Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients
Abstract
1. Introduction
2. Impact of the Gluten-Free Diet on the Microbiome
3. Methods
4. Dietary Supplements Beyond the Gluten-Free Diet
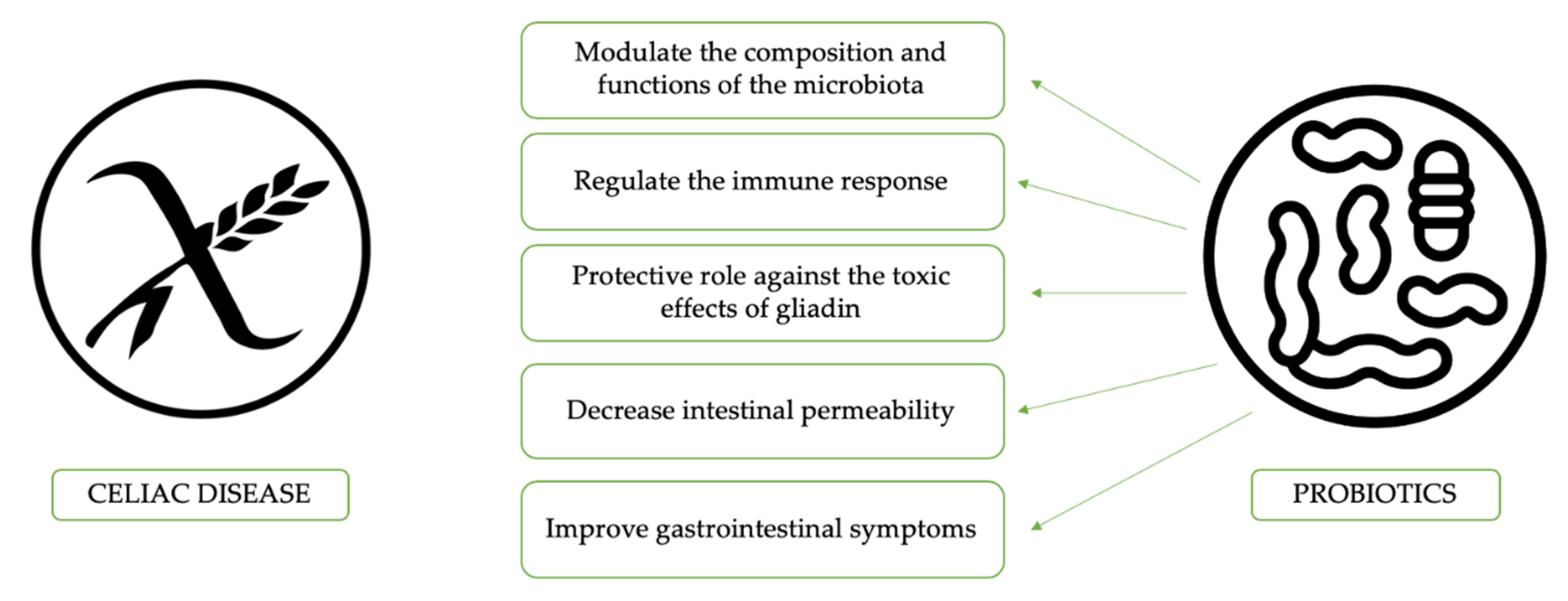
4.1. Probiotics
4.2. Prebiotics
4.3. Synbiotic in Celiac Disease
4.4. Other Dietary Supplements
5. Impact of Oat Intake
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rubio-Tapia, A.; Murray, J.A. Updated guidelines by the European Society for the Study of Coeliac Disease. United Eur. Gastroenterol. J. 2019, 7, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Araya, M.; Roncoroni, L.; Doneda, L.; Elli, L. Dietary Gluten as a Conditioning Factor of the Gut Microbiota in Celiac Disease. Adv. Nutr. 2020, 11, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Gambuti, E.; Alfano, F.; Strada, A.; Ciccocioppo, R.; Lungaro, L.; Zoli, G.; Volta, U.; De Giorgio, R.; Caio, G. Life-threatening onset of coeliac disease: A case report and literature review. BMJ Open Gastroenterol. 2020, 7, e000406. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Ursini, F.; Manfredini, R.; De Giorgio, R. Small bowel adenocarcinoma as a complication of celiac disease: Clinical and diagnostic features. BMC Gastroenterol. 2019, 19, 1–19. [Google Scholar] [CrossRef]
- Vanoli, A.; Di Sabatino, A.; Furlan, D.; Klersy, C.; Grillo, F.; Fiocca, R.; Mescoli, C.; Rugge, M.; Nesi, G.; Fociani, P.; et al. Small bowel carcinomas in coeliac or Crohn’s disease: Clinico-pathological, molecular, and prognostic features. A study from the small bowel cancer Italian consortium. J. Crohn’s Colitis 2017, 11, 942–953. [Google Scholar] [CrossRef]
- Harnett, J.; Myers, S.P.; Rolfe, M. Probiotics and the Microbiome in Celiac Disease: A Randomised Controlled Trial. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Caio, G.; Riegler, G.; Patt Urelli, M.; Facchiano, A.; De Magistris, L.; Sapone, A. Pathophysiology of non-celiac gluten sensitivity: Where are we now? Minerva Gastroenterol. Dietol. 2017, 63, 16–21. [Google Scholar]
- Volta, U.; Caio, G.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Boschetti, E.; Stanghellini, V.; De Giorgio, R. Features and Progression of Potential Celiac Disease in Adults. Clin. Gastroenterol. Hepatol. 2016, 14, 686–693. [Google Scholar] [CrossRef]
- Kaukinen, K.; Lindfors, K.; Mäki, M. Advances in the treatment of coeliac disease: An immunopathogenic perspective. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabó, I.R.; Kovács, J.B.; Lorincz, M.; Gorácz, G.; Szabados, K.; Balogh, M. Prospective significance of antiendomysium antibody positivity in subsequently verified celiac disease. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Ciclitira, P.; Hadjivassiliou, M.; Kaukinen, K.; Ludvigsson, J.F.; McGough, N.; Sanders, D.S.; Woodward, J.; Leonard, J.N.; Swift, G.L. The gluten-Free diet and its current application in coeliac disease and dermatitis Herpetiformis. United Eur. Gastroenterol. J. 2015, 3, 121–135. [Google Scholar] [CrossRef] [PubMed]
- ÁRnason, A.; Skaftadóttir, I.; Sigmundsson, J.; Mooney, E.; Björnsson, J.; Cariglia, N.; Pálsson, G.; Gudjónsson, H. The association between coeliac disease, dermatitis herpetiformis and certain hla-antigens in icelanders. Int. J. Immunogenet. 1994, 21, 457–460. [Google Scholar] [CrossRef]
- Diez-Sampedro, A.; Olenick, M.; Maltseva, T.; Flowes, M. A Gluten-Free Diet, Not an Appropriate Choice Without a Medical Diagnosis. J. Nutr. Metab. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Davis, M.A.; Karagas, M.R.; Ahsan, H.; Argos, M. The unintended consequences of a gluten-free diet. Epidemiology 2017, 28, e24–e25. [Google Scholar] [CrossRef][Green Version]
