi, M.; Miwa, Y.; Fujiwara, T.; Kusumi, A. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 2012, 8, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Won, H.; Adams, D.J.; Lee, S.K. CD55 regulates bone mass in mice by modulating RANKL-mediated Rac signaling and osteoclast function. J. Bone Miner. Res. 2020, 35, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Bressendorff, S.; Troelsen, J.T.; Olsen, J. Differentiation-dependent activation of the human intestinal alkaline phosphatase promoter by HNF-4 in intestinal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Carr, A.; Corner, G.A.; Tögel, L.; Dávaos-Salas, M.; Tran, H.; Chueh, A.C.; Al-Obaidi, S.; Chionh, F.; Ahmad, N.; et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like Factor 5 (KLF5)-dependent manner. J. Biol. Chem. 2014, 289, 25306–25316. [Google Scholar] [CrossRef]
- Malo, M.S.; Zhang, W.; Alkhoury, F.; Pushpakaran, P.; Abedrapo, M.A.; Mozumder, M.; Fleming, E.; Siddique, A.; Henderson, J.W.; Hodin, R.A. Thyroid hormone positively regulates the enterocyte differentiation marker intestinal alkaline phosphatase gene via an atypical response element. Mol. Endocrinol. 2004, 18, 1941–1962. [Google Scholar] [CrossRef][Green Version]
- Malo, M.S.; Mozumder, M.; Zhang, X.B.; Biswas, S.; Chen, A.; Bai, L.; Merchant, J.L.; Hodin, R.A. Intestinal alkaline phosphatase gene expression is activated by ZBP-89. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 737–746. [Google Scholar] [CrossRef][Green Version]
- Alkhoury, F.; Malo, M.S.; Mozumder, M.; Mostafa, G.; Hodin, R.A. Differential regulation of intestinal alkaline phosphatase gene expression by Cdx1 and Cdx2. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, 285–290. [Google Scholar] [CrossRef][Green Version]
- Narisawa, S.; Huang, L.; Iwasaki, A.; Hasegawa, H.; Alpers, D.H.; Millán, J.L. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol. Cell. Biol. 2003, 23, 7525–7530. [Google Scholar] [CrossRef]
- Chen, K.T.; Malo, M.S.; Moss, M.S.; Zeller, S.; Johnson, P.; Ebrahimi, F.; Mostafa, G.; Alam, S.N.; Ramasamy, S.; Warren, H.S.; et al. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 467–475. [Google Scholar] [CrossRef]
- Moss, A.K.; Hamarneh, S.R.; Mohamed, M.M.R.; Ramasamy, S.; Yammine, H.; Patel, P.; Kaliannan, K.; Alam, S.N.; Muhammad, N.; Moaven, O.; et al. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Mizumori, M.; Ham, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D.; Akiba, Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J. Physiol. 2009, 587, 3651–3663. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, D.; Huo, H.; Zhang, W.; Adiliaghdam, F.; Morrison, S.; Ramirez, J.M.; Gul, S.S.; Hamarneh, S.R.; Hodin, R.A. Intestinal alkaline phosphatase regulates tight junction protein levels. J. Am. Coll. Surg. 2016, 222, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Widmaier, E.P. Involvement of CD36 and intestinal alkaline phosphatases in fatty acid transport in enterocytes, and the response to a high-fat diet. Life Sci. 2011, 88, 384–391. [Google Scholar] [CrossRef]
- Kaliannan, K.; Hamarneh, S.R.; Economopoulos, K.P.; Alam, S.N.; Moaven, O.; Patel, P.; Malo, N.S.; Ray, M.; Abtahi, S.M.; Muhammad, N.; et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 7003–7008. [Google Scholar] [CrossRef]
- Kühn, F.; Adiliaghdam, F.; Cavallaro, P.M.; Hamarneh, S.R.; Tsurumi, A.; Hoda, R.S.; Munoz, A.R.; Dhole, Y.; Ramirez, J.M.; Liu, E.; et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 2020, 5, e134049. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, A.; Hisamatsu, K.; Okumura, N.; Sugimitsu, Y.; Yanase, E.; Ueno, Y.; Nagaoka, S. IIAEK Targets Intestinal Alkaline Phosphatase (IAP) to Improve Cholesterol Metabolism with a Specific Activation of IAP and Downregulation of ABCA1. Nutrients 2020, 12, 2859. https://doi.org/10.3390/nu12092859
Takeuchi A, Hisamatsu K, Okumura N, Sugimitsu Y, Yanase E, Ueno Y, Nagaoka S. IIAEK Targets Intestinal Alkaline Phosphatase (IAP) to Improve Cholesterol Metabolism with a Specific Activation of IAP and Downregulation of ABCA1. Nutrients. 2020; 12(9):2859. https://doi.org/10.3390/nu12092859
Chicago/Turabian StyleTakeuchi, Asahi, Kentaro Hisamatsu, Natsuki Okumura, Yuki Sugimitsu, Emiko Yanase, Yoshihito Ueno, and Satoshi Nagaoka. 2020. "IIAEK Targets Intestinal Alkaline Phosphatase (IAP) to Improve Cholesterol Metabolism with a Specific Activation of IAP and Downregulation of ABCA1" Nutrients 12, no. 9: 2859. https://doi.org/10.3390/nu12092859
APA StyleTakeuchi, A., Hisamatsu, K., Okumura, N., Sugimitsu, Y., Yanase, E., Ueno, Y., & Nagaoka, S. (2020). IIAEK Targets Intestinal Alkaline Phosphatase (IAP) to Improve Cholesterol Metabolism with a Specific Activation of IAP and Downregulation of ABCA1. Nutrients, 12(9), 2859. https://doi.org/10.3390/nu12092859





