Abstract
To date, the only available treatment for celiac disease (CD) patients is a life-lasting gluten-free diet (GFD). Lack of adherence to the GFD leads to a significant risk of adverse health consequences. Food cross-contamination, nutritional imbalances, and persistent gastrointestinal symptoms are the main concerns related to GFD. Moreover, despite rigid compliance to GFD, patients struggle in achieving a full restoring of the gut microbiota, which plays a role in the nutritive compounds processing, and absorption. Pivotal studies on the supplementation of GFD with probiotics, such as Bifidobacterium and Lactobacilli, reported a potential to restore gut microbiota composition and to pre-digest gluten in the intestinal lumen, reducing the inflammation associated with gluten intake, the intestinal permeability, and the cytokine and antibody production. These findings could explain an improvement in symptoms and quality of life in patients treated with GFD and probiotics. On the other hand, the inclusion of prebiotics in GFD could also be easy to administer and cost-effective as an adjunctive treatment for CD, having the power to stimulate the growth of potentially health-promoting bacteria strains. However, evidence regarding the use of prebiotics and probiotics in patients with CD is still insufficient to justify their use in clinical practice.
1. Introduction
Celiac disease (CD) is a common systemic disorder mainly affecting the small intestine [1], due to the abnormal response of human immunity to gluten ingestion. CD onset is favored in subjects carrying genetic susceptibility (HLA-DQ2/DQ8 positivity and non-HLA genes), under the influence of triggering environmental factors, such as viral infections and dysbiosis of the gut microbiota [2]. Although 30–40% of the global population carries the HLA DQ2/DQ8 genotype, only 1–1.5% of them express the CD phenotype, meaning that other factors, such as diet and environment, take part in the illness outbreak [2,3]. The term “gluten” comprises more than 100 ethanol-soluble proteins, (i.e., prolamins and glutelins), derived from wheat, rye, and barley. These proteins show specific features, such as hydrophobicity and repeated amino-acid sequences rich in glutamine and proline residues, which enhance protein resistance to human intestinal proteases digestion [2,3,4]. To date, the unique treatment that has proven to be effective in CD patients is strictly life-long adherence to the gluten-free diet (GFD), i.e., a diet containing less than 10 mg per day of gluten [1,2]. Lack of adherence to the GFD leads to a significant risk of adverse health consequences [5] and increases mortality from malignancies (e.g., small bowel adenocarcinoma, cancer of the esophagus, B-cell and T-cell non-Hodgkin lymphomas), and in particular intestinal T-cell lymphomas; the risk reduces with strict adherence to the diet [6,7]. Despite rigid compliance to GFD, several studies showed that patients struggle in achieving a full restoring of the gut microbiota; the cause of this phenomenon could be inferred from a persistent genetic influence than to the lack of prebiotics usually ingested with gluten assumption [8]. The supplementation of GFD with probiotics, such as Bifidobacterium and Lactobacilli, could help to restore altered gut microbiota, reducing both gliadin toxicity and immune activation [9], while improving the daily ingestible gluten amount to better tolerate the GFD. Besides, considering the alterations of the gut microbiota as an environmental factor promoting the CD pathogenesis, and considering that the genetic background may influence its composition in subjects at high risk, probiotics administration may have a role in primary prevention for subjects at high risk for CD [10]. Today, research is seeking new nutritional possibilities to improve the life of CD patients, suggesting innovative therapeutic approaches, such as supplements able to reduce intestinal permeability or to suppress the inflammatory immune response, such as probiotics and prebiotics [11]. Thus, this review aims to summarize the recent advances in probiotics, prebiotics, and other dietary supplements for gut microbiota modulation in CD patients.
2. Impact of the Gluten-Free Diet on the Microbiome
The only effective treatment for CD is a life-long strict observance of GFD, which means not only avoiding gluten-containing foods but also gluten contaminations [12]. Among cereals, gluten is present in durum wheat, bread wheat, barley, rye, Khorasan wheat, the three species of spelt, and triticale; other gluten-containing wheat derivates are bulgur and seitan. Gluten has a low nutritional value, but it confers important qualities to foods, improving palatability; it is almost ubiquitous in refined foods that are not declared gluten-free (GF), and that thereby should be avoided by celiac patients [13]. Although the range of high-quality GF products has increased in recent years, and the GFD has gained broad acceptance, it still shows many downsides [13]. First of all, the major issue of GFD is the gluten-free foods’ cross-contamination, which can occur both in the production lines or during the preparation of gluten-free foods at home or when eating out [12]. For this reason, it is suggested to provide CD patients with the knowledge and the skills to adhere to a correct GFD, advising them about “how to read” food labels [14]. Secondly, the GFD is not a complete and balanced diet, but it leads to micro- and macronutrients deficiencies, which is of particular importance when affecting children’s metabolism. In particular, gluten-free foods lack minerals (calcium, iron, magnesium, and zinc), vitamins (vitamin B12, folate, and vitamin D), and fibers [15]. Some authors propose dietary education for CD patients to promote “nutritional awareness”, as many of the inadequacies of dietary intakes, such as the insufficient intake of fiber and folate, may depend on individual food choices. In contrast, some deficiencies, such as thiamin, are CD specific [16]. Moreover, GFD is also a threat to health because it increases dietary exposure to arsenic and other toxic contaminants [17]. Finally, CD patients are nowadays exposed to nutritional imbalances by assuming foods that are high in sugars, fats, and calories, or rich in proteins, like eggs and meat, in addition to snacks with a high content of lipids and low fibers [18,19], which may additionally lead to irritable bowel syndrome-like symptoms [20]. Indeed, the majority of gluten-free commercial grain-based products contain less fiber than their gluten-containing equivalents so patients may report weight gain and constipation [21].