- Bouguerra, F.; Babron, M.C.; Eliaou, J.F.; Debbabi, A.; Clot, J.; Khaldi, F.; Greco, L.; Clerget-Darpoux, F. Synergistic Effect of Two HLA Heterodimers in the Susceptibility to Celiac Disease in Tunisia. Genet. Epidemiol. 1997, 14, 413–422. [Google Scholar] [CrossRef]
- Pasqui, F.; Poli, C.; Colecchia, A.; Marasco, G.; Festi, D. Adverse food reaction and functional gastrointestinal disorders: Role of the dietetic approach. J. Gastrointest. Liver Dis. 2015, 24, 319–327. [Google Scholar] [CrossRef]
- Marasco, G.; Colecchia, A.; Festi, D. Dysbiosis in celiac disease patients with persistent symptoms on gluten-free diet: A condition similar to that present in irritable bowel syndrome patients? Am. J. Gastroenterol. 2015, 110, 598. [Google Scholar] [CrossRef]
- Boy, M.F.; La Nasa, G.; Balestrieri, A.; Cherchi, M.V.; Usai, P. Distribution of HLA-DPBl,-DQBI-DQAl alleles among sardinian celiac patients. Dis. Markers 1994, 12, 199–204. [Google Scholar] [CrossRef]
- Nadal, I.; Donat, E.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Herrán, A.R.; Arias, L.; Vivas, S.; de Morales, J.M.R.; Calleja, S.; de Miera, L.E.S.; Arroyo, P.; Casqueiro, J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 2012, 18, 649–656. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef]
- Jackson, F.W. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects—Comment by Jackson. Br. J. Nutr. 2010, 104, 773. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, C.G.; Gagliardi, F.; Ricciuti, P.; Ndagijimana, M.; Francavilla, R.; Guerzoni, M.E.; Crecchio, C.; Gobbetti, M.; De Angelis, M. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 2009, 75, 3963–3971. [Google Scholar] [CrossRef]
- Schippa, S.; Iebba, V.; Barbato, M.; Di Nardo, G.; Totino, V.; Checchi, M.P.; Longhi, C.; Maiella, G.; Cucchiara, S.; Conte, M.P. A distinctive “microbial signature” in celiac pediatric patients. BMC Microbiol. 2010, 10, 175. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. BioMed Res. Int. 2016, 2016, 4740907. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. Corrigendum to “A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation”. BioMed Res. Int. 2019, 2019, 9042956. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; Hyett, B.; Kelly, E.; Schuppan, D.; Kelly, C.P. A figure is presented}Etiologies and Predictors of Diagnosis in Nonresponsive Celiac Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 445–450. [Google Scholar] [CrossRef]
- Dewar, D.H.; Donnelly, S.C.; McLaughlin, S.D.; Johnson, M.W.; Ellis, H.J.; Ciclitira, P.J. Celiac disease: Management of persistent symptoms in patients on a gluten-free diet. World J. Gastroenterol. 2012, 18, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Ciccocioppo, R.; Zoli, G.; De Giorgio, R.; Volta, U. Therapeutic options for coeliac disease: What else beyond gluten-free diet? Dig. Liver Dis. 2020, 52, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Congia, M.; Frau, F.; Lampis, R.; Frau, R.; Mele, R.; Cucca, F.; Muntoni, F.; Porcu, S.; Boi, F.; Contu, L.; et al. A high frequency of the A30, B18, DR3, DRw52, DQw2 extended haplotype in Sardinian celiac disease patients: Further evidence that disease susceptibility is conferred by DQ A1*0501, B1*0201. Tissue Antigens 1992, 39, 78–83. [Google Scholar] [CrossRef]
- Djilali-Saiah, I.; Caillat-Zucman, S.; Schmitz, J.; Laise Chaves-Vieira, M.; Bach, J.F. Polymorphism of antigen processing (TAP, LMP) and HLA class II genes in celiac disease. Hum. Immunol. 1994, 40, 8–16. [Google Scholar] [CrossRef]
