Urinary Sodium Excretion and Blood Pressure Relationship across Methods of Evaluating the Completeness of 24-h Urine Collections
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Blood Pressure Measurement
2.3. Demographic, Cardiovascular Risk Factor, and Physical Measures Data
2.4. 24-h Urinary Sodium and Creatinine
2.5. Methods of Evaluating the Completeness of 24-h Urine Collections
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Karppanen, H.; Karppanen, P.; Mervaala, E. Why and how to implement sodium, potassium, calcium, and magnesium changes in food items and diets? J. Hum. Hypertens. 2005, 19, S10–S19. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Mugavero, K.; Bowman, B.A.; Frieden, T.R. Dietary sodium and cardiovascular disease risk—Measurement matters. N. Engl. J. Med. 2016, 375, 580. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Salt reduction lowers cardiovascular risk: Meta-analysis of outcome trials. Lancet 2011, 378, 380–382. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Tan, M.; He, F.J.; MacGregor, G.A. Salt and cardiovascular disease in PURE: A large sample size cannot make up for erroneous estimations. J. Renin-Angiotensin-Aldosterone Syst. 2018, 19. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef]
- Santos, J.A.; Sparks, E.; Thout, S.R.; McKenzie, B.; Trieu, K.; Hoek, A.; Johnson, C.; McLean, R.; Arcand, J.; Campbell, N.R. The Science of Salt: A global review on changes in sodium levels in foods. J. Clin. Hypertens. 2019, 21, 1043–1056. [Google Scholar] [CrossRef]
- World Health Organization. The SHAKE Technical Package for Salt Reduction; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Campbell, N.R.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar] [CrossRef]
- Wielgosz, A.; Robinson, C.; Mao, Y.; Jiang, Y.; Campbell, N.R.; Muthuri, S.; Morrison, H. The impact of using different methods to assess completeness of 24-hour urine collection on estimating dietary sodium. J. Clin. Hypertens. 2016, 18, 581–584. [Google Scholar] [CrossRef]
- John, K.A.; Cogswell, M.E.; Campbell, N.R.; Nowson, C.A.; Legetic, B.; Hennis, A.J.; Patel, S.M. Accuracy and usefulness of select methods for assessing complete collection of 24-hour urine: A systematic review. J. Clin. Hypertens. 2016, 18, 456–467. [Google Scholar] [CrossRef]