GFD induces microbial shifts with possible consequent impairment of the immune-metabolic homeostasis, which may contribute to functional symptoms’ persistence. Only a few studies longitudinally evaluated gut microbiota before and after long-term GFD. Pivotal studies on duodenal mucosal and fecal microbiota showed an incomplete restoration of the microbial composition after two years of GFD [22,23], with persistently low levels of Bifidobacteria and Lactobacilli along with reduced bacterial diversity [24,25]. Persistently impaired microbial shifts were subsequently found by the same research group [24] focused on Bifidobacterial strains and by another research group [25]. Di Cagno et al. [26] found that CD children treated with GFD had lower levels of Lactobacillus, Enterococcus, and Bifidobacteria, and increased levels of Bacteroides, Staphylococcus, Salmonella, Shigella, and Klebsiella in comparison to healthy controls. Moreover, CD children on a GFD showed lower levels of short-chain fatty acids (SCFAs) with respect to those patients on the gluten-containing diet. Thus, it is possible to conclude that an incomplete microbial restoration is present in CD patients despite the instauration of a GFD. This finding is not surprising since a previous study [27] on healthy subjects undergoing GFD reported a decrease in Bifidobacterium, Bifidobacterium longum, Clostridium lituseburense, Lactobacillus, and Faecalibacterium prausnitzii, and an increase in Enterobacteriaceae and E. coli strains. Moreover, the inflammatory pathway was consistently imbalanced due to GFD, with a proinflammatory shift. This shift might be explained by the reduction in prebiotics included in the gluten-containing diet [28]. Longitudinal evaluation in the same patients before and after GFD confirmed these results, reporting reduced abundances of Lactobacillus, Bifidobacterium, and Bacteroides [29]. Similar results were also reported in a small study on children [30]. Besides a significant impairment in gut microbial restoration characterized by reduced bacterial richness, reduced levels of Bacteroidetes and Firmicutes and higher levels of Proteobacteria have also been found after three years of GFD in CD patients complaining of irritable bowel syndrome-like symptoms [20]. Notably, dysbiosis, namely a microbial imbalance, is also linked to several conditions among which irritable bowel syndrome (IBS) is one of the most common and, as CD, is characterized by different gastrointestinal symptoms. In this setting, several probiotic strains, such as L. plantarum PBS067, B. lactis BL050, L. acidophilus PBS066, L. rhamnosus LRH020, and L. reuteri PBS072, have been demonstrated to improve gastrointestinal distresses thanks to gut eubiosis recovery [31,32].
3. Methods
This narrative review aims to describe the effects of probiotics, prebiotics, and other dietary supplements on the gut microbiota of CD patients in GFD. In the preparation of this manuscript, we followed the narrative review checklist by the Academy of Nutrition and Dietetics. We conducted a PubMed, MEDLINE, and Scopus search from inception to March 2020 using the search terms ‘gluten’ AND ‘celiac disease’ AND ‘microbiota’ AND ‘gluten-free diet’ OR ‘probiotics’ OR ‘prebiotics’ followed by a manual review of the literature to select relevant articles for this clinical review. Literature researches were carried out based on title and abstract; the reference list of each relevant article was evaluated in order to find any other relevant articles. Randomized controlled trials, cross-sectional studies, and eminent reviews on the topic were included in the present narrative review if they reported data on the microbiota composition or metabolomic of celiac disease patients during GFD or probiotic or prebiotic or other dietary compound supplementation. We restricted the search strategy for language to English articles. The article search was carried out independently by two authors (GM and GGC).
4. Dietary Supplements Beyond the Gluten-Free Diet
Despite strict GFD adherence, a certain degree of mucosal inflammation and symptoms may persist in up to 5% of CD patients. Non-responsive CD (NRCD), unlike refractory CD (RCD), has a higher prevalence, affecting from 7% to 30% of celiac patients [33,34]. Therefore, a supportive pharmacological treatment would be suggested [16,17,35,36,37]. Besides, several researchers focused their studies on finding additional dietary compounds able to increase gluten tolerability; among these, prebiotics and probiotics play a leading role.
4.1. Probiotics
The evidence of dysbiosis in CD patients [38,39,40] has gained more and more research on the use of probiotics for gut microbiota restoration and modulation. Indeed, the composition of gut microbiota influences the spectrum of gastrointestinal symptoms of this disease [40]; microbiological studies [26,41,42] showed a different abundance of Lactobacillus and Bifidobacterium strains in CD patients at disease diagnosis, other than a reduction in several ‘health’-promoting bacterial strains, such as Akkermansia muciniphila, demonstrating an association with intestinal dysbiosis [43]. The unbalanced gut microbiota may indeed promote CD, influencing the mutualistic relationship between the colonic microbiota, their metabolic products, and the host immune system; in order to maintain immunological homeostasis, it is essential to establish a “healthy relationship” since the first years of life [44]. Supporting these hypotheses, a study by Wacklin et al. [38] showed that intestinal dysbiosis is linked with refractory gastrointestinal symptoms, iron deficiency, low bone density, and anemia in CD patients on GFD. As a matter of fact, CD is strongly influenced by dysbiosis, and several theories postulate that it could facilitate a loss of gluten tolerance in genetically predisposed subjects, increasing the gut mucosal permeability, with the leakage of tight junctions during inflammation and the recruitment of T cells [41,45,46,47].