- Wacklin, P.; Kaukinen, K.; Tuovinen, E.; Collin, P.; Lindfors, K.; Partanen, J.; Mäki, M.; Mättuö, J. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm. Bowel Dis. 2013, 19, 934–941. [Google Scholar] [CrossRef]
- Marasco, G.; Di Biase, A.R.; Schiumerini, R.; Eusebi, L.H.; Iughetti, L.; Ravaioli, F.; Scaioli, E.; Colecchia, A.; Festi, D. Gut Microbiota and Celiac Disease. Dig. Dis. Sci. 2016, 61, 1461–1472. [Google Scholar] [CrossRef]
- Di Biase, A.R.; Marasco, G.; Ravaioli, F.; Dajti, E.; Colecchia, L.; Righi, B.; D’Amico, V.; Festi, D.; Iughetti, L.; Colecchia, A. Gut microbiota signatures and clinical manifestations in celiac disease children at onset: A pilot study. J. Gastroenterol. Hepatol. 2020, jgh.15183. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Vivas, S.; Ruiz De Morales, J.M.; Sáenz De Miera, L.E.; Rodríguez-Aparicio, L.B.; Casqueiro, J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Drabińska, N.; Jarocka-Cyrta, E. The effect of oligofructose-enriched inulin supplementation on gut microbiota, nutritional status and gastrointestinal symptoms in paediatric coeliac disease patients on a gluten-free diet: Study protocol for a pilot randomized controlled trial. Nutr. J. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Heal Dis. 2015, 26. [Google Scholar] [CrossRef]
- Ou, G.; Hedberg, M.; Hörstedt, P.; Baranov, V.; Forsberg, G.; Drobni, M.; Sandström, O.; Wai, S.N.; Johansson, I.; Hammarström, M.L.; et al. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am. J. Gastroenterol. 2009, 104, 3058–3067. [Google Scholar] [CrossRef]
- Adebola, O.O.; Corcoran, O.; Morgan, W.A. Synbiotics: The impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. J. Funct. Foods 2014, 10, 75–84. [Google Scholar] [CrossRef]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef]
- Lindfors, K.; Blomqvist, T.; Juuti-Uusitalo, K.; Stenman, S.; Venäläinen, J.; Mäki, M.; Kaukinen, K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 2008, 152, 552–558. [Google Scholar] [CrossRef]
- D’Arienzo, R.; Stefanile, R.; Maurano, F.; Mazzarella, G.; Ricca, E.; Troncone, R.; Auricchio, S.; Rossi, M. Immunomodulatory effects of Lactobacillus casei administration in a mouse model of gliadin-sensitive enteropathy. Scand. J. Immunol. 2011, 74, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Olivares, M.; Gallina, O.; Sanz, Y. Bifidobacterium longum CECT 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PLoS ONE 2012, 7, e30744. [Google Scholar] [CrossRef] [PubMed]
- Papista, C.; Gerakopoulos, V.; Kourelis, A.; Sounidaki, M.; Kontana, A.; Berthelot, L.; Moura, I.C.; Monteiro, R.C.; Yiangou, M. Gluten induces coeliac-like disease in sensitised mice involving IgA, CD71 and transglutaminase 2 interactions that are prevented by probiotics. Lab. Investig. 2012, 92, 625–635. [Google Scholar] [CrossRef]
- De Angelis, M.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; De Simone, C.; Silano, M.; De Vincenzi, M.; Losito, I.; Gobbetti, M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim. Biophys. Acta-Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Medina, M.; Izquierdo, E.; Ennahar, S.; Sanz, Y. Differential immunomodulatory properties of Bifidobacterium logum strains: Relevance to probiotic selection and clinical applications. Clin. Exp. Immunol. 2007, 150, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Hwang, H.J.; Sugai, E.; Corso, L.; Cherñavsky, A.C.; Bellavite, F.P.; González, A.; Vodánovich, F.; Moreno, M.L.; Vázquez, H.; et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J. Clin. Gastroenterol. 2013, 47, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Castillejo, G.; Varea, V.; Sanz, Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br. J. Nutr. 2014, 112, 30–40. [Google Scholar] [CrossRef]
