Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly
Abstract
1. Introduction
2. Materials and Methods
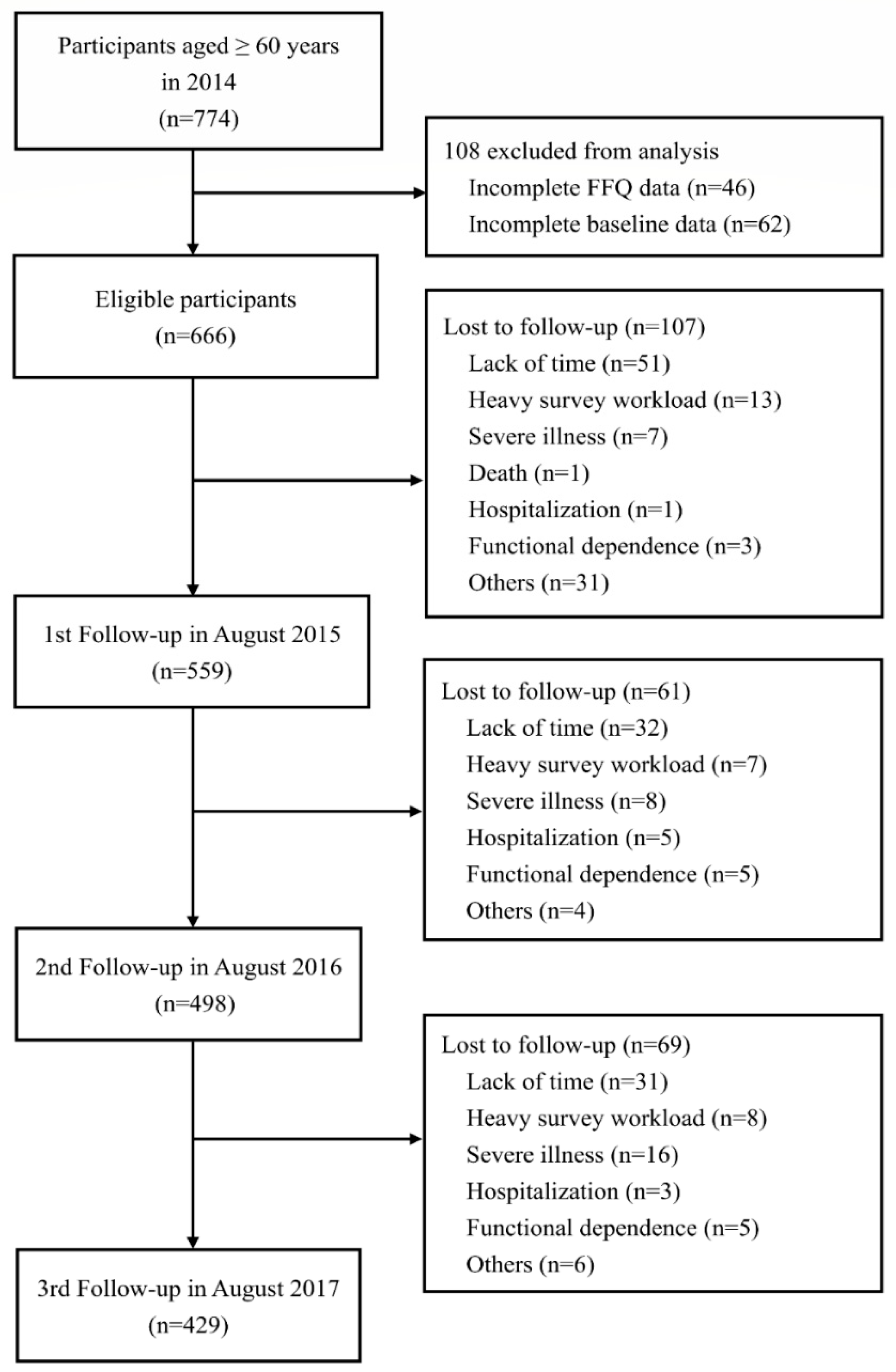
2.1. Study Design and Participants
2.2. Definition of Social Frailty
2.3. Measures
2.4. Dietary Assessment
2.5. Diet Quality and Diet Quantity
2.6. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
3.2. Effects of Social Frailty on Diet Quality and Diet Quantity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Makizako, H.; Tsutsumimoto, K.; Shimada, H.; Arai, H. Social frailty among community-dwelling older adults: Recommended assessments and implications. Ann. Geriatr. Med. Res. 2018, 22, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bunt, S.; Steverink, N.; Olthof, J.; van der Schans, C.P.; Hobbelen, J.S.M. Social frailty in older adults: A scoping review. Eur. J. Ageing 2017, 14, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Nakakubo, S.; Hotta, R.; Suzuki, T. Social frailty in community-dwelling older adults as a risk factor for disability. J. Am. Med. Dir. Assoc. 2015, 16, 1003.e7–1003.e11. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Arai, H. Social frailty predicts incident disability and mortality among community-dwelling japanese older adults. J. Am. Med. Dir. Assoc. 2018, 19, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, F.; Tang, Z. Social frailty is associated with physical functioning, cognition, and depression, and predicts mortality. J. Nutr. Health Aging 2018, 22, 989–995. [Google Scholar] [CrossRef]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Association of social frailty with both cognitive and physical deficits among older people. J. Am. Med. Dir. Assoc. 2017, 18, 603–607. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Lee, S. Social frailty leads to the development of physical frailty among physically non-frail adults: A four-year follow-up longitudinal cohort study. Int. J. Environ. Res. Public Health 2018, 15, 490. [Google Scholar] [CrossRef]
- Andrew, M.K.; Mitnitski, A.B.; Rockwood, K. Social vulnerability, frailty and mortality in elderly people. PLoS ONE 2008, 3, e2232. [Google Scholar] [CrossRef]
- Wallace, L.M.; Theou, O.; Pena, F.; Rockwood, K.; Andrew, M.K. Social vulnerability as a predictor of mortality and disability: Cross-country differences in the survey of health, aging, and retirement in Europe (SHARE). Aging Clin. Exp. Res. 2015, 27, 365–372. [Google Scholar] [CrossRef]