- Nowson, C.A.; Lim, K.; Campbell, N.R.; O’Connell, S.L.; He, F.J.; Daly, R.M. Impact of fractional excretion of sodium on a single morning void urine collection as an estimate of 24-hour urine sodium. J. Clin. Hypertens. 2019, 21, 1763–1770. [Google Scholar] [CrossRef]
- Naser, A.M.; Rahman, M.; Unicomb, L.; Doza, S.; Gazi, M.S.; Alam, G.R.; Karim, M.R.; Uddin, M.N.; Khan, G.K.; Ahmed, K.M.; et al. Drinking water salinity, urinary macro-mineral excretions, and blood pressure in the southwest coastal population of Bangladesh. J. Am. Heart Assoc. 2019, 8, e012007. [Google Scholar] [CrossRef]
- Khan, A.E.; Ireson, A.; Kovats, S.; Mojumder, S.K.; Khusru, A.; Rahman, A.; Vineis, P. Drinking water salinity and maternal health in coastal Bangladesh: Implications of climate change. Environ. Health Perspect. 2011, 119, 1328–1332. [Google Scholar] [CrossRef]
- Khan, A.E.; Scheelbeek, P.F.D.; Shilpi, A.B.; Chan, Q.; Mojumder, S.K.; Rahman, A.; Haines, A.; Vineis, P. Salinity in drinking water and the risk of (pre) eclampsia and gestational hypertension in coastal Bangladesh: A case-control study. PLoS ONE 2014, 9, e108715. [Google Scholar] [CrossRef]
- Scheelbeek, P.F.; Chowdhury, M.A.; Haines, A.; Alam, D.S.; Hoque, M.A.; Butler, A.P.; Khan, A.E.; Mojumder, S.K.; Blangiardo, M.A.; Elliott, P.; et al. Drinking water salinity and raised blood pressure: Evidence from a cohort study in coastal Bangladesh. Environ. Health Perspect. 2017, 125. [Google Scholar] [CrossRef]
- Talukder, M.R.R.; Rutherford, S.; Phung, D.; Islam, M.Z.; Chu, C. The effect of drinking water salinity on blood pressure in young adults of coastal Bangladesh. Environ. Pollut. 2016, 248–254. [Google Scholar] [CrossRef]
- Sultana, S.; Ahmed, K.; Mahtab-Ul-Alam, S.; Hasan, M.; Tuinhof, A.; Ghosh, S.; Rahman, M.; Ravenscroft, P.; Zheng, Y. Low-cost aquifer storage and recovery: Implications for improving drinking water access for rural communities in coastal Bangladesh. J. Hydrol. Eng. 2015, 20, B5014007. [Google Scholar] [CrossRef]
- Atikul Islam, M.; Sakakibara, H.; Karim, M.R.; Sekine, M. Potable water scarcity: Options and issues in the coastal areas of Bangladesh. J. Water Health 2013, 11, 532–542. [Google Scholar] [CrossRef]
- Naser, A.M.; Rahman, M.; Unicomb, L.; Doza, S.; Anand, S.; Chang, H.; Luby, S.; Clasen, T.; Narayan, K. Comparison of Urinary Sodium and Blood Pressure Relationship from the Spot Versus 24-Hour Urine Samples. J. Am. Heart Assoc. 2019, 8, e013287. [Google Scholar] [CrossRef]