- Pisarello, M.L.J.; Vintiñi, E.O.; González, S.N.; Pagani, F.; Medina, M.S. Decrease in lactobacilli in the intestinal microbiota of celiac children with a gluten-free diet, And selection of potentially probiotic strains. Can. J. Microbiol. 2014, 61, 32–37. [Google Scholar] [CrossRef]
- Golfetto, L.; de Senna, F.D.; Hermes, J.; Beserra, B.T.S.; da Silva França, F.; Martinello, F. Baixa contagem de bifidobactérias em pacientes adultos com doença celíaca, em dieta isenta de glúten. Arq. Gastroenterol. 2014, 51, 139–143. [Google Scholar] [CrossRef]
- Klemenak, M.; Dolinšek, J.; Langerholc, T.; Di Gioia, D.; Mičetić-Turk, D. Administration of Bifidobacterium breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig. Dis. Sci. 2015, 60, 3386–3392. [Google Scholar] [CrossRef]
- Quagliariello, A.; Aloisio, I.; Bozzicionci, N.; Luiselli, D.; D’Auria, G.; Martinez-Priego, L.; Pérez-Villarroya, D.; Langerholc, T.; Primec, M.; Mičetić-Turk, D.; et al. Effect of bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: A pilot study. Nutrients 2016, 8, 660. [Google Scholar] [CrossRef]
- Martinello, F.; Roman, C.F.; de Souza, P.A. Efeitos do consumo de probióticos sobre as bifidobactérias intestinais de pacientes celíacos. Arq. Gastroenterol. 2017, 54, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sánchez, M.I.; Smecuol, E.C.; Temprano, M.P.; Sugai, E.; González, A.; Moreno, M.L.; Huang, X.; Bercik, P.; Cabanne, A.; Vázquez, H.; et al. Bifidobacterium infantis NLS Super Strain Reduces the Expression of α-Defensin-5, a Marker of Innate Immunity, in the Mucosa of Active Celiac Disease Patients. J. Clin. Gastroenterol. 2017, 51, 814–817. [Google Scholar] [CrossRef]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019, 53, E117–E125. [Google Scholar] [CrossRef] [PubMed]
- Primec, M.; Klemenak, M.; Di Gioia, D.; Aloisio, I.; Bozzi Cionci, N.; Quagliariello, A.; Gorenjak, M.; Mičetić-Turk, D.; Langerholc, T. Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial modulators of TNF-α and short-chain fatty acids. Clin. Nutr. 2019, 38, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, U.; Aronsson, C.A.; Liu, X.; Kurppa, K.; Yang, J.; Liu, E.; Skidmore, J.; Winkler, C.; Rewers, M.J.; Hagopian, W.A.; et al. Early probiotic supplementation and the risk of celiac disease in children at genetic risk. Nutrients 2019, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.; Samson, S.; Bourdet-Sicard, R.; Rubio, M.; Sarfati, M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J. Leukoc. Biol. 2008, 84, 468–476. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J. Cell. Biochem. 2010, 109, 801–807. [Google Scholar] [CrossRef]
- Laparra, J.M.; Olivares, M.; Sanz, Y. Oral administration of Bifidobacterium longum CECT 7347 ameliorates gliadin-induced alterations in liver iron mobilisation. Br. J. Nutr. 2013, 110, 1828–1836. [Google Scholar] [CrossRef]
- De Palma, G.; Cinova, J.; Stepankova, R.; Tuckova, L.; Sanz, Y. Pivotal Advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J. Leukoc. Biol. 2010, 87, 765–778. [Google Scholar] [CrossRef]
- World Gastroenterology Organisation Global Guidelines Probiotics and Prebiotics. World Gastroenterology Organisation, 2017. Available online: www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf (accessed on 30 June 2020).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. Intestinal permeability in children with celiac disease after the administration of oligofructose-enriched inulin into a gluten-free diet—Results of a randomized, placebo-controlled, pilot trial. Nutrients 2020, 12, 1736. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–549. [Google Scholar] [CrossRef]