- Armstrong, J.J.; Andrew, M.K.; Mitnitski, A.; Launer, L.J.; White, L.R.; Rockwood, K. Social vulnerability and survival across levels of frailty in the honolulu-asia aging study. Age Ageing 2015, 44, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Pek, K.; Chew, J.; Lim, J.P.; Yew, S.; Tan, C.N.; Yeo, A.; Ding, Y.Y.; Lim, W.S. Social frailty is independently associated with mood, nutrition, physical performance, and physical activity: Insights from a theory-guided approach. Int. J. Environ. Res. Public Health 2020, 17, 4239. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Verlaan, S.; Ligthart-Melis, G.C.; Wijers, S.L.J.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. High prevalence of physical frailty among community-dwelling malnourished older adults-a systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2017, 18, 374–382. [Google Scholar] [CrossRef]
- Streicher, M.; van Zwienen-Pot, J.; Bardon, L.; Nagel, G.; Teh, R.; Meisinger, C.; Colombo, M.; Torbahn, G.; Kiesswetter, E.; Flechtner-Mors, M.; et al. Determinants of incident malnutrition in community-dwelling older adults: A manuel multicohort meta-analysis. J. Am. Geriatr. Soc. 2018, 66, 2335–2343. [Google Scholar] [CrossRef]
- Fekete, C.; Weyers, S. Social inequalities in nutrition: Evidence, causes and interventions. Bundesgesundheitsblatt Gesundh. Gesundh. 2016, 59, 197–205. [Google Scholar] [CrossRef]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Social isolation and risk for malnutrition among older people. Geriatr. Gerontol. Int. 2017, 17, 286–294. [Google Scholar] [CrossRef]
- Arai, K.; Sakakibara, H. Malnutrition and social isolation among elderly residents of city public housing. Nihon Koshu Eisei Zasshi 2015, 62, 379–389. [Google Scholar] [CrossRef]
- Rashidi Pour Fard, N.; Amirabdollahian, F.; Haghighatdoost, F. Dietary patterns and frailty: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 498–513. [Google Scholar] [CrossRef]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. Dietary patterns and risk of frailty in chinese community-dwelling older people in Hong Kong: A prospective cohort study. Nutrients 2015, 7, 7070–7084. [Google Scholar] [CrossRef]
- Fukuda, Y.; Ishikawa, M.; Yokoyama, T.; Hayashi, T.; Nakaya, T.; Takemi, Y.; Kusama, K.; Yoshiike, N.; Nozue, M.; Yoshiba, K.; et al. Physical and social determinants of dietary variety among older adults living alone in Japan. Geriatr. Gerontol. Int. 2017, 17, 2232–2238. [Google Scholar] [CrossRef]
- Yoshiba, K.; Takemi, Y.; Ishikawa, M.; Yokoyama, T.; Nakaya, T.; Murayama, N. Relationship between dietary diversity and food access among elderly living alone in Saitama Prefecture. Nihon Koshu Eisei Zasshi 2015, 62, 707–718. [Google Scholar] [CrossRef]
- Arakawa Martins, B.; Visvanathan, R.; Barrie, H.; Huang, C.H.; Matsushita, E.; Okada, K.; Satake, S.; Uno, C.; Kuzuya, M. Frailty prevalence using frailty index, associated factors and level of agreement among frailty tools in a cohort of Japanese older adults. Arch. Gerontol. Geriatr. 2019, 84. [Google Scholar] [CrossRef]