- Naser, A.M.; Unicomb, L.; Doza, S.; Ahmed, K.M.; Rahman, M.; Uddin, M.N.; Quraishi, S.B.; Selim, S.; Shamsudduha, M.; Burgess, W.; et al. Stepped-wedge cluster-randomised controlled trial to assess the cardiovascular health effects of a managed aquifer recharge initiative to reduce drinking water salinity in southwest coastal Bangladesh: Study design and rationale. BMJ Open 2017, 7, e015205. [Google Scholar] [CrossRef]
- Naser, A.M.; Doza, S.; Rahman, M.; Unicomb, L.; Ahmed, K.M.; Anand, S.; Selim, S.; Shamsudduha, M.; Narayan, K.; Chang, H.; et al. Consequences of access to water from managed aquifer recharge systems for blood pressure and proteinuria in south-west coastal Bangladesh: A stepped-wedge cluster-randomized trial. Int. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Naser, A.M.; Rahman, M.; Unicomb, L.; Parvez, S.M.; Islam, S.; Doza, S.; Khan, G.K.; Ahmed, K.M.; Anand, S.; Luby, S.P.; et al. Associations of drinking rainwater with macro-mineral intake and cardiometabolic health: A pooled cohort analysis in Bangladesh, 2016–2019. npj Clean Water 2020, 1–11. [Google Scholar] [CrossRef]
- Gunnsteinsson, S.; Labrique, A.B.; West Jr, K.P.; Christian, P.; Mehra, S.; Shamim, A.A.; Rashid, M.; Katz, J.; Klemm, R.D. Constructing indices of rural living standards in Northwestern Bangladesh. J. Health Popul. Nutr. 2010, 28, 509. [Google Scholar]
- Taussky, H.H.; Kurzmann, G. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J. Biol. Chem. 1954, 208, 853–861. [Google Scholar]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef]
- Walser, M. Creatinine excretion as a measure of protein nutrition in adults of varying age. J. Parenter. Enter. Nutr. 1987, 11, 73S–78S. [Google Scholar] [CrossRef]
- Kawasaki, T.; Uezono, K.; Itoh, K.; Ueno, M. Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application. Jpn. J. Public Health 1991, 38, 567–574. [Google Scholar]
- Orsini, N.; Greenland, S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011, 11, 1. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Berlin, Germany, 2015. [Google Scholar]
- Smith, Z.; Knight, T.; Sahota, P.; Kernohan, E.; Baker, M. Dietary patterns in Asian and Caucasian men in Bradford: Differences and implications for nutrition education. J. Hum. Nutr. Diet. 1993, 6, 323–333. [Google Scholar] [CrossRef]
- Aggarwal, B.; Makarem, N.; Shah, R.; Emin, M.; Wei, Y.; St-Onge, M.P.; Jelic, S. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: Findings from the American Heart Association go red for women strategically focused Research network. J. Am. Heart Assoc. 2018, 7, e008590. [Google Scholar] [CrossRef]