Probiotics are defined according to the World Health Organization as live microorganisms, which, if administered in adequate amounts, can confer health benefits to the host [48]. Probiotics use in CD could modulate the composition and functions of the microbiota; this may delay the onset of the disease or prevent it. Probiotics are also able to regulate the immune response, the degradation of toxin receptors, the competition for nutrients, the blockage of adhesion sites, and the production of inhibitory substances against pathogens [49]. Studies evaluating the effect of probiotics in CD patients are summarized in Table 1 and Table 2 [8,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. In particular, a study by Lindfors et al. [50] highlighted that specific probiotics, such as Lactobacillus fermentum or Bifidobacterium lactis, have a protective role against the toxic effects of gliadin in intestinal cell cultures (human colon Caco-2 cells), with the result of dose-dependent inhibition of increased epithelial gliadin-induced permeability and stimulation of IL-10 production by regulatory T-cells [55,67]. Indeed, the activation of inflammation through induction of the cytokines cascade by the NFkB pathway is one of the leading causes of symptoms. Other Bifidobacterial strains are able to improve the altered gut bacterial composition in CD, reducing inflammation, as demonstrated by Laparra et al. [68]. In another study, the same group found that gliadin-digested fragments and Bifidobacteria (specifically B. longum CECT 7347) induce a downregulation of the mRNA expression of proinflammatory cytokines (NFkB, TNF-alpha, and IL-1beta) [52]. The same species were also able to restore the expression of liver transferrin receptor (TfR)-2, lowered by gliadin, and to improve gliadin-mediated perturbations, such as liver iron deposition and mobilization [69]. Moreover, Lactobacillus casei seemed to be useful in CD for the recovery of gut-associated lymphoid tissue (GALT) homeostasis and a healthy mucosal structure [51].

Table 1.
Studies evaluating probiotic use in celiac disease in vitro, ex vivo, and in animal models.

Table 2.
Studies evaluating probiotic use in celiac disease patients.
Probiotics effects in the specific setting of CD patients are summarized in Figure 1. To understand the role of some bacterial clusters in the modulation of the immune response, the effect of Bifidobacterium bifidum and Bifidobacterium longum on peripheral blood mononuclear cells, alone or with CD triggers, were compared to Gram-negative bacteria, such as Bacteroides fragilis and Escherichia coli. It was found that Gram-negative bacteria induce a higher secretion of TH-1 proinflammatory cytokines and activation mechanisms (HLA-DR, CD40, IL-12, and IFN-c) with respect to the Bifidobacterium strains [70]. The latter strain, on the other hand, was able to upregulate CD83 expression, which is a marker of mature dendritic cells. Thus, the authors concluded that microbiota could regulate monocytes and the IFN reaction to gliadin locally. Other common topics among the studies about probiotics and the amelioration of intestinal symptoms in celiac patients is the impact of “good” bacteria on the microbiota. Previous studies using VSL#3 in CD showed that Streptococcus thermophilus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii spp. Bulgaricus, Bifidobacterium breve, Bifidobacterium longum, and Bifidobacterium infantis were able to decrease the toxicity of wheat flour during long-term fermentation, due to complete hydrolysis of the gliadin [54]. Additionally, a reduced reorganization of intracellular F-actin with decreased release of zonulin led to decreased intestinal permeability [54]. Conversely, Smecuol et al. [56] studied the effect of Bifidobacterium infantis natren life start strain administration on CD patients on a gluten-containing diet, observing an increase of the intestinal permeability. It is possible that probiotic administration did not modify the intestinal permeability probably due to an insufficient dose or to the short time of administration; however, it improved gastrointestinal symptoms, mainly referred to as better digestion and reduced constipation. A similar study evaluated the effects of 3 months of GFD supplemented with Bifidobacterium longum CECT 7347 in newly diagnosed CD children, showing a decrease in CD3 T-cells, a reduction in the Bacteroides fragilis group and in the content of IgA in stools, and, eventually, an improvement of the symptoms in CD patients [57]. As a confirmation of probiotics’ effect on CD symptoms, a study carried out in Argentina identified significant alterations in the amount of Lactobacillus strains in symptom-free CD children; five different Lactobacilli were isolated in the stools of healthy children and Lactobacillus rhamnosus and Lactobacillus paracasei were proposed as potential probiotic strains since they show high resistance to gastrointestinal tract conditions [58].
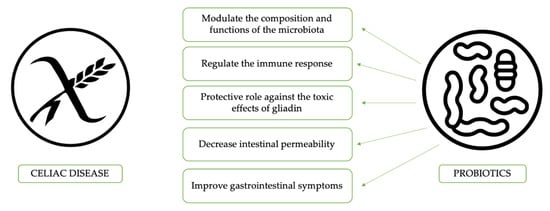
Figure 1.
The potential benefits of probiotics use in celiac disease patients.
In conclusion, Bifidobacteria and Lactobacilli administration seems to have the potential to restore gut microbiota composition and to predigest gluten in the intestinal lumen, reducing the inflammation associated with gluten intake, intestinal permeability, and cytokine and antibody production. These findings could explain an improvement in symptoms and quality of life in patients treated with GFD and probiotics. However, since evidence on this topic is still scarce, routine use in the clinical practice of probiotics is still not advised by international guidelines for the use of probiotics [71].