- Kanauchi, O.; Andoh, A.; Iwanaga, T.; Fujiyama, Y.; Mitsuyama, K.; Toyonaga, A.; Bamba, T. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J. Gastroenterol. Hepatol. 1999, 14, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Finlay, R.K.; Wynne, A.G.; Gibson, G.R. A human volunteer study on the prebiotic effects of HP-inulin—Faecal bacteria enumerated using fluorescent In situ hybridisation (FISH). Anaerobe 2001, 7, 113–118. [Google Scholar] [CrossRef]
- Nilsson, U.; Nyman, M. Short-chain Fatty Acid Formation in the Hindgut of Rats Fed Oligosaccharides Varying in Monomeric Composition, Degree of Polymerisation and Solubility. Br. J. Nutr. 2005, 94, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Abela, A.G.; Fava, S. Does the level of bacterial exposure in early life impact the risk of type 1 diabetes? Expert Rev. Clin. Immunol. 2013, 9, 695–697. [Google Scholar] [CrossRef]
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The effect of oligofructose-enriched inulin on faecal bacterial counts and microbiota-associated characteristics in celiac disease children following a gluten-free diet: Results of a randomized, placebo-controlled trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef]
- Feruś, K.; Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. A randomized, placebo-controlled, pilot clinical trial to evaluate the effect of supplementation with prebiotic synergy 1 on iron homeostasis in children and adolescents with celiac disease treated with a gluten-free diet. Nutrients 2018, 10, 1818. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Ciska, E.; Jarocka-Cyrta, E. Plasma profile and urine excretion of amino acids in children with celiac disease on gluten-free diet after oligofructose-enriched inulin intervention: Results of a randomised placebo-controlled pilot study. Amino Acids 2018, 50, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Markiewicz, L.H.; Lamparski, G.; Juśkiewicz, J. Administration of inulin-supplemented gluten-free diet modified calcium absorption and caecal microbiota in rats in a calcium-dependent manner. Nutrients 2017, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Świątecka, D.; Bączek, N.; Brzóska, M.M. Inulin and fructooligosaccharide affect: In vitro calcium uptake and absorption from calcium-enriched gluten-free bread. Food Funct. 2016, 7, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Capriles, V.D.; Arêas, J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Korus, J.; Grzelak, K.; Achremowicz, K.; Sabat, R. Influence of Prebiotic Additions on the Quality of Gluten-free Bread and on the Content of Inulin and Fructooligosaccharides. Food Sci. Technol. Int. 2006, 12, 489–495. [Google Scholar] [CrossRef]
- Fuller, Z.; Louis, P.; Mihajlovski, A.; Rungapamestry, V.; Ratcliffe, B.; Duncan, A.J. Influence of cabbage processing methods and prebiotic manipulationof colonic microflora on glucosinolate breakdown in man. Br. J. Nutr. 2007, 98, 364–372. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: A review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-free diet: Gaps and needs for a healthier diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.D.; Chen, Y. Oat safety for celiac disease patients: Theoretical analysis correlates adverse symptoms in clinical studies to contaminated study oats. Nutr. Res. 2018, 60, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Högberg, L.; Laurin, P.; Fâlth-Magnusson, K.; Grant, C.; Grodzinsky, E.; Jansson, G.; Ascher, H.; Browaldh, L.; Hammersjö, J.