- Matsushita, E.; Okada, K.; Ito, Y.; Satake, S.; Shiraishi, N.; Hirose, T.; Kuzuya, M. Characteristics of physical prefrailty among Japanese healthy older adults. Geriatr. Gerontol. Int. 2017, 17, 1568–1574. [Google Scholar] [CrossRef]
- Huang, C.H.; Martins, B.A.; Okada, K.; Matsushita, E.; Uno, C.; Satake, S.; Kuzuya, M. A 3-year prospective cohort study of dietary patterns and frailty risk among community-dwelling older adults. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psych. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Cereda, E. Mini nutritional assessment. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 29–41. [Google Scholar] [CrossRef]
- Takahashi, K.; Yoshimura, Y.; Kaimoto, T.; Kunii, D.; Komatsu, T.; Yamamoto, S. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake. Jpn. J. Nutr. Diet. 2001, 59, 221–232. [Google Scholar] [CrossRef]
- Maruyama, K.; Ikeda, A.; Ishihara, J.; Takachi, R.; Sawada, N.; Shimazu, T.; Nakamura, K.; Tanaka, J.; Yamaji, T.; Iwasaki, M.; et al. Food frequency questionnaire reproducibility for middle-aged and elderly Japanese. Asia Pac. J. Clin. Nutr. 2019, 28, 362–370. [Google Scholar] [CrossRef]
- Ministry of Education, Culture, Sports, Science and Technology. Standard Tables of Food Composition in Japan 2015, 7th ed.; Office for Resources, Policy Division Science and Technology Policy Bureau: Tokyo, Japan, 2015.
- Kumagai, S.; Watanabe, S.; Shibata, H.; Amano, H.; Fujiwara, Y.; Shinkai, S.; Yoshida, H.; Suzuki, T.; Yukawa, H.; Yasumura, S.; et al. Effects of dietary variety on declines in high-level functional capacity in elderly people living in a community. Nihon Koshu Eisei Zasshi (Jpn. J. Public Health) 2003, 50, 1117–1124. [Google Scholar] [CrossRef]
- Motokawa, K.; Watanabe, Y.; Edahiro, A.; Shirobe, M.; Murakami, M.; Kera, T.; Kawai, H.; Obuchi, S.; Fujiwara, Y.; Ihara, K.; et al. Frailty severity and dietary variety in Japanese older persons: A cross-sectional study. J. Nutr. Health Aging 2018, 22, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef]
- Manore, M.M. Exercise and the Institute of Medicine recommendations for nutrition. Curr. Sp. Med. Rep. 2005, 4, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Food composition tables of Japan and the nutrient table/database. J. Nutr. Sci. Vitaminol. 2015, 61, S25–S27. [Google Scholar] [CrossRef]
- Lemmens, H.J.; Brodsky, J.B.; Bernstein, D.P. Estimating ideal body weight—A new formula. Obes. Surg. 2005, 15, 1082–1083. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y.; Albert, P.S. Models for longitudinal data: A generalized estimating equation approach. Biometrics 1988, 44, 1049–1060. [Google Scholar] [CrossRef]
- Huque, M.H.; Carlin, J.B.; Simpson, J.A.; Lee, K.J. A comparison of multiple imputation methods for missing data in longitudinal studies. BMC Med. Res. Methodol. 2018, 18, 168. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81, 258p. [Google Scholar]
