Impact of Ascorbic Acid on the In Vitro Iron Bioavailability of a Casein-Based Iron Fortificant
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the ICC
2.2. Solubility and Iron Dissociation at Acidic pH
2.3. Digestibility and Solubility of the ICC under Simulated Gastric Digestion
2.4. In Vitro-Simulated Digestion Coupled with the Caco-2 Cell Model
2.5. Statistics
3. Results
3.1. Solubility and Iron Dissociation at Acidic pH
3.2. Digestibility and Solubility of the ICC under Simulated Gastric Digestion
3.3. In Vitro Iron Bioavailability with and without AA
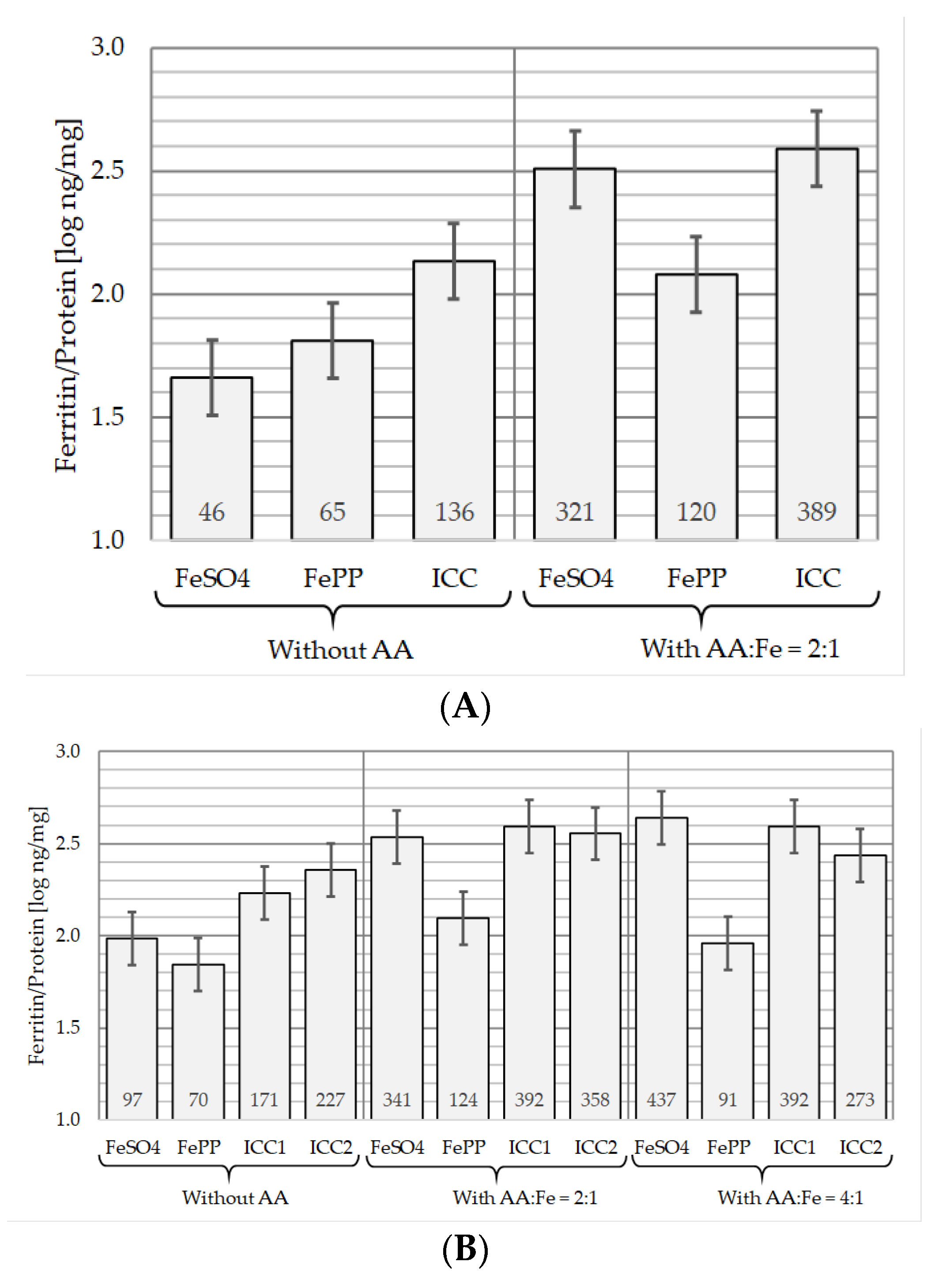
3.3.1. In the Absence of AA
3.3.2. In the Presence of AA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of nutrition for development (BOND)-iron review. J. Nutr. 2018, 148, 1001S–1067S. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Donahue Angel, M.; Rohner, F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: A systematic analysis of national surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO. Guidelines on Food Fortification with Micronutrients; World Health Organization/Food and Agricultural Organization of the United Nations: Geneva, Switzerland, 2006. [Google Scholar]
- Bedard, M.; Husny, J. Composition in Powder Form Comprising Iron-Casein Complexes and Compounds Sensitive to Oxidation. Patent AU2017376591A1, 5 December 2019. [Google Scholar]
- Mittal, V.A.; Ellis, A.; Das, S.; Ye, A.; Singh, H. Mineral Fortification Process and Its Uses. Patent WO/2013/191568 A1, 30 November 2017. [Google Scholar]
- Mittal, V.A.; Ellis, A.; Ye, A.; Edwards, P.J.B.; Das, S.; Singh, H. Iron binding to caseins in the presence of orthophosphate. Food Chem. 2016, 190, 128–134. [Google Scholar] [CrossRef]
- Mittal, V.A.; Ellis, A.; Ye, A.; Edwards, P.J.B.; Singh, H. The adsorption of orthophosphate onto casein-iron precipitates. Food Chem. 2018, 239, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Basch, J.J.; Jones, S.B.; Kalan, E.B.; Wondolowski, M.V. Distribution of added iron and polyphosphate phosphorus in cow’s milk. J. Dairy Sci. 1974, 57, 545–550. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J. Iron. J. Nutr. 2001, 131, 1383S–1386S. [Google Scholar] [CrossRef]
- Henare, S.J.; Nur Singh, N.; Ellis, A.M.; Moughan, P.J.; Thompson, A.K.; Walczyk, T. Iron bioavailability of a casein-based iron fortificant compared with that of ferrous sulfate in whole milk: A randomized trial with a crossover design in adult women. Am. J. Clin. Nutr. 2019, 110, 1362–1369. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Erlandsson, M.; Sandberg, A.S.; Rossander-Hulten, L. Calcium: Effect of different amounts on nonheme- and heme-iron absorption in humans. Am. J. Clin. Nutr. 1991, 53, 112–119. [Google Scholar] [CrossRef]