4.2. Prebiotics
Prebiotics are defined according to the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement as a substrate that is selectively utilized by host microorganisms conferring a health benefit [72]. Among the new therapies recently proposed, prebiotics are a promising and safe additive to GFD with a beneficial influence on human health [73]. Prebiotics have the power to stimulate the growth and activity of potentially health-promoting bacteria strains in the intestine, mainly Bifidobacterium and Lactobacillus. For this reason, their ability to regulate the activity of gut microbiota could be used to address CD-related symptoms. Literature data lead to the hypothesis that the inclusion of prebiotics in GFD could also be easy to administer and cost-effective as an adjunctive treatment for CD [73]. Only a few pilot human studies have been carried out to clarify the impact of prebiotics on the intestinal inflammation in general and, in particular, on CD [43,56]; most of them include formulations of prebiotics combined with other ingredients. Krupa-Kozak et al. [43] carried out one of the first studies on this subject: a randomized placebo-controlled clinical trial aiming to assess the influence of an oligofructose-enriched inulin, named “Orafti®-Synergy1”(Tienen, Belgium), on pediatric CD patients following GFD. The authors found an increase in the count of Bifidobacterium and a decrease of Lactobacillus in the pediatric population analyzed. This result was different from CD patients assuming the placebo, in which a decrease in the count and diversity of Bifidobacterium species was reported. Indeed, Bifidobacterium can prevent entomopathogen infections and reduce gastrointestinal mucosal inflammation [74,75]. In parallel, Adebola et al. [46] demonstrated that inulin is not able to directly stimulate any of the five probiotic strains of Lactobacillus, whereas other potential prebiotics, including lactulose and lactobionic acid, may exert this effect and represent an optimal substrate for bacteria to minimize the adverse effects of bile acid stress. Indeed, these authors found that Lactobacillus strains, in particular Lactobacillus acidophilus NCFM and Lactobacillus reuteri NCIMB 11951, have stimulating effects only in specific formulations of probiotics and prebiotics with lactulose or lactobionic acid [46]. A similar study by Tuohy et al. [76] observed a significant increase in the Bifidobacterium count in healthy volunteers receiving inulin for two weeks. Furthermore, another important study [43] showed that the addition of oligofructose-enriched inulin to GFD improved the fecal microbiota, increasing the total SCFAs, such as propionate and butyrate, remarkably. This effect could be attributed to the fermentation of inulin-type fructans (ITFs) as a readily available source of energy for gut microbiota present on the prebiotic formula, which is usually a mixture of short-chain fructo-oligosaccharides and long-chain inulin. In particular, butyric acid is the primary fermentation product of oligosaccharides, whereas inulin fermentation leads to the formation of propionic acid; the latter has a role in the proliferation and differentiation of colon epithelial cells (tight-junction proteins, claudin-1, zonula-occludens-2) [77,78]. Thus, supplementation with “Orafti®-Synergy1” could be a promising therapeutic approach for modulating intestinal microbiota and it could also have future employment in other autoimmune diseases like diabetes type I, considering the putative role of intestinal microbiota in their development [79,80]. Drabisnka et al. [80,81,82] conducted additional studies on the utility of “Orafti®-Synergy1” and its effects on CD children’s metabolism and microbiota. They reported that “Orafti®-Synergy1” had an effect on iron homeostasis in CD patients treated with GFD, bringing about a significant decrease in the plasma hepcidin concentration, which is a key regulator for duodenal iron, resulting in a positive effect on its absorption. Moreover, since the risk of an adverse calcium balance and reduced bone density in CD patients is induced both by the disease and by the GFD, they assessed the effects of gluten-free bread with additional calcium (Ca) in a rat model [83]. As a result, they found that the dietary inulin in GFD influences the intestinal microbiota positively by the stimulation of Ca absorption, especially in conditions of Ca malnutrition. They concluded that the addition of inulin to a GFD could be a promising strategy for beneficial modulation of intestinal microbiota and the improvement of Ca absorption. The same authors [84] evaluated, through an in vitro study on Caco-2 cells treated with a human intestinal bacteria suspension, whether inulin and fructo-oligosaccharides (FOSs) can influence Ca uptake. They found that the addition of ITF might enhance cellular Ca uptake by altering the rate and amount of organic acids, such as butyric, valeric, and lactic acids (produced by the intestinal microbiota) and stimulating cellular Ca retention, reducing its transport. In another study, Capriles et al. [85] tried to investigate the impact of ITFs, added in different quantities (0%, 4%, 8%, 10%, and 12%) to GFD, on the sensory and nutritional quality of the diet. Indeed, ITFs can cause gas retention during baking, improving GFD quality by yielding a better specific volume, softer crumb, and improved crust and crumb browning with ameliorated sensory acceptance. The addition of 12% ITFs to the standard formulation is required in order to obtain GFD enriched with 8% ITFs (4 g of fructans per 50-g bread serving size), and can provide health benefits: Decrease of the glycemic index (from 71 to 48) and glycemic load (from 12 to 8) and improved Ca absorption. The results of this study are in line with those reported by Korus et al. [86] describing the partial replacement of starch in GFD recipes for 3.5% and 8% inulin. The effects of prebiotics on gut microbiota have also been elucidated by a crossover study by Fuller et al. [87], who tried to enhance the concentration of colonic Bifidobacterial populations with the use of a commercially available prebiotic (inulin). They found that after 16 days of treatment, the fecal Bifidobacterial population was significantly higher. In conclusion, prebiotic use in CD patients has largely not been investigated and their use, mainly that of inulin, has been proved to enhance the abundances of ‘beneficial’ bacterial strains, such as Bifidobacterium and Lactobacillus strains, other than enhancing the homeostatic and metabolic activity of these strains. However, to date, sufficient data are not currently available to definitely recommend their use in the routine clinical practice.
4.3. Synbiotic in Celiac Disease
The growth of probiotics in the large bowel could be improved through the synergistic combination with prebiotics; for this purpose, it has been created the term “synbiotic”, which defines a product in which a prebiotic is specifically added to favour the growth of the wanted probiotic [88]. As an example, a study by Furrie et al. [47] developed a synbiotic for patients with ulcerative colitis (UC). It combines a probiotic, the Bifidobacterium longum isolated from healthy rectal epithelium, with the prebiotic “Orafti®-Synergy1” (a preferential inulin-oligofructose growth substrate for the probiotic strain), achieving proper compliance and symptoms improvement in the acute phase [47]. Moreover, a study by Adebola et al. Ref. [46] examined the ability of three potential prebiotics, inulin, lactulose and lactobionic acid to support the growth of five probiotics lactobacilli cultures and provide protection from bile acid stress. Of the five tested probiotics, only Lactobacillus acidophilus NCFM and Lactobacillus reuteri NCIMB 11951 utilized lactulose. Similar variability was observed with the ability of the prebiotics to protect probiotics from bile acid stress: both Lactobacillus acidophilus NCFM and Lactobacillus reuteri NCIMB 11951 were able to grow in 2 mM cholic and taurocholic acid when incubated in synbiotic combinations with lactulose (1%) or lactobionic acid (1%). Although synbiotic preparations are increasingly used, the potential benefits to gut health may be limited, as only specific combinations may enhance probiotics survival and growth. For this reason, nowadays, prebiotics are increasingly added to probiotic food preparations (synbiotic) to enhance probiotics survival and growth [46]. However, in the specific CD setting, no studies are available exploring the combined effect of probiotics and prebiotics.