- Bennett, E.; Peters, S.A.E.; Woodward, M. Sex differences in macronutrient intake and adherence to dietary recommendations: Findings from the UK biobank. BMJ Open. 2018, 8, e020017. [Google Scholar] [CrossRef]
- Kalousova, L. Social isolation as a risk factor for inadequate diet of older Eastern Europeans. Int. J. Public Health 2014, 59, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.; Kitamura, A.; Seino, S.; Nishi, M.; Tomine, Y.; Taniguchi, Y.; Yokoyama, Y.; Narita, M.; Shinkai, S. Relationship between eating alone and dietary variety among urban older Japanese adults. Nihon Koshu Eisei Zasshi 2018, 65, 744–754. [Google Scholar] [CrossRef]
- Otto, M.C.d.O.; Padhye, N.S.; Bertoni, A.G.; Jacobs, D.R., Jr.; Mozaffarian, D. Everything in moderation—Dietary diversity and quality, central obesity and risk of diabetes. PLoS ONE 2015, 10, e0141341. [Google Scholar] [CrossRef]
- Jayawardena, R.; Byrne, N.M.; Soares, M.J.; Katulanda, P.; Yadav, B.; Hills, A.P. High dietary diversity is associated with obesity in Sri Lankan adults: An evaluation of three dietary scores. BMC Public Health 2013, 13, 314. [Google Scholar] [CrossRef]
- Narita, M.; Kitamura, A.; Takemi, Y.; Yokoyama, Y.; Morita, A.; Shinkai, S. Food diversity and its relationship with nutrient intakes and meal days involving staple foods, main dishes, and side dishes in community-dwelling elderly adults. Nihon Koshu Eisei Zasshi 2020, 67, 171–182. [Google Scholar] [CrossRef]
- Donkin, A.J.M.; Johnson, A.E.; Lilley, J.M.; Morgan, K.; Neale, R.J.; Page, R.M.; Silburn, R.L. Gender and living alone as determinants of fruit and vegetable consumption among the elderly living at home in urban nottingham. Appetite 1998, 30, 39–51. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Poscia, A.; Stojanovic, J.; La Milia, D.I.; Duplaga, M.; Grysztar, M.; Moscato, U.; Onder, G.; Collamati, A.; Ricciardi, W.; Magnavita, N. Interventions targeting loneliness and social isolation among the older people: An update systematic review. Exp. Gerontol. 2018, 102, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Chipps, J.; Jarvis, M.A.; Ramlall, S. The effectiveness of e-interventions on reducing social isolation in older persons: A systematic review of systematic reviews. J. Telemed. Telecare 2017, 23, 817–827. [Google Scholar] [CrossRef]
- Rugel, E.J.; Carpiano, R.M. Gender differences in the roles for social support in ensuring adequate fruit and vegetable consumption among older adult Canadians. Appetite 2015, 92, 102–109. [Google Scholar] [CrossRef]
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variable † | Social Robustness(N = 194) | Social Prefrailty and Frailty (N = 97) | p Value * | Social Robustness(N = 222) | Social Prefrailty and Frailty (N = 153) | p Value * |