- Shawki, A.; Mackenzie, B. Interaction of calcium with the human divalent metal-ion transporter-1. Biochem. Biophys. Res. Commun. 2010, 393, 471–475. [Google Scholar] [CrossRef]
- Pauline, M.; Verghese, S.T.; Srinivasu, B.Y.; Bose, B.; Thomas, T.; Mandal, A.K.; Thankachan, P.; Kurpad, A.V. Effect of ascorbic acid rich, micro-nutrient fortified supplement on the iron bioavailability of ferric pyrophosphate from a milk based beverage in Indian school children. Asia Pac. J. Clin. Nutr. 2018, 27, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Stekel, A.; Olivares, M.; Pizarro, F.; Chadud, P.; Lopez, I.; Amar, M. Absorption of fortification iron from milk formulas in infants. Am. J. Clin. Nutr. 1986, 43, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, T.; Muthayya, S.; Wegmuller, R.; Thankachan, P.; Sierksma, A.; Frenken, L.G.; Thomas, T.; Kurpad, A.; Hurrell, R.F. Inhibition of iron absorption by calcium is modest in an iron-fortified, casein- and whey-based drink in Indian children and is easily compensated for by addition of ascorbic acid. J. Nutr. 2014, 144, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Coad, J. Dairy product (calcium) consumption and iron nutrition. In Nutrients in Dairy and Their Implications for Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: London, UK, 2017; pp. 149–160. [Google Scholar]
- SACN. The Scientific Advisory Committee on Nutrition. Iron and Health; TSO: London, UK, 2010. [Google Scholar]
- Lonnerdal, B. Calcium and iron absorption—Mechanisms and public health relevance. Int. J. Vitam. Nutr. Res. 2010, 80, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Grinder-Pedersen, L.; Bukhave, K.; Jensen, M.; Hojgaard, L.; Hansen, M. Calcium from milk or calcium-fortified foods does not inhibit nonheme-iron absorption from a whole diet consumed over a 4-d period. Am. J. Clin. Nutr. 2004, 80, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B.; Cook, J.D. Effect of calcium intake on nonheme-iron absorption from a complete diet. Am. J. Clin. Nutr. 1997, 65, 1820–1825. [Google Scholar] [CrossRef]
- Snedeker, S.M.; Smith, S.A.; Greger, J.L. Effect of dietary calcium and phosphorus levels on the utilization of iron, copper, and zinc by adult males. J. Nutr. 1982, 112, 136–143. [Google Scholar] [CrossRef]
- Tidehag, P.; Sandberg, A.S.; Hallmans, G.; Wing, K.; Turk, M.; Holm, S.; Grahn, E. Effect of milk and fermented milk on iron absorption in ileostomy subjects. Am. J. Clin. Nutr. 1995, 62, 1234–1238. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Smith, R.G.; Kretsch, M.J.; Keyes, W.R.; Shah, A.G. Milk’s effect on the bioavailability of iron from cereal-based diets in young women by use of in vitro and in vivo methods. Am. J. Clin. Nutr. 1990, 52, 373–378. [Google Scholar] [CrossRef]