4.4. Other Dietary Supplements
Recent studies showed that diet and dietary compounds are playing an increasing role in the modulation of the metabolic activity of the intestinal microbiota. As example beyond CD, the randomized trial by Beaumont et al. [89] in overweight patients analyzed the effects of a high-protein diet (HPD) on gut microbiota composition, metabolic activity, and large intestine mucosal gene expression. The authors reported that a three-week administration of HPD was sufficient to alter the bacterial metabolite production and to modify gene expression in the rectal mucosa. These findings could be explained as a consequence of the increased level of the amino acid-derived bacterial metabolites’ concentration and of the decreased butyrate production, two factors leading to the modification of several key homeostatic processes at the gene expression level in the rectal mucosa. On the other hand, according to the experience by Wu et al. [90] comparing the metabolomic activity of two groups of subjects with agrarian or ‘Westernized’ diet, dietary delivery of substrates to the gut microbiota is not enough for the control of the production of metabolites, which needs the coexistence of specific bacterial lineages [90]. As a matter of fact, focusing on CD patients, a recent systematic review by Rondanelli et al. [91] underlined that in CD patients on a long-term gluten-free diet with good compliance, a micronutrient deficiency was detected in up to 30% of subjects for vitamin B12, 40% for iron and zinc, 20% for folic acid and in children for magnesium, and 25% for vitamin D; thus, the authors suggested that dietary supplementation of these micronutrients would be useful. This evidence may be explained by several factors, such as the GFD itself, the unbalance of GFD on a more ‘agrarian’ or ‘Westernized’ pattern, and the residual dysbiosis of CD patients which together obstacles the presence of specific bacterial lineages able to guarantee an adequate production of metabolites and absorption of micronutrients. However, a recent systematic review by Melini et al. [92] found that in the last decade, GF products are increasingly richer in fiber content, which may be helpful in the shaping of an adequate microbial profile of these patients.
5. Impact of Oat Intake
Another dietary controversy is the inclusion of oats in the diet of CD patients. Oats are a great source of nutrients, often lacking in the gluten-free diet, such as iron and fiber; however, several studies have shown the possibility of cross-reactivity: The avenin (a protein similar in function to gluten) in oats can activate gluten-reactive T cells [93]. A randomized double-blind multicenter study [94] focused on the effect of a GFD containing oats (GFD-oats) compared to a standard GFD (GFD-std) in 116 children (GFD-oats, n = 57; GFD-std, n = 59) with newly diagnosed CD. The authors found a significant decrease in the total SCFA concentration in the GFD-std group during a year of GFD but not in the GFD-oats group, in which the proinflammatory acetic acid and total SCFA concentration remained high throughout the diet period. An explanation could be the higher fiber content, due to the addition of oats to the GFD, thus providing more substrate for fermentation. Lundin et al. [95] raised some concerns regarding the safety of oats in adults with CD; in fact, they concluded that several CD adults exposed to oats in their GFD appear to experience abdominal symptoms or even produce avenin-reactive T-cells in the small intestinal mucosa [96]. On the other hand, Koskinen et al. [97] reported that oats did not induce transglutaminase-2 autoantibody production at the intestinal mucosal level in CD children over a 2-year follow-up [97]. In the groups analyzed, CD children had similar serum anti-avenin antibody titers following 1-year GFD-std or GFD-oats treatment, but some of the CD children in both study groups continued to excrete high amounts of urinary nitrite/nitrate, an indicator of upregulation of inducible nitric oxide synthase, after proinflammatory cytokine stimuli [98,99]. Considering the positive nutritional effect of oats, further studies are needed to deeply evaluate the use of this cereal, which, however, needs to be strictly wheat free [100].
6. Conclusions
Different dietary and supplementary strategies are being explored, aiming to support the compliance and the response of CD patients to GFD and to avoid complications and severe progression of the disease. The synergic use of prebiotics, together with prebiotics, could be a promising therapeutic approach for modulating intestinal microbiota composition and function. Nevertheless, evidence regarding the use of prebiotics and probiotics in patients with CD is still insufficient to justify their use in routine clinical practice. Well-designed randomized controlled trials are needed to clarify their role in CD patients.