| Age, years | 69.9 (4.3) | 69.5 (4.5) | 0.45 | 68.7 (4.5) | 69.9 (4.4) | 0.01 |
| Educational level, N (%) | ||||||
| ≤9 years | 8 (4.2) | 6 (6.3) | 0.18 | 11 (5.0) | 13 (8.7) | 0.11 |
| 10–12 years | 63 (33) | 40 (42.1) | 113 (51.6) | 86 (57.3) | ||
| >12 years | 120 (62.8) | 49 (51.6) | 95 (43.4) | 51 (34.0) | ||
| Economic status, N (%) | ||||||
| Need support | 0 (0) | 2 (2.1) | <0.01 | 0 (0) | 9 (6.0) | <0.01 |
| Self-supporting | 157 (82.8) | 88 (92.6) | 175 (79.9) | 112 (74.7) | ||
| Well off | 34 (17.8) | 5 (5.3) | 44 (20.1) | 29 (19.3) | ||
| BMI, kg/m2 | 23.2 (2.6) | 23.3 (2.3) | 0.95 | 22.1 (2.6) | 21.7 (2.7) | 0.07 |
| MNA, scores | 26.8 (2.1) | 26.2 (2.1) | 0.04 | 26.0 (2.3) | 25.2 (2.3) | <0.01 |
| Dietary diversity score § | 1.7 (1.4) | 1.3 (1.3) | 0.01 | 1.8 (1.2) | 1.9 (1.3) | 0.71 |
| DQI-I | 47.9 (10.2) | 47.7 (9.1) | 0.87 | 47.5 (9.5) | 47.9 (9.0) | 0.70 |
| CCI, scores | 3.5 (1.4) | 3.4 (1.2) | 0.74 | 2.9 (1.0) | 3.2 (1.1) | 0.02 |
| Polypharmacy (≥5 medications), N (%) | 30 (15.7) | 20 (20.1) | 0.31 | 15 (6.8) | 15 (10.0) | 0.24 |
| Dietary intake | ||||||
| Energy intake, kcal/day | 2015.3 (476.7) | 1854.3 (402.0) | 0.01 | 1862.4 (409.9) | 1867.2 (403.9) | 0.91 |
| Energy intake, kcal/kg/day ‡ | 33.5 (8.2) | 31.0 (7.3) | 0.01 | 36.3 (8.3) | 36.7 (8.5) | 0.67 |
| Carbohydrate intake, g/kg/day ‡ | 4.3 (1.1) | 4.0 (1.0) | 0.04 | 4.7 (1.1) | 4.7 (1.1) | 0.79 |
| Fat intake, g/kg/day ‡ | 1.1 (0.4) | 1.0 (0.3) | 0.03 | 1.3 (0.4) | 1.3 (0.4) | 0.61 |
| Protein intake, g/kg/day ‡ | 1.2 (0.3) | 1.1 (0.3) | 0.01 | 1.4 (0.4) | 1.4 (0.4) | 0.62 |
| Fiber intake, g/kg/day ‡ | 0.2 (0.1) | 0.2 (0.1) | 0.01 | 0.3 (0.1) | 0.3 (0.1) | 0.93 |
| Retinol activity equivalent, μg/day | 284.5 (77.4) | 268.4 (84) | 0.01 | 336.6 (77.7) | 340.4 (86.8) | 0.42 |
| Vitamin D, μg/day | 4.2 (1.5) | 3.9 (1.6) | 0.04 | 4.7 (1.7) | 4.6 (1.7) | 0.45 |
| Tocopherol, mg/day | 4.1 (0.6) | 3.9 (0.7) | 0.01 | 4.5 (0.6) | 4.4 (0.7) | 0.04 |
| Vitamin K, μg/day | 103.4 (32.2) | 95.7 (33.9) | <0.01 | 123.3 (33.8) | 121.1 (34.7) | 0.25 |
| Vitamin B1, mg/day | 0.5 (0.1) | 0.5 (0.1) | 0.02 | 0.5 (0.1) | 0.5 (0.1) | 0.21 |
| Vitamin B2, mg/day | 0.6 (0.1) | 0.6 (0.1) | 0.78 | 0.6 (0.1) | 0.6 (0.1) | <0.01 |
| Niacin, mg/day | 7.8 (1.6) | 7.4 (1.7) | <0.01 | 8.1 (1.6) | 7.9 (1.7) | 0.09 |
| Vitamin B6, mg/day | 0.6 (0.1) | 0.6 (0.1) | <0.01 | 0.6 (0.1) | 0.6 (0.1) | 0.05 |
| Vitamin B12, mg/day | 3.8 (1.2) | 3.6 (1.3) | 0.07 | 4.2 (1.3) | 4.1 (1.3) | 0.39 |
| Folate, μg/day | 143 (35.1) | 133.6 (33.8) | <0.01 | 164.1 (37.7) | 161.2 (37.3) | 0.18 |
| Vitamin C, mg/day | 48.9 (17.1) | 43.2 (16.7) | <0.01 | 60.1 (18.1) | 58.3 (17.7) | 0.08 |
| Sodium, mg/day | 3859.6 (1324.7) | 3372.2 (1129.9) | <0.01 | 3940.7 (1280.3) | 3742.1 (1238.3) | 0.14 |