Å.; Lindberg, E.; et al. Oats to children with newly diagnosed coeliac disease: A randomised double blind study. Gut 2004, 53, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lundin, K.E.A.; Nilsen, E.M.; Scott, H.G.; Løberg, E.M.; Gjøen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Loøvik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.A.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, O.; Villanen, M.; Korponay-Szabo, I.; Lindfors, K.; Mäki, M.; Kaukinen, K. Oats Do Not Induce Systemic or Mucosal Autoantibody Response in Children With Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 559–565. [Google Scholar] [CrossRef]
- Hollén, E.; Peterson, K.H.; Sundqvist, T.; Grodzinsky, E.; Högberg, L.; Laurin, P.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.E. Coeliac children on a gluten-free diet with or without oats display equal anti-avenin antibody titres. Scand. J. Gastroenterol. 2006, 41, 42–47. [Google Scholar] [CrossRef]
- Hollén, E.; Forslund, T.; Högberg, L.; Laurin, P.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.E.; Sundqvist, T. Urinary nitric oxide during one year of gluten-free diet with or without oats in children with coeliac disease. Scand. J. Gastroenterol. 2006, 41, 1272–1278. [Google Scholar] [CrossRef]
- Troncone, R.; Ivarsson, A.; Szajewska, H.; Mearin, M.L. Review article: Future research on coeliac disease—A position report from the European multistakeholder platform on coeliac disease (CDEUSSA). Aliment. Pharmacol. Ther. 2008, 27, 1030–1043. [Google Scholar] [CrossRef]
| Author, Year | Composition, Strains | Duration of Administration | Study Design | Aims and Findings | Meaning |
|---|---|---|---|---|---|
| Lindfors K. et al., 2008 [50]. | Bifidobacterium lactis | In vitro study | - | Inhibit the gluten/gliadin-induced damage in the small-intestinal mucosa. | Inhibition dose-dependent to increased epithelial gliadin-induced permeability and stimulation of IL-10 production by regulatory T-cells. |
| D’Arienzo et al., 2011 [51]. | Lactobacillus casei ATCC 9595 | 35 days | Animal study | Complete recovery of villous blunting, decreased weight loss and recovered basal TNF-α levels. | L. casei was effective in rescuing the normal mucosal architecture and Gut associated lymphoid tissue homeostasis. |
| Laparra et al., 2012 [52]. | Bifidobacterium longum CECT 7347 | 10 days from birth | Animal study | In gluten-sensitized animals B. longum administration increased NFκB expression, IL-10, CD8+, but reduced TNF-α expression, CD4+ and CD4+/Fox3+ cell populations. | B. longum regulates inflammatory cytokine production and CD4+ T cell mediated immune response in an animal model of gliadin induced enteropathy. |
| Papista et al., 2012 [53]. | Saccharomyces boulardi KK1 strain, hydrolyzed the 28-kDa gliadin fraction | 30 days | Animal study | S. boulardi administration improved enteropathy development, decreased epithelial cell expression of CD71 and localized cytokine production. | A new mouse model for human CD based on histopathological features and common biomarkers. S. boulardi showed activity in the treatment of CD by reversing disease development. |
| Author, Year | Composition, Strains | Duration of Administration | Study Design | Number of Participants | Aims and Findings | Meaning |
|---|---|---|---|---|---|---|
| De Angelis et al., 2006 [54]. | VSL#3 | - | Comparative study | - | VSL#3 can largely colonize the intestine for a long period. | VSL#3 treatment would eliminate any traces of toxic peptides in processed foods minimizing the long-term risks and improving the quality life. |