Author Contributions
Conceptualization, G.M., G.C.; writing—original draft preparation, G.M., G.G.C., G.C.; writing—review and editing, G.M., G.G.C., B.R., L.L., G.C.; supervision, G.M., A.R.D.B., A.C., R.D.G., U.V., D.F. and G.C.; funding acquisition, G.C. and R.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
Fondi Ateneo Ricerca 2020 from University of Ferrara (FAR 2020) (to G.C.) Ricerca Finalizzata 2016 (RF16MINT) (Project title: “Intestinal microbiome biomarkers for non-celiac gluten sensitivity: New diagnostic and therapeutic targets”) from Italian Ministry of Public Health (to G.C. and R.D.G).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rubio-Tapia, A.; Murray, J.A. Updated guidelines by the European Society for the Study of Coeliac Disease. United Eur. Gastroenterol. J. 2019, 7, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Araya, M.; Roncoroni, L.; Doneda, L.; Elli, L. Dietary Gluten as a Conditioning Factor of the Gut Microbiota in Celiac Disease. Adv. Nutr. 2020, 11, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Gambuti, E.; Alfano, F.; Strada, A.; Ciccocioppo, R.; Lungaro, L.; Zoli, G.; Volta, U.; De Giorgio, R.; Caio, G. Life-threatening onset of coeliac disease: A case report and literature review. BMJ Open Gastroenterol. 2020, 7, e000406. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Ursini, F.; Manfredini, R.; De Giorgio, R. Small bowel adenocarcinoma as a complication of celiac disease: Clinical and diagnostic features. BMC Gastroenterol. 2019, 19, 1–19. [Google Scholar] [CrossRef]
- Vanoli, A.; Di Sabatino, A.; Furlan, D.; Klersy, C.; Grillo, F.; Fiocca, R.; Mescoli, C.; Rugge, M.; Nesi, G.; Fociani, P.; et al. Small bowel carcinomas in coeliac or Crohn’s disease: Clinico-pathological, molecular, and prognostic features. A study from the small bowel cancer Italian consortium. J. Crohn’s Colitis 2017, 11, 942–953. [Google Scholar] [CrossRef]
- Harnett, J.; Myers, S.P.; Rolfe, M. Probiotics and the Microbiome in Celiac Disease: A Randomised Controlled Trial. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Caio, G.; Riegler, G.; Patt Urelli, M.; Facchiano, A.; De Magistris, L.; Sapone, A. Pathophysiology of non-celiac gluten sensitivity: Where are we now? Minerva Gastroenterol. Dietol. 2017, 63, 16–21. [Google Scholar]
- Volta, U.; Caio, G.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Boschetti, E.; Stanghellini, V.; De Giorgio, R. Features and Progression of Potential Celiac Disease in Adults. Clin. Gastroenterol. Hepatol. 2016, 14, 686–693. [Google Scholar] [CrossRef]
- Kaukinen, K.; Lindfors, K.; Mäki, M. Advances in the treatment of coeliac disease: An immunopathogenic perspective. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabó, I.R.; Kovács, J.B.; Lorincz, M.; Gorácz, G.; Szabados, K.; Balogh, M. Prospective significance of antiendomysium antibody positivity in subsequently verified celiac disease. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Ciclitira, P.; Hadjivassiliou, M.; Kaukinen, K.; Ludvigsson, J.F.; McGough, N.; Sanders, D.S.; Woodward, J.; Leonard, J.N.; Swift, G.L. The gluten-Free diet and its current application in coeliac disease and dermatitis Herpetiformis. United Eur. Gastroenterol. J. 2015, 3, 121–135. [Google Scholar] [CrossRef] [PubMed]
- ÁRnason, A.; Skaftadóttir, I.; Sigmundsson, J.; Mooney, E.; Björnsson, J.; Cariglia, N.; Pálsson, G.; Gudjónsson, H. The association between coeliac disease, dermatitis herpetiformis and certain hla-antigens in icelanders. Int. J. Immunogenet. 1994, 21, 457–460. [Google Scholar] [CrossRef]
- Diez-Sampedro, A.; Olenick, M.; Maltseva, T.; Flowes, M. A Gluten-Free Diet, Not an Appropriate Choice Without a Medical Diagnosis. J. Nutr. Metab. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Davis, M.A.; Karagas, M.R.; Ahsan, H.; Argos, M. The unintended consequences of a gluten-free diet. Epidemiology 2017, 28, e24–e25. [Google Scholar] [CrossRef][Green Version]
- Bouguerra, F.; Babron, M.C.; Eliaou, J.F.; Debbabi, A.; Clot, J.; Khaldi, F.; Greco, L.; Clerget-Darpoux, F. Synergistic Effect of Two HLA Heterodimers in the Susceptibility to Celiac Disease in Tunisia. Genet. Epidemiol. 1997, 14, 413–422. [Google Scholar] [CrossRef]
- Pasqui, F.; Poli, C.; Colecchia, A.; Marasco, G.; Festi, D. Adverse food reaction and functional gastrointestinal disorders: Role of the dietetic approach. J. Gastrointest. Liver Dis. 2015, 24, 319–327. [Google Scholar] [CrossRef]
- Marasco, G.; Colecchia, A.; Festi, D. Dysbiosis in celiac disease patients with persistent symptoms on gluten-free diet: A condition similar to that present in irritable bowel syndrome patients? Am. J. Gastroenterol. 2015, 110, 598. [Google Scholar] [CrossRef]
- Boy, M.F.; La Nasa, G.; Balestrieri, A.; Cherchi, M.V.; Usai, P. Distribution of HLA-DPBl,-DQBI-DQAl alleles among sardinian celiac patients. Dis. Markers 1994, 12, 199–204. [Google Scholar] [CrossRef]
- Nadal, I.; Donat, E.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Herrán, A.R.; Arias, L.; Vivas, S.; de Morales, J.M.R.; Calleja, S.; de Miera, L.E.S.; Arroyo, P.; Casqueiro, J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 2012, 18, 649–656. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef]
- Jackson, F.W. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects—Comment by Jackson. Br. J. Nutr. 2010, 104, 773. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, C.G.; Gagliardi, F.; Ricciuti, P.; Ndagijimana, M.; Francavilla, R.; Guerzoni, M.E.; Crecchio, C.; Gobbetti, M.; De Angelis, M. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 2009, 75, 3963–3971. [Google Scholar] [CrossRef]