- Gaucheron, F.; LeGraet, Y.; Boyaval, E.; Piot, M. Binding of cations to casein molecules: Importance of physico-chemical conditions. Milchwissenschaft 1997, 52, 322–326. [Google Scholar]
- Raouche, S.; Naille, S.; Dobenesque, M.; Bot, A.; Jumas, J.; Cuq, J.; Marchesseau, S. Iron fortification of skim milk: Minerals and 57Fe Mössbauer study. Int. Dairy J. 2009, 19, 56–63. [Google Scholar] [CrossRef]
- Bouhallab, S.; Cinga, V.; Ait-Oukhatar, N.; Bureau, F.; Neuville, D.; Arhan, P.; Maubois, J.L.; Bougle, D. Influence of various phosphopeptides of caseins on iron absorption. J. Agric. Food Chem. 2002, 50, 7127–7130. [Google Scholar] [CrossRef]
- Kibangou, I.B.; Bouhallab, S.; Henry, G.; Bureau, F.; Allouche, S.; Blais, A.; Guerin, P.; Arhan, P.; Bougle, D.L. Milk proteins and iron absorption: Contrasting effects of different caseinophosphopeptides. Pediatr. Res. 2005, 58, 731–734. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Lynch, S.R.; Trinidad, T.P.; Dassenko, S.A.; Cook, J.D. Iron absorption in humans as influenced by bovine milk proteins. Am. J. Clin. Nutr. 1989, 49, 546–552. [Google Scholar] [CrossRef]
- Ait-Oukhatar, N.; Bouhallab, S.; Arhan, P.; Maubois, J.L.; Drosdowsky, M.; Bougle, D. Iron tissue storage and hemoglobin levels of deficient rats repleted with iron bound to the caseinophosphopeptide 1–25 of beta-casein. J. Agric. Food Chem. 1999, 47, 2786–2790. [Google Scholar] [CrossRef] [PubMed]
- Bouhallab, S.; Bougle, D. Biopeptides of milk: Caseinophosphopeptides and mineral bioavailability. Reprod. Nutr. Dev. 2004, 44, 493–498. [Google Scholar] [CrossRef]
- Pérès, J.M.; Bouhallab, N.; Bureau, F.; Bouglè, D. Mechanisms of absorption of caseinophosphopeptide bound iron. J. Nutr. Biochem. 1999, 10, 215–222. [Google Scholar] [CrossRef]
- Ait-Oukhatar, N.; Peres, J.M.; Bouhallab, S.; Neuville, D.; Bureau, F.; Bouvard, G.; Arhan, P.; Bougle, D. Bioavailability of caseinophosphopeptide-bound iron. J. Lab. Clin. Med. 2002, 140, 290–294. [Google Scholar] [CrossRef]
- Tetens, I. EFSA panel on dietetic products, nutrition and allergies (NDA); scientific opinion on the substantiation of a health claim related to glucose and contribution to energy-yielding metabolism pursuant to article 13 (5) of regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1226. [Google Scholar]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Zimmermann, M.B.; Wegmuller, R.; Walczyk, T.; Zeder, C.; Hurrell, R.F. Iron status and food matrix strongly affect the relative bioavailability of ferric pyrophosphate in humans. Am. J. Clin. Nutr. 2006, 83, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.C.; Davidsson, L.; Zeder, C.; Walczyk, T.; Marti, I.; Hurrell, R.F. Effect of ascorbic acid and particle size on iron absorption from ferric pyrophosphate in adult women. Int. J. Vitam. Nutr. Res. 2004, 74, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Habicht, J.P.; Miller, D.D.; Glahn, R.P. An in vitro digestion/Caco-2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. J. Nutr. 2004, 134, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Bothwell, T.; Campbell, L. A comparison of physical properties, screening procedures and a human efficacy trial for predicting the bioavailability of commercial elemental iron powders used for food fortification. Int. J. Vitam. Nutr. Res. 2007, 77, 107–124. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Dave, A.C.; Loveday, S.M.; Anema, S.G.; Loo, T.S.; Norris, G.E.; Jameson, G.B.; Singh, H. Beta-lactoglobulin self-assembly: Structural changes in early stages and disulfide bonding in fibrils. J. Agric. Food Chem. 2013, 61, 7817–7828. [Google Scholar] [CrossRef]
- Mason, R.L.; Gunst, R.F.; Hess, J.L. Statistical Design and Analysis of Experiments with Applications to Engineering and Science, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2003. [Google Scholar]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Sabatier, M.; Grathwohl, D.; Beaumont, M.; Groulx, K.; Guignard, L.F.; Kastenmayer, P.; Dubascoux, S.; Richoz, J.; Habeych, E.; Zeder, C.; et al. The bioavailability of iron picolinate is comparable to iron sulfate when fortified into a complementary fruit yogurt: A stable iron isotope study in young women. Eur. J. Nutr. 2020, 59, 1371–1378. [Google Scholar] [CrossRef]
- Walczyk, T.; Kastenmayer, P.; Storcksdieck Genannt Bonsmann, S.; Zeder, C.; Grathwohl, D.; Hurrell, R.F. Ferrous ammonium phosphate (FeNH(4)PO(4)) as a new food fortificant: Iron bioavailability compared to ferrous sulfate and ferric pyrophosphate from an instant milk drink. Eur. J. Nutr. 2013, 52, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.L.; Arnaud, M.J.; Chichester, C.O.; Cook, J.D.; Harrison, B.N.; Hurrell, R.F.; Kahn, S.G.; Morris, E.R.; Tanner, J.T.; Whittaker, P.; et al. Comparison of in vitro, animal, and clinical determinations of iron bioavailability: International nutritional anemia consultative group task force report on iron bioavailability. Am. J. Clin. Nutr. 1989, 49, 225–238. [Google Scholar] [CrossRef]