- Wilcox, R. Trimming and winsorization. Encycl. Biostat. 2005, 8. [Google Scholar] [CrossRef]
- Hubert, L.J. The use of orthogonal polynomials for trend analysis. Am. Educ. Res. J. 1973, 10, 241–244. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- De Keyzer, W.; Huybrechts, I.; Dekkers, A.L.; Geelen, A.; Crispim, S.; Hulshof, P.J.; Andersen, L.F.; Řehůřková, I.; Ruprich, J.; Volatier, J.-L.; et al. Predicting urinary creatinine excretion and its usefulness to identify incomplete 24 h urine collections. Br. J. Nutr. 2012, 108, 1118–1125. [Google Scholar] [CrossRef]
- He, J.; Gu, D.; Chen, J.; Jaquish, C.E.; Rao, D.C.; Hixson, J.E.; Chen, J.-C.; Duan, X.; Huang, J.-F.; Chen, C.-S.; et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J. Hypertens. 2009, 27, 48. [Google Scholar] [CrossRef]
- Mill, J.G.; Baldo, M.P.; Molina, M.D.C.B.; Schmidt, M.I.; Barreto, S.M.; Chor, D.; Griep, R.H.; Matos, S.M.; Ribeiro, A.L.P.; Duncan, B.B.; et al. Sex-specific patterns in the association between salt intake and blood pressure: The ELSA-Brasil study. J. Clin. Hypertens. 2019, 21, 502–509. [Google Scholar] [CrossRef]
- Pechère-Bertschi, A.; Burnier, M. Female sex hormones, salt, and blood pressure regulation. Am. J. Hypertens. 2004, 17, 994–1001. [Google Scholar] [CrossRef]
- Lermen, D.; Bartel-Steinbach, M.; Gwinner, F.; Conrad, A.; Weber, T.; von Briesen, H.; Kolossa-Gehring, M. Trends in characteristics of 24-h urine samples and their relevance for human biomonitoring studies—20 years of experience in the German Environmental Specimen Bank. Int. J. Hyg. Environ. Health 2019, 222, 831–839. [Google Scholar] [CrossRef]
- Aubinière-Robb, L.; Jeemon, P.; Hastie, C.E.; Patel, R.K.; McCallum, L.; Morrison, D.; Walters, M.; Dawson, J.; Sloan, W.; Muir, S.; et al. Blood pressure response to patterns of weather fluctuations and effect on mortality. Hypertension 2013, 62, 190–196. [Google Scholar] [CrossRef]
- Brook, R.D.; Weder, A.B.; Rajagopalan, S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J. Clin. Hypertens. 2011, 13, 836–842. [Google Scholar] [CrossRef]