| Medina M. et al., 2007 [55]. | Bifidobacterium longum | 4 months | Comparative study | - | Genomic DNA of some strains stimulated the production of Th1 and pro-inflammatory cytokines, interferon-gamma and TNF-a, instead of IL-10. | Immunomodulatory activity of B. longum. |
| Smecuol et al., 2013 [56]. | Bifidobacterium natren life start | 3 weeks treatment, follow up on day 50 | Double blind, randomize, placebo-controlled trial | 22 (n = 12 B. NLS, n = 10 placebo) | Effect on intestinal permeability; outcome of clinical symptoms by GSRS questionnaire; modification of immunologic indicators influenced by gluten. | Administration of Bifidobacterium NLS to untreated CD patients does not modify protein abnormalities but might improve symptoms and produce immunologic changes. |
| Olivares et al., 2014 [57]. | Bifidobacterium longum CECT 7347 | 3 months | Double blind, randomized, placebo-controlled trial | 33 (n = 17 B. longum CECT 7347, n = 16 placebo) | Baseline and post-intervention outcomes (immune phenotype of peripheral blood cells, serum cytokine, fecal secretory IgA, anthropometric parameters and intestinal microbiota composition). | Patients undergoing probiotic treatment showed greater height percentile, decreased peripheral CD3+ T lymphocytes, and slightly reduced TNF-α concentration. Additionally, reduced B. fragilis and secretory IgA in the stool. |
| Pisarello et al., 2014 [58]. | Lactobacillus rhamnosus; Lactobacillus paracasei | 11 months | Comparative study | 30 (n = 15 healthy, n = 15 CD children) | Lactobacillus counts in the CD children on a GFD group revealed significantly lower values than those in the healthy controls group. | Treatment with probiotics cannot replace GFD but is able to attenuate the altered inflammatory parameters in celiac individuals and to modify the composition of the intestinal microbiota. |
| Golfetto et al., 2014 [59]. | Bifidobacteria spp. | - | Comparative study | 14 CD patients, 42 health control | The concentration of Bifidobacteria per gram of feces was significantly higher in healthy subjects (controls) (1.5 ± 0.63 × 108 CFU/g) when compared to celiac patients (2.5 ± 1.5 × 107 CFU/g). | Lower levels of Bifidobacterial can provide an imbalance in the intestinal microbiota of CD patients, regardless of pH, even while on a gluten-free diet. |
| Klemenak et al., 2015 [60]. | Bifidobacterium breve BRO3 and B. breve B632 | 3 months | Double-blinded, placebo-controlled trial | 49 CD children (n = 24 B. breve BRO3 and B. breve B632, n = 25 placebo), 18 healthy control | Outcomes: level of Serum production of IL-10; TNF-α. | TNF-α levels decreased after 3 months of probiotic treatment, however on follow up after 3 months, the levels increased. The IL-10 levels were below detection. |
| Quagliariello et al., 2016 [61]. | Bifidbacterium breve strains B632 and BRO3 | 3 months | Double-blinded, placebo-controlled study | 40 CD children, 16 healthy control | Determination of microbiome after probiotic treatment. | 3-month administration of probiotic can restore the microbiota of CD patients similar to healthy children. |
| Harnett et al., 2016 [8]. | A proprietary blend of 450 billion viable lyophilized bacteria (9 strains) known as the De Simone formulation, previously VSL#3. | 12 weeks | Multicenter randomized Placebo-controlled trial | 45 (n = 23 VSL#3, n = 22 placebo) | Microbial counts and comparison between baseline and end-of-study of predominant, pathogenic and opportunistic bacteria. Urinary metabolomics and fecal lactoferrin. | No significant changes in the gastrointestinal microbial counts in CD individuals with persistent symptoms over 12 weeks period. |