- Schippa, S.; Iebba, V.; Barbato, M.; Di Nardo, G.; Totino, V.; Checchi, M.P.; Longhi, C.; Maiella, G.; Cucchiara, S.; Conte, M.P. A distinctive “microbial signature” in celiac pediatric patients. BMC Microbiol. 2010, 10, 175. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. BioMed Res. Int. 2016, 2016, 4740907. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. Corrigendum to “A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation”. BioMed Res. Int. 2019, 2019, 9042956. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; Hyett, B.; Kelly, E.; Schuppan, D.; Kelly, C.P. A figure is presented}Etiologies and Predictors of Diagnosis in Nonresponsive Celiac Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 445–450. [Google Scholar] [CrossRef]
- Dewar, D.H.; Donnelly, S.C.; McLaughlin, S.D.; Johnson, M.W.; Ellis, H.J.; Ciclitira, P.J. Celiac disease: Management of persistent symptoms in patients on a gluten-free diet. World J. Gastroenterol. 2012, 18, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Ciccocioppo, R.; Zoli, G.; De Giorgio, R.; Volta, U. Therapeutic options for coeliac disease: What else beyond gluten-free diet? Dig. Liver Dis. 2020, 52, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Congia, M.; Frau, F.; Lampis, R.; Frau, R.; Mele, R.; Cucca, F.; Muntoni, F.; Porcu, S.; Boi, F.; Contu, L.; et al. A high frequency of the A30, B18, DR3, DRw52, DQw2 extended haplotype in Sardinian celiac disease patients: Further evidence that disease susceptibility is conferred by DQ A1*0501, B1*0201. Tissue Antigens 1992, 39, 78–83. [Google Scholar] [CrossRef]
- Djilali-Saiah, I.; Caillat-Zucman, S.; Schmitz, J.; Laise Chaves-Vieira, M.; Bach, J.F. Polymorphism of antigen processing (TAP, LMP) and HLA class II genes in celiac disease. Hum. Immunol. 1994, 40, 8–16. [Google Scholar] [CrossRef]
- Wacklin, P.; Kaukinen, K.; Tuovinen, E.; Collin, P.; Lindfors, K.; Partanen, J.; Mäki, M.; Mättuö, J. The duodenal microbiota composition of adult celiac disease patients is associated with the clinical manifestation of the disease. Inflamm. Bowel Dis. 2013, 19, 934–941. [Google Scholar] [CrossRef]
- Marasco, G.; Di Biase, A.R.; Schiumerini, R.; Eusebi, L.H.; Iughetti, L.; Ravaioli, F.; Scaioli, E.; Colecchia, A.; Festi, D. Gut Microbiota and Celiac Disease. Dig. Dis. Sci. 2016, 61, 1461–1472. [Google Scholar] [CrossRef]
- Di Biase, A.R.; Marasco, G.; Ravaioli, F.; Dajti, E.; Colecchia, L.; Righi, B.; D’Amico, V.; Festi, D.; Iughetti, L.; Colecchia, A. Gut microbiota signatures and clinical manifestations in celiac disease children at onset: A pilot study. J. Gastroenterol. Hepatol. 2020, jgh.15183. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Vivas, S.; Ruiz De Morales, J.M.; Sáenz De Miera, L.E.; Rodríguez-Aparicio, L.B.; Casqueiro, J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Drabińska, N.; Jarocka-Cyrta, E. The effect of oligofructose-enriched inulin supplementation on gut microbiota, nutritional status and gastrointestinal symptoms in paediatric coeliac disease patients on a gluten-free diet: Study protocol for a pilot randomized controlled trial. Nutr. J. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Heal Dis. 2015, 26. [Google Scholar] [CrossRef]
- Ou, G.; Hedberg, M.; Hörstedt, P.; Baranov, V.; Forsberg, G.; Drobni, M.; Sandström, O.; Wai, S.N.; Johansson, I.; Hammarström, M.L.; et al. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am. J. Gastroenterol. 2009, 104, 3058–3067. [Google Scholar] [CrossRef]
- Adebola, O.O.; Corcoran, O.; Morgan, W.A. Synbiotics: The impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. J. Funct. Foods 2014, 10, 75–84. [Google Scholar] [CrossRef]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Vanderpool, C.; Yan, F.; Polk, D.B. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef]
- Lindfors, K.; Blomqvist, T.; Juuti-Uusitalo, K.; Stenman, S.; Venäläinen, J.; Mäki, M.; Kaukinen, K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 2008, 152, 552–558. [Google Scholar] [CrossRef]
- D’Arienzo, R.; Stefanile, R.; Maurano, F.; Mazzarella, G.; Ricca, E.; Troncone, R.; Auricchio, S.; Rossi, M. Immunomodulatory effects of Lactobacillus casei administration in a mouse model of gliadin-sensitive enteropathy. Scand. J. Immunol. 2011, 74, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Olivares, M.; Gallina, O.; Sanz, Y. Bifidobacterium longum CECT 7347 modulates immune responses in a gliadin-induced enteropathy animal model. PLoS ONE 2012, 7, e30744. [Google Scholar] [CrossRef] [PubMed]
- Papista, C.; Gerakopoulos, V.; Kourelis, A.; Sounidaki, M.; Kontana, A.; Berthelot, L.; Moura, I.C.; Monteiro, R.C.; Yiangou, M. Gluten induces coeliac-like disease in sensitised mice involving IgA, CD71 and transglutaminase 2 interactions that are prevented by probiotics. Lab. Investig. 2012, 92, 625–635. [Google Scholar] [CrossRef]
- De Angelis, M.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; De Simone, C.; Silano, M.; De Vincenzi, M.; Losito, I.; Gobbetti, M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim. Biophys. Acta-Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Medina, M.; Izquierdo, E.; Ennahar, S.; Sanz, Y. Differential immunomodulatory properties of Bifidobacterium logum strains: Relevance to probiotic selection and clinical applications. Clin. Exp. Immunol. 2007, 150, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Hwang, H.J.; Sugai, E.; Corso, L.; Cherñavsky, A.C.; Bellavite, F.P.; González, A.; Vodánovich, F.; Moreno, M.L.; Vázquez, H.; et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J. Clin. Gastroenterol. 2013, 47, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Castillejo, G.; Varea, V.; Sanz, Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br. J. Nutr. 2014, 112, 30–40. [Google Scholar] [CrossRef]
- Pisarello, M.L.J.; Vintiñi, E.O.; González, S.N.; Pagani, F.; Medina, M.S. Decrease in lactobacilli in the intestinal microbiota of celiac children with a gluten-free diet, And selection of potentially probiotic strains. Can. J. Microbiol. 2014, 61, 32–37. [Google Scholar] [CrossRef]