- Modesti, P.A. Season, temperature and blood pressure: A complex interaction. Eur. J. Intern. Med. 2013, 24, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.P.; Miller, V.S. Sweat rate and sodium loss during work in the heat. J. Occup. Med. Toxicol. 2008, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Kolding, M.; Leslie, E.; Moreno, D.; Norwood, S.; Ordille, A.; Weller, R. Heat acclimation causes a linear decrease in sweat sodium ion concentration. J. Therm. Biol. 2018, 71, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.; Racinais, S.; Sawka, M.N. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand. J. Med. Sci. Sports 2015, 25, 20–38. [Google Scholar] [CrossRef]
- White, W.B. Importance of blood pressure control over a 24-hour period. J. Manag. Care Pharm. 2007, 13 (Suppl. B), 34–39. [Google Scholar] [CrossRef]
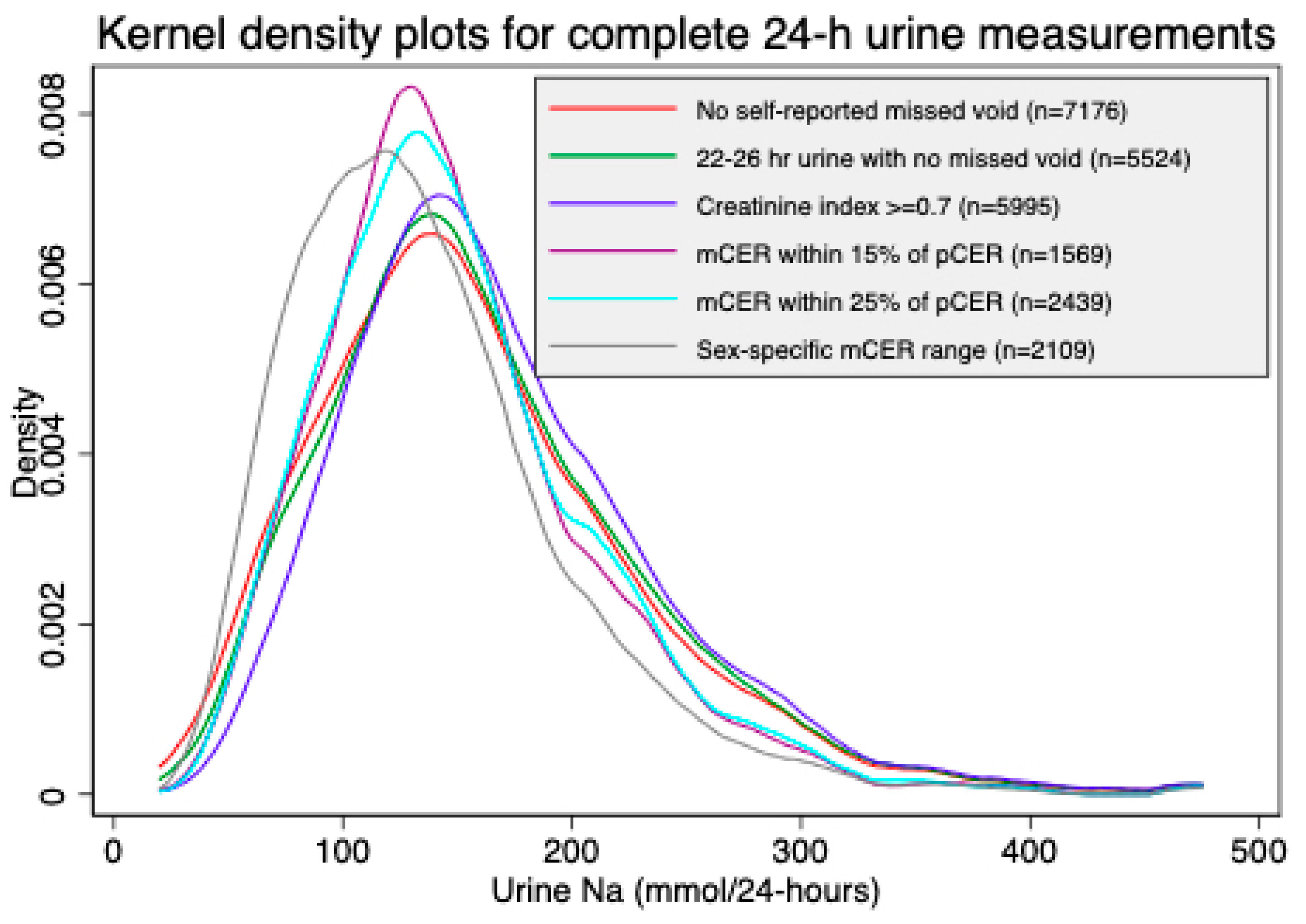
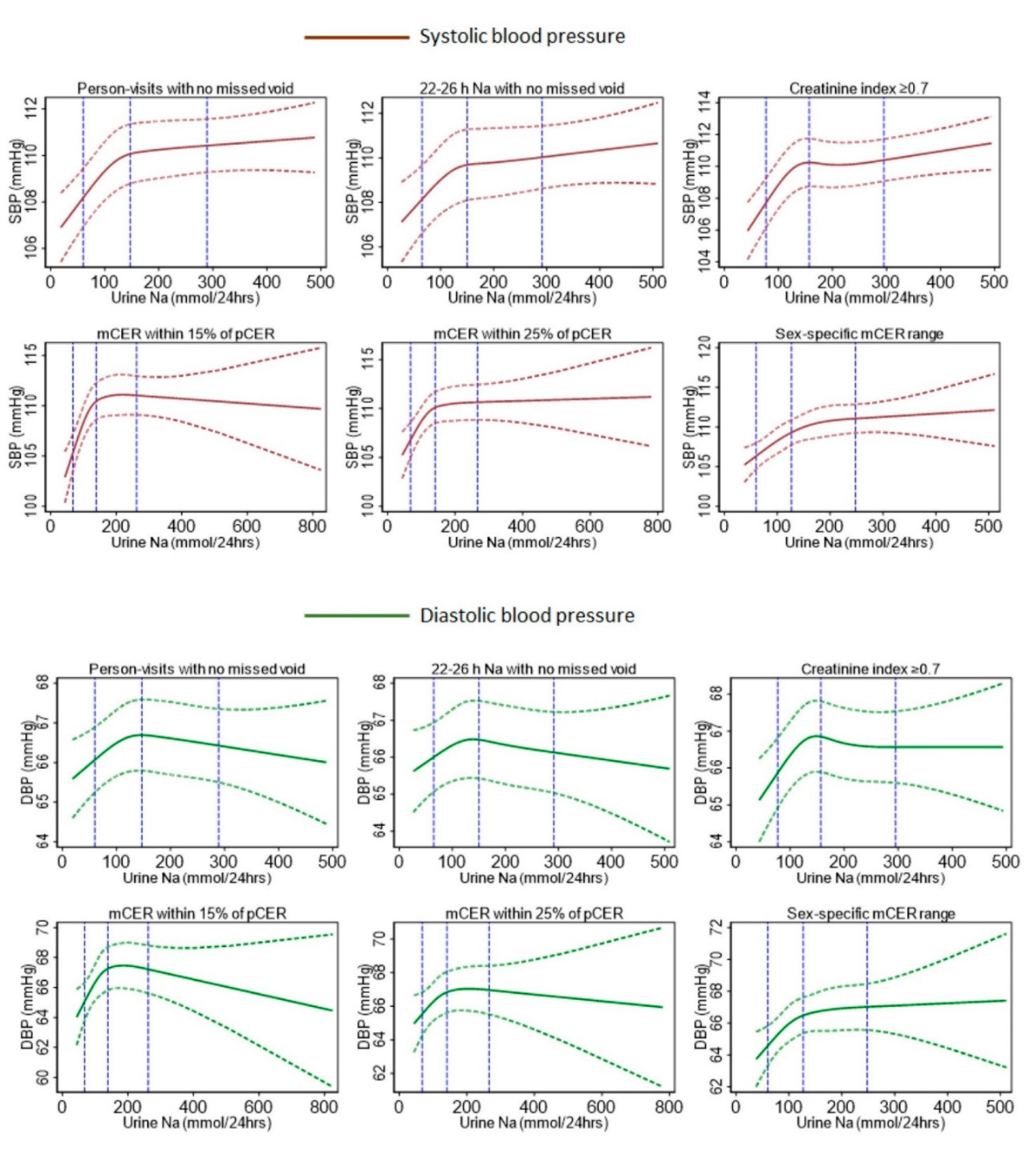

| Characteristics | All Person-Visits of 24-h Urine Collected | Self-Reported No Missed Voids | 22–26 h Urine Samples with No Missed Voids | Creatinine Index ≥0.7 & No Missed Voids | mCER within 15% of Kawasaki pCER and No Missed Voids | mCER within 25% of Kawasaki pCER and No Missed Voids | mCER 15–25 mg/kg/24 h (Men) and 10–20 mg/kg/24 h (Women), and No Missed Voids |
|---|---|---|---|---|---|---|---|
| Person-visits included, N (%) | 9804 (100%) | 7176 (73%) | 5524 (56%) | 5995 (61%) | 1569 (16%) | 2439 (25%) | 2109 (22%) |
| Age, mean (SD) | 42.7 (14.4) | 42.8 (14.5) | 42.8 (14.4) | 42.3 (14.2) | 42.9 (14.6) | 43.0 (14.5) | 44.3 (14.6) |
| Age category, % (n) | |||||||
| 20- < 30 years | 20 (1974) | 20 (1490) | 20 (1110) | 21 (1300) | 18 (295) | 18 (457) | 16 (342) |
| 30- < 40 years | 29 (2829) | 28 (2093) | 28 (1560) | 29 (1777) | 30 (484) | 30 (731) | 29 (605) |
| 40- < 50 years | 20 (1937) | 20 (1468) | 20 (1109) | 20 (1270) | 18 (286) | 18 (456) | 19 (392) |
| 50- < 60 years | 16 (1617) | 16 (1224) | 17 (909) | 16 (1003) | 15 (249) | 16 (391) | 19 (391) |
| 60- < 70 years | 10 (1006) | 11 (785) | 11 (604) | 10 (630) | 12 (186) | 12 (303) | 13 (274) |
| ≥70 years | 5 (441) | 5 (372) | 4 (239) | 4 (275) | 7 (108) | 6 (140) | 5 (105) |
| Male sex, % (n) | 39 (3802) | 36 (2677) | 34 (1893) | 35 (2198) | 30 (473) | 30 (742) | 31 (664) |
| BMI, mean (SD) | 22.4 (3.9) | 22.5 (4.1) | 22.6 (3.8) | 22.4 (4.1) | 22.9 (4.0) | 22.9 (4.0) | 23.3 (4.1) |
| BMI categories, % | |||||||
| Underweight | 15 (1445) | 14 (1070) | 14 (748) | 15 (910) | 14 (218) | 13 (331) | 11 (227) |
| Normal weight | 43 (4251) | 43 (3169) | 42 (2309) | 43 (2674) | 38 (609) | 39 (963) | 39 (821) |