| Martinello et al., 2017 [62]. | Yogurt with probiotic from PIA, Nova Petropolis-RS (undetermined microbial concentration). | 30 days | Case-control study | 14 CD patients, 17 healthy control | Fecal bifidobacteria concentration after consuming 100 g of yogurt in the morning. | Fecal Bifidobacteria count was higher in healthy patients compared to CD patients. Probiotic yogurt consumption increased the Bifidobacteria number in CD patients but not in healthy participants. |
| Pinto-Sanchez et al., 2017 [63] | B. infantis Natren Life Start super strain. | 6 weeks | Double-blinded, randomized, placebo-controlled study | 41 (n = 24 active no treatment CD, n = 12 active CD B. NLS, n = 5 GFD) | Determine mucosal expression of innate immune markers: number of macrophages, Paneth cells and α-defensin-5 expression by immunohistochemistry in duodenal biopsies. | Duodenal biopsies revealed that B. infantis NLS-SS decreased all the three markers in CD patients. However, the decrease in macrophage counts was higher in GFD. |
| Francavilla et al., 2019 [64]. | A product containing five strains: L. casei, L. plantarum, B. animalis subsp. Lacti, B. breve Bbr8 LMG P-17501 and B. breve B110 LMG P-17500. | A 6-week treatment period, precede by 2-week run in period followed by a 6 week follow up phase for a total of 14 weeks. | Prospective, double- blind, randomized placebo-controlled parallel group study | 109 (n = 54 probiotics, n = 55 placebo) | Determine if probiotics improve GI symptoms as assessed by IBS-SSS. | Probiotics significantly decreased the IBS-SSS and GSRS scores compared to the placebo, reduced IBS-type symptoms. Probiotics in CD patients on strict GFD diet modified the gut microbiota (increase the Bifidobacteria). |
| Primec et al., 2019 [65]. | Bifidobacterium breve strains B632 and BRO3. | 3 months. | Double-blinded, placebo-controlled study | 40 CD children (n = 20 probiotics, n = 20 placebo), 16 healthy control | Evaluate the influence of probiotics on the fecal microbiome, SCFA and serum TNF-α. | Verrucomicrobia, Paracubacteria and some yet unknown phyla of bacteria and archaea showed a strong correlation to CD. |
| Uusitalo et al., 2019 [66]. | L. reuteri; L. rhamnosus, and some unidentified. | Different time periods | Prospective study | 6520 | To study the association between the exposure of probiotics via dietary supplements or by infant formula since 1 year old for the development of CDA or CD. | Overall exposure of probiotics during the first year of age was not associated with CDA or CD. However, intake of probiotics via dietary supplements was associated with increased risk of CDA. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasco, G.; Cirota, G.G.; Rossini, B.; Lungaro, L.; Di Biase, A.R.; Colecchia, A.; Volta, U.; De Giorgio, R.; Festi, D.; Caio, G. Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients 2020, 12, 2674. https://doi.org/10.3390/nu12092674
Marasco G, Cirota GG, Rossini B, Lungaro L, Di Biase AR, Colecchia A, Volta U, De Giorgio R, Festi D, Caio G. Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients. 2020; 12(9):2674. https://doi.org/10.3390/nu12092674
Chicago/Turabian StyleMarasco, Giovanni, Giovanna Grazia Cirota, Benedetta Rossini, Lisa Lungaro, Anna Rita Di Biase, Antonio Colecchia, Umberto Volta, Roberto De Giorgio, Davide Festi, and Giacomo Caio. 2020. "Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients" Nutrients 12, no. 9: 2674. https://doi.org/10.3390/nu12092674
APA StyleMarasco, G., Cirota, G. G., Rossini, B., Lungaro, L., Di Biase, A. R., Colecchia, A., Volta, U., De Giorgio, R., Festi, D., & Caio, G. (2020). Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients, 12(9), 2674. https://doi.org/10.3390/nu12092674