- Golfetto, L.; de Senna, F.D.; Hermes, J.; Beserra, B.T.S.; da Silva França, F.; Martinello, F. Baixa contagem de bifidobactérias em pacientes adultos com doença celíaca, em dieta isenta de glúten. Arq. Gastroenterol. 2014, 51, 139–143. [Google Scholar] [CrossRef]
- Klemenak, M.; Dolinšek, J.; Langerholc, T.; Di Gioia, D.; Mičetić-Turk, D. Administration of Bifidobacterium breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig. Dis. Sci. 2015, 60, 3386–3392. [Google Scholar] [CrossRef]
- Quagliariello, A.; Aloisio, I.; Bozzicionci, N.; Luiselli, D.; D’Auria, G.; Martinez-Priego, L.; Pérez-Villarroya, D.; Langerholc, T.; Primec, M.; Mičetić-Turk, D.; et al. Effect of bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: A pilot study. Nutrients 2016, 8, 660. [Google Scholar] [CrossRef]
- Martinello, F.; Roman, C.F.; de Souza, P.A. Efeitos do consumo de probióticos sobre as bifidobactérias intestinais de pacientes celíacos. Arq. Gastroenterol. 2017, 54, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sánchez, M.I.; Smecuol, E.C.; Temprano, M.P.; Sugai, E.; González, A.; Moreno, M.L.; Huang, X.; Bercik, P.; Cabanne, A.; Vázquez, H.; et al. Bifidobacterium infantis NLS Super Strain Reduces the Expression of α-Defensin-5, a Marker of Innate Immunity, in the Mucosa of Active Celiac Disease Patients. J. Clin. Gastroenterol. 2017, 51, 814–817. [Google Scholar] [CrossRef]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019, 53, E117–E125. [Google Scholar] [CrossRef] [PubMed]
- Primec, M.; Klemenak, M.; Di Gioia, D.; Aloisio, I.; Bozzi Cionci, N.; Quagliariello, A.; Gorenjak, M.; Mičetić-Turk, D.; Langerholc, T. Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial modulators of TNF-α and short-chain fatty acids. Clin. Nutr. 2019, 38, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, U.; Aronsson, C.A.; Liu, X.; Kurppa, K.; Yang, J.; Liu, E.; Skidmore, J.; Winkler, C.; Rewers, M.J.; Hagopian, W.A.; et al. Early probiotic supplementation and the risk of celiac disease in children at genetic risk. Nutrients 2019, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.; Samson, S.; Bourdet-Sicard, R.; Rubio, M.; Sarfati, M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J. Leukoc. Biol. 2008, 84, 468–476. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J. Cell. Biochem. 2010, 109, 801–807. [Google Scholar] [CrossRef]
- Laparra, J.M.; Olivares, M.; Sanz, Y. Oral administration of Bifidobacterium longum CECT 7347 ameliorates gliadin-induced alterations in liver iron mobilisation. Br. J. Nutr. 2013, 110, 1828–1836. [Google Scholar] [CrossRef]
- De Palma, G.; Cinova, J.; Stepankova, R.; Tuckova, L.; Sanz, Y. Pivotal Advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J. Leukoc. Biol. 2010, 87, 765–778. [Google Scholar] [CrossRef]
- World Gastroenterology Organisation Global Guidelines Probiotics and Prebiotics. World Gastroenterology Organisation, 2017. Available online: www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf (accessed on 30 June 2020).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. Intestinal permeability in children with celiac disease after the administration of oligofructose-enriched inulin into a gluten-free diet—Results of a randomized, placebo-controlled, pilot trial. Nutrients 2020, 12, 1736. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–549. [Google Scholar] [CrossRef]
- Kanauchi, O.; Andoh, A.; Iwanaga, T.; Fujiyama, Y.; Mitsuyama, K.; Toyonaga, A.; Bamba, T. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J. Gastroenterol. Hepatol. 1999, 14, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Finlay, R.K.; Wynne, A.G.; Gibson, G.R. A human volunteer study on the prebiotic effects of HP-inulin—Faecal bacteria enumerated using fluorescent In situ hybridisation (FISH). Anaerobe 2001, 7, 113–118. [Google Scholar] [CrossRef]
- Nilsson, U.; Nyman, M. Short-chain Fatty Acid Formation in the Hindgut of Rats Fed Oligosaccharides Varying in Monomeric Composition, Degree of Polymerisation and Solubility. Br. J. Nutr. 2005, 94, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Abela, A.G.; Fava, S. Does the level of bacterial exposure in early life impact the risk of type 1 diabetes? Expert Rev. Clin. Immunol. 2013, 9, 695–697. [Google Scholar] [CrossRef]
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The effect of oligofructose-enriched inulin on faecal bacterial counts and microbiota-associated characteristics in celiac disease children following a gluten-free diet: Results of a randomized, placebo-controlled trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef]
- Feruś, K.; Drabińska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. A randomized, placebo-controlled, pilot clinical trial to evaluate the effect of supplementation with prebiotic synergy 1 on iron homeostasis in children and adolescents with celiac disease treated with a gluten-free diet. Nutrients 2018, 10, 1818. [Google Scholar] [CrossRef]
- Drabińska, N.; Krupa-Kozak, U.; Ciska, E.; Jarocka-Cyrta, E. Plasma profile and urine excretion of amino acids in children with celiac disease on gluten-free diet after oligofructose-enriched inulin intervention: Results of a randomised placebo-controlled pilot study. Amino Acids 2018, 50, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Markiewicz, L.H.; Lamparski, G.; Juśkiewicz, J. Administration of inulin-supplemented gluten-free diet modified calcium absorption and caecal microbiota in rats in a calcium-dependent manner. Nutrients 2017, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Świątecka, D.; Bączek, N.; Brzóska, M.M. Inulin and fructooligosaccharide affect: In vitro calcium uptake and absorption from calcium-enriched gluten-free bread. Food Funct. 2016, 7, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Capriles, V.D.; Arêas, J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Korus, J.; Grzelak, K.; Achremowicz, K.; Sabat, R. Influence of Prebiotic Additions on the Quality