| Overweight | 31 (3081) | 32 (2348) | 33 (1852) | 32 (1995) | 34 (550) | 34 (846) | 35 (740) |
| Obese | 11 (1027) | 11 (854) | 11 (622) | 11 (676) | 14 (231) | 14 (338) | 15 (321) |
| 24-h urine Na for both sexes, mean(SD) [p-value ¥] | 155 (76) [0.011] | 158 (76) [reference] | 162 (76) [0.003] | 169 (74) [<0.001] | 151 (79) [<0.001] | 152 (74) [<0.001] | 137 (66) [<0.001] |
| 24-h urine Na for males, mean(SD) [p-value ¥] | 149 (79) [0.003] | 155 (81) [reference] | 161 (84) [0.016] | 169 (80) [<0.001] | 151 (99) [0.344] | 151 (87) [0.246] | 133 (57) [<0.001] |
| 24-h urine Na for females, mean(SD) [p-value ¥] | 158 (74) [0.1646] | 160 (73) [reference] | 163 (72) [0.062] | 169 (71) [<0.001] | 151 (68) [<0.001] | 153 (68) [<0.001] | 139 (70) [<0.001] |
| Methods of Evaluating 24-h Urine Samples | Urine Volume in L, Median (IQR) | Urine Creatinine Concentration in mg/dL, Median (IQR) | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| No self-reported missed voids | 1.93 (1.37–2.65) | 1.92 (1.41–2.57) | 65 (48–92) | 53 (39–72) |
| 22–26 h urine samples with no missed voids | 1.95 (1.40–2.69) | 1.95 (1.45–2.60) | 66 (49–92) | 53 (39–72) |
| Creatinine index ≥0.7 and no missed voids | 2.09 (1.55–2.81) | 2.03 (1.54–2.67) | 68 (51–96) | 54 (40–75) |
| mCER within 15% of Kawasaki pCER and no missed voids | 1.87 (1.32, 2.63) | 1.83 (1.36–2.47) | 68 (52–94) | 52 (39–68) |
| mCER within 25% of Kawasaki pCER and no missed voids | 1.80 (1.32–2.53) | 1.85 (1.35–2.49) | 67 (52–95) | 52 (39–69) |
| mCER 15–25 mg/kg/24 h (men) and 10–20 mg/kg/24 h (women), and no missed voids | 1.74 (1.24–2.33) | 1.65 (1.23–2.18) | 62 (49–85) | 50 (39–65) |
| Methods for Complete 24-h Urine Assessment | Systolic BP (mmHg) | Diastolic BP (mmHg) | ||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-Value for Trend | Tertile 1 | Tertile 2 | Tertile 3 | p-Value for Trend | |
| No self-reported missed voids | ||||||||
| Model 1 | Ref | 0.60 (0.01, 1.19) | 0.98 (0.36, 1.60) | 0.002 | Ref | −0.04 (−0.42, 0.35) | 0.14 (−0.24, 0.52) | 0.463 |
| Model 2 | Ref | 0.75 (0.15, 1.35) | 1.14 (0.57, 1.71) | <0.001 | Ref | 0.01 (−0.34, 0.36) | 0.12 (−0.26, 0.49) | 0.545 |
| Model 3 | Ref | 0.73 (0.14, 1.33) | 1.15 (0.58, 1.72) | <0.001 | Ref | −0.01 (−0.35, 0.33) | 0.13 (−0.25, 0.50) | 0.508 |
| 22–26 h urine samples with no missed voids | ||||||||
| Model 1 | Ref | 0.62 (0.06, 1.17) | 1.03 (0.35, 1.70) | 0.003 | Ref | 0.01 (−0.48, 0.48) | 0.12 (−0.37, 0.61) | 0.625 |