| Potassium, mg/day | 2383.5 (746.6) | 2074.7 (686.8) | <0.01 | 2453.2 (697.4) | 2432.8 (674.0) | 0.78 |
| Calcium, mg/day | 619.6 (221.9) | 555.9 (184.6) | 0.02 | 632.1 (186.6) | 655.5 (195.4) | 0.25 |
| Magnesium, mg/day | 255.5 (75.7) | 225.1 (65.2) | <0.01 | 251.8 (69.7) | 251.3 (67.0) | 0.94 |
| Phosphate, mg/day | 1078.1 (298.6) | 960.3 (257.5) | <0.01 | 1070.0 (281.6) | 1085.7 (269.1) | 0.59 |
| Iron, mg/day | 7.8 (2.7) | 6.9 (2.1) | 0.01 | 7.8 (2.2) | 7.9 (2.3) | 0.93 |
| Zinc, mg/day | 8.1 (2.1) | 7.2 (1.8) | <0.01 | 8.0 (2.1) | 8.1 (1.9) | 0.82 |
| Copper, mg/day | 1.1 (0.3) | 1.0 (0.3) | <0.01 | 1.1 (0.3) | 1.1 (0.3) | 0.75 |
| Manganese, mg/day | 2.6 (0.6) | 2.3 (0.6) | <0.01 | 2.5 (0.6) | 2.4 (0.6) | 0.71 |
| Macronutrient composition | ||||||
| Carbohydrate intake, % total energy | 57.5 (5.4) | 58.3 (6.1) | 0.27 | 54 (4.7) | 53.7 (5.6) | 0.62 |
| Fat intake, % total energy | 28.5 (4.2) | 28.0 (4.9) | 0.41 | 30.9 (3.8) | 31.0 (4.6) | 0.82 |
| Protein intake, % total energy | 14.0 (1.9) | 13.7 (2.0) | 0.18 | 15.0 (1.9) | 15.2 (1.9) | 0.37 |
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | GEE β Estimates * | p Value | 95% CI | GEE β Estimates * | p Value | 95% CI | ||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||
| Energy intake, kcal/kg/day ‡ | −1.59 | <0.01 | −2.49 | −0.69 | 0.41 | 0.47 | −0.68 | 1.49 |
| Protein intake, g/kg/day ‡ | −0.08 | <0.01 | −0.12 | −0.04 | 0.03 | 0.15 | −0.01 | 0.08 |
| Fat intake, g/kg/day ‡ | −0.06 | 0.01 | −0.10 | −0.02 | 0.03 | 0.26 | −0.02 | 0.08 |
| Carbohydrate intake, g/kg/day ‡ | −0.18 | 0.01 | −0.31 | −0.05 | 0.01 | 0.89 | −0.14 | 0.16 |
| Fiber intake, g/kg/day ‡ | −0.01 | 0.01 | −0.02 | −0.003 | <0.01 | 0.82 | −0.01 | 0.01 |
| Macronutrient composition | ||||||||
| Protein intake, % total energy | −0.17 | 0.32 | −0.50 | 0.16 | 0.20 | 0.15 | −0.07 | 0.46 |
| Fat intake, % total energy | −0.12 | 0.74 | −0.80 | 0.57 | 0.31 | 0.28 | −0.26 | 0.88 |
| Carbohydrate intake, % total energy | 0.27 | 0.56 | −0.65 | 1.19 | −0.52 | 0.15 | −1.22 | 0.18 |
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | GEE β Estimates * | p Value | 95% CI | GEE β Estimates * | p Value | 95% CI | ||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||
| a. Factors influencing longitudinal changes based on the dietary diversity score by sex | ||||||||
| Age, years | 0.04 | 0.01 | 0.01 | 0.07 | 0.03 | 0.02 | <0.01 | 0.06 |
| BMI, kg/m2 | −0.01 | 0.81 | −0.05 | 0.04 | −0.03 | 0.16 | −0.07 | 0.01 |
| Educational level | ||||||||
| ≤9 years | 0.00 | 0.00 | ||||||
| 10–12 years | −0.36 | 0.23 | −0.95 | 0.23 | 0.11 | 0.54 | −0.25 | 0.47 |
| >12 years | −0.10 | 0.73 | −0.70 | 0.49 | 0.25 | 0.21 | −0.14 | 0.64 |
| GDS | −0.01 | 0.70 | −0.05 | 0.04 | −0.03 | 0.04 | −0.07 | −0.002 |
| CCI | 0.09 | 0.02 | 0.02 | 0.16 | 0.08 | 0.03 | 0.01 | 0.16 |