- Chaud, M.V.; Izumi, C.; Nahaal, Z.; Shuhama, T.; Bianch Mde, L.; de Freitas, O. Iron derivatives from casein hydrolysates as a potential source in the treatment of iron deficiency. J. Agric. Food Chem. 2002, 50, 871–877. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Ferrous bisglycinate as a source of iron for use in the manufacturing of foods and in food supplements. EFSA J. 2006, 299, 1–17. [Google Scholar]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Pizarro, F.; Pineda, O.; Name, J.J.; Hertrampf, E.; Walter, T. Milk inhibits and ascorbic acid favors ferrous bis-glycine chelate bioavailability in humans. J. Nutr. 1997, 127, 1407–1411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kloots, W.; Op den Kamp, D.; Abrahamse, L. In vitro iron availability from iron-fortified whole-grain wheat flour. J. Agric. Food Chem. 2004, 52, 8132–8136. [Google Scholar] [CrossRef]
- Wortley, G.; Leusner, S.; Good, C.; Gugger, E.; Glahn, R. Iron availability of a fortified processed wheat cereal: A comparison of fourteen iron forms using an in vitro digestion/human colonic adenocarcinoma (CaCo-2) cell model. Br. J. Nutr. 2005, 93, 65–71. [Google Scholar] [CrossRef]
- Zhu, L.; Glahn, R.P.; Nelson, D.; Miller, D.D. Comparing soluble ferric pyrophosphate to common iron salts and chelates as sources of bioavailable iron in a Caco-2 cell culture model. J. Agric. Food Chem. 2009, 57, 5014–5019. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Biebinger, R.; Egli, I.; Zeder, C.; Hurrell, R.F. Iron deficiency up-regulates iron absorption from ferrous sulphate but not ferric pyrophosphate and consequently food fortification with ferrous sulphate has relatively greater efficacy in iron-deficient individuals. Br. J. Nutr. 2011, 105, 1245–1250. [Google Scholar] [CrossRef]
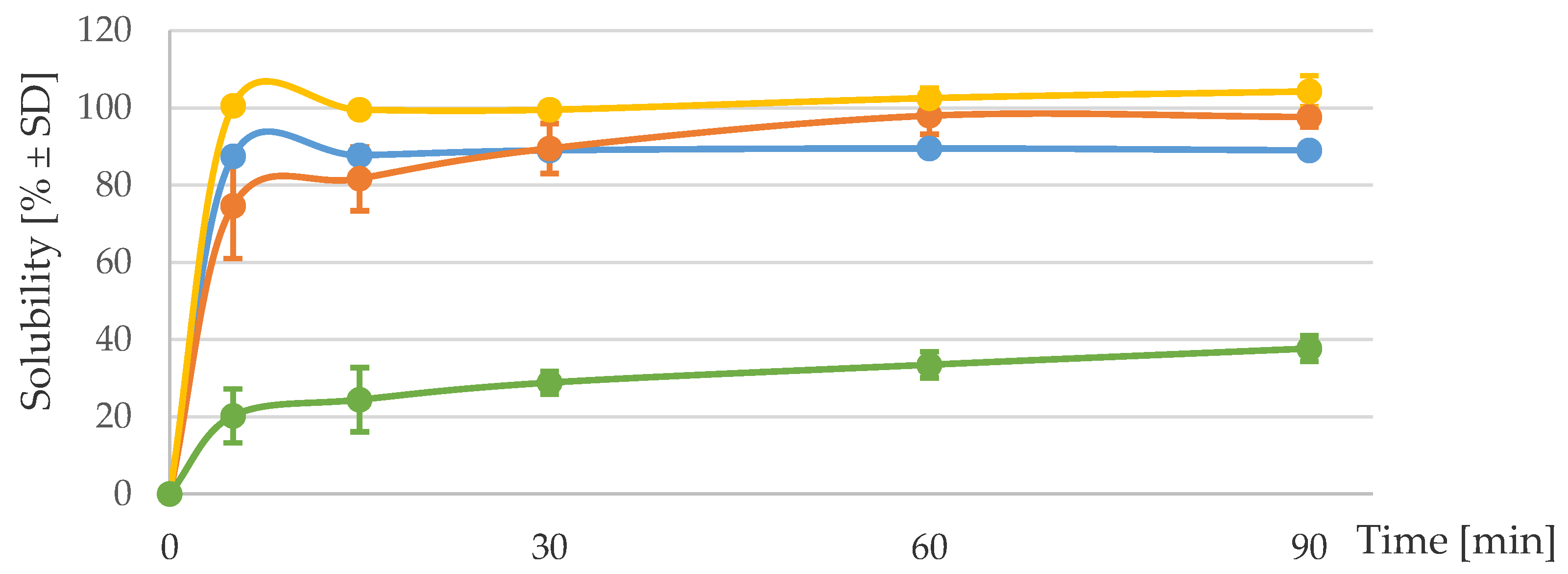

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatier, M.; Rytz, A.; Husny, J.; Dubascoux, S.; Nicolas, M.; Dave, A.; Singh, H.; Bodis, M.; Glahn, R.P. Impact of Ascorbic Acid on the In Vitro Iron Bioavailability of a Casein-Based Iron Fortificant. Nutrients 2020, 12, 2776. https://doi.org/10.3390/nu12092776
Sabatier M, Rytz A, Husny J, Dubascoux S, Nicolas M, Dave A, Singh H, Bodis M, Glahn RP. Impact of Ascorbic Acid on the In Vitro Iron Bioavailability of a Casein-Based Iron Fortificant. Nutrients. 2020; 12(9):2776. https://doi.org/10.3390/nu12092776
Chicago/Turabian StyleSabatier, Magalie, Andreas Rytz, Joeska Husny, Stéphane Dubascoux, Marine Nicolas, Anant Dave, Harjinder Singh, Mary Bodis, and Raymond P. Glahn. 2020. "Impact of Ascorbic Acid on the In Vitro Iron Bioavailability of a Casein-Based Iron Fortificant" Nutrients 12, no. 9: 2776. https://doi.org/10.3390/nu12092776
APA StyleSabatier, M., Rytz, A., Husny, J., Dubascoux, S., Nicolas, M., Dave, A., Singh, H., Bodis, M., & Glahn, R. P. (2020). Impact of Ascorbic Acid on the In Vitro Iron Bioavailability of a Casein-Based Iron Fortificant. Nutrients, 12(9), 2776. https://doi.org/10.3390/nu12092776