| Model 2 | Ref | 0.75 (0.22, 1.27) | 1.09 (0.44, 1.73) | 0.001 | Ref | 0.03 (−0.39, 0.45) | 0.01 (−0.46, 0.47) | 0.984 |
| Model 3 | Ref | 0.74 (0.22, 1.26) | 1.10 (0.47, 1.73) | <0.001 | Ref | 0.02 (−0.39, 0.43) | 0.01 (−0.44, 0.46) | 0.963 |
| Creatinine index ≥0.7 & no missed voids | ||||||||
| Model 1 | Ref | 1.13 (0.55, 1.72) | 1.31 (0.71, 1.92) | <0.001 | Ref | 0.51 (0.12, 0.89) | 0.53 (0.18, 0.88) | 0.003 |
| Model 2 | Ref | 1.24 (0.65, 1.84) | 1.33 (0.75, 1.91) | <0.001 | Ref | 0.52 (0.15, 0.90) | 0.41 (0.02, 0.80) | 0.037 |
| Model 3 | Ref | 1.21 (0.62, 1.81) | 1.33 (0.74, 1.92) | <0.001 | Ref | 0.50 (0.12, 0.87) | 0.41 (0.01, 0.81) | 0.047 |
| mCER within 15% of Kawasaki predicted daily creatinine | ||||||||
| Model 1 | Ref | 2.21 (0.82, 3.60) | 2.56 (1.03, 4.09) | 0.001 | Ref | 0.95 (−0.01, 1.91) | 1.38 (0.41, 2.35) | 0.005 |
| Model 2 | Ref | 2.49 (1.02, 3.95) | 2.96 (1.45, 4.46) | 0.001 | Ref | 0.84 (−0.13, 1.81) | 1.88 (0.24, 2.14) | 0.014 |
| Model 3 | Ref | 2.49 (1.02, 3.97) | 2.97 (1.48, 4.46) | <0.001 | Ref | 0.87 (−0.11, 1.84) | 1.20 (0.25, 2.15) | 0.013 |
| mCER within 25% of Kawasaki predicted daily creatinine | ||||||||
| Model 1 | Ref | 0.63 (−0.44, 1.69) | 1.41 (0.17, 2.64) | 0.025 | Ref | 0.18 (−0.51, 0.88) | 0.72 (−0.12, 1.56) | 0.095 |
| Model 2 | Ref | 0.80 (−0.28, 1.88) | 1.54 (0.34, 2.74) | 0.012 | Ref | 0.08 (−0.63, 0.80) | 0.46 (−0.40, 1.31) | 0.295 |
| Model 3 | Ref | 0.79 (−0.28, 1.85) | 1.55 (0.37, 2.72) | 0.010 | Ref | 0.08 (−0.65, 0.80) | 0.46 (−0.40, 1.31) | 0.295 |
| mCER 15–25 mg/kg/24 h for men and 10–20 mg/kg/24 h for women | ||||||||
| Model 1 | Ref | 2.31 (1.09, 3.52) | 3.83 (2.19, 5.46) | <0.001 | Ref | 1.59 (0.81, 2.38) | 2.29 (1.33, 3.25) | <0.001 |
| Model 2 | Ref | 2.01 (0.88, 3.15) | 3.34 (1.76, 4.91) | <0.001 | Ref | 1.23 (0.45, 2.02) | 1.62 (0.63, 2.61) | 0.001 |
| Model 3 | Ref | 2.01 (0.85, 3.16) | 3.36 (1.75, 4.96) | <0.001 | Ref | 1.23 (0.45, 2.02) | 1.66 (0.66, 2.66) | 0.001 |
| Methods for Complete 24-h Urine Assessment | Model 1 β (95% CI) | Model 2 β (95% CI) | Model 3 β (95% CI) |
|---|---|---|---|
| Systolic blood pressure | |||
| No self-reported missed voids (n = 7176) | 0.57 (0.28, 0.86) | 0.64 (0.34, 0.94) | 0.64 (0.34, 0.94) |
| 22–26 h urine samples with no missed voids (n = 5524) | 0.54 (0.21, 0.87) | 0.53 (0.19, 0.87) | 0.54 (0.21, 0.87) |