| Social frailty status | ||||||||
| Social robustness | 0.00 | 0.00 | ||||||
| Social prefrailty and frailty | −0.25 | 0.01 | −0.44 | −0.05 | 0.08 | 0.41 | −0.11 | 0.27 |
| b. Factors influencing longitudinal change based on the Diet Quality Index-International by sex | ||||||||
| Age, years | 0.07 | 0.60 | −0.18 | 0.31 | 0.09 | 0.37 | −0.10 | 0.28 |
| BMI, kg/m2 | −0.11 | 0.56 | −0.50 | 0.27 | −0.12 | 0.44 | −0.42 | 0.18 |
| Educational level | ||||||||
| ≤9 years | 0.00 | 0.00 | ||||||
| 10–12 years | 2.15 | 0.24 | −1.41 | 5.70 | 0.29 | 0.84 | −2.54 | 3.12 |
| >12 years | 0.28 | 0.88 | −3.44 | 3.99 | −1.18 | 0.43 | −4.11 | 1.75 |
| GDS score | −0.02 | 0.92 | −0.36 | 0.33 | 0.10 | 0.49 | −0.18 | 0.38 |
| CCI score | −0.14 | 0.53 | −0.58 | 0.30 | −0.31 | 0.25 | −0.83 | 0.21 |
| Social frailty status | ||||||||
| Social robustness | 0.00 | 0.00 | ||||||
| Social prefrailty and frailty | −0.26 | 0.71 | −1.64 | 1.12 | −0.08 | 0.90 | −1.35 | 1.19 |
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | GEE β Estimates * | p Value | 95% CI | GEE β Estimates * | p Value | 95% CI | ||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | |||||
| Age, years | 0.01 | 0.73 | −0.03 | 0.04 | 0.00 | 0.86 | −0.03 | 0.04 |
| BMI, kg/m2 | 0.38 | <0.01 | 0.31 | 0.45 | 0.47 | <0.01 | 0.41 | 0.53 |
| Educational level | ||||||||
| ≤9 years | 0.00 | 0.00 | ||||||
| 10–12 years | −0.50 | 0.18 | −1.23 | 0.22 | 0.06 | 0.84 | −0.58 | 0.70 |
| >12 years | −0.03 | 0.94 | −0.74 | 0.68 | 0.22 | 0.51 | −0.43 | 0.86 |
| GDS score | −0.16 | <0.01 | −0.23 | −0.09 | −0.22 | <0.01 | −0.27 | −0.16 |
| CCI score | −0.22 | <0.01 | −0.30 | −0.14 | −0.25 | <0.01 | −0.37 | −0.14 |
| Social frailty status | ||||||||
| Social robustness | 0.00 | 0.00 | ||||||
| Social prefrailty and frailty | −0.32 | 0.04 | −0.62 | −0.02 | −0.23 | 0.12 | −0.51 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.H.; Okada, K.; Matsushita, E.; Uno, C.; Satake, S.; Arakawa Martins, B.; Kuzuya, M. Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly. Nutrients 2020, 12, 2845. https://doi.org/10.3390/nu12092845
Huang CH, Okada K, Matsushita E, Uno C, Satake S, Arakawa Martins B, Kuzuya M. Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly. Nutrients. 2020; 12(9):2845. https://doi.org/10.3390/nu12092845
Chicago/Turabian StyleHuang, Chi Hsien, Kiwako Okada, Eiji Matsushita, Chiharu Uno, Shosuke Satake, Beatriz Arakawa Martins, and Masafumi Kuzuya. 2020. "Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly" Nutrients 12, no. 9: 2845. https://doi.org/10.3390/nu12092845
APA StyleHuang, C. H., Okada, K., Matsushita, E., Uno, C., Satake, S., Arakawa Martins, B., & Kuzuya, M. (2020). Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly. Nutrients, 12(9), 2845. https://doi.org/10.3390/nu12092845