| Creatinine index ≥0.7 and no missed voids (n = 5995) | 0.67 (0.29, 1.06) | 0.68 (0.28, 1.09) | 0.68 (0.27, 1.08) |
| mCER within 15% of Kawasaki pCER and no missed voids (n = 1569) | 0.86 (−0.11, 1.84) | 0.97 (−0.08, 2.02) | 0.98 (−0.07, 2.02) |
| mCER within 25% of Kawasaki pCER and no missed voids (n = 2439) | 0.81 (0.05, 1.58) | 0.87 (0.06, 1.67) | 0.87 (0.07, 1.67) |
| mCER 15–25 mg/kg/24 h (men) and 10–20 mg/kg/24 h (women), and no missed voids (n = 2109) | 2.25 (1.10, 3.39) | 1.94 (0.90, 2.98) | 1.96 (0.93, 2.99) |
| Diastolic blood pressure | |||
| No self-reported missed voids (n = 7176) | 0.06 (−0.15, 0.27) | 0.02 (−0.18, 0.23) | 0.03 (−0.18, 0.24) |
| 22–26 h urine samples with no missed voids (n = 5524) | 0.04 (−0.22, 0.30) | −0.04 (−0.30, 0.21) | −0.04 (−0.30, 0.22) |
| Creatinine index ≥0.7 and no missed voids (n = 5995) | 0.16 (−0.09, 0.40) | 0.09 (−0.16, 0.35) | 0.09 (−0.17, 0.36) |
| mCER within 15% of Kawasaki pCER and no missed voids (n = 1569) | 0.38 (−0.07, 0.83) | 0.29 (−0.1, 0.69) | 0.30 (−0.09, 0.70) |
| mCER within 25% of Kawasaki pCER and no missed voids (n = 2439) | 0.41 (−0.03, 0.84) | 0.29 (−0.12, 0.71) | 0.29 (−0.12, 0.71) |
| mCER 15–25 mg/kg/24 h (men) and 10–20 mg/kg/24 h (women), and no missed voids (n = 2109) | 1.30 (0.53, 2.08) | 0.85 (0.13, 1.56) | 0.88 (0.16, 1.59) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naser, A.M.; He, F.J.; Rahman, M.; Narayan, K.M.V.; Campbell, N.R.C. Urinary Sodium Excretion and Blood Pressure Relationship across Methods of Evaluating the Completeness of 24-h Urine Collections. Nutrients 2020, 12, 2772. https://doi.org/10.3390/nu12092772
Naser AM, He FJ, Rahman M, Narayan KMV, Campbell NRC. Urinary Sodium Excretion and Blood Pressure Relationship across Methods of Evaluating the Completeness of 24-h Urine Collections. Nutrients. 2020; 12(9):2772. https://doi.org/10.3390/nu12092772
Chicago/Turabian StyleNaser, Abu Mohd, Feng J. He, Mahbubur Rahman, K. M. Venkat Narayan, and Norm R. C. Campbell. 2020. "Urinary Sodium Excretion and Blood Pressure Relationship across Methods of Evaluating the Completeness of 24-h Urine Collections" Nutrients 12, no. 9: 2772. https://doi.org/10.3390/nu12092772
APA StyleNaser, A. M., He, F. J., Rahman, M., Narayan, K. M. V., & Campbell, N. R. C. (2020). Urinary Sodium Excretion and Blood Pressure Relationship across Methods of Evaluating the Completeness of 24-h Urine Collections. Nutrients, 12(9), 2772. https://doi.org/10.3390/nu12092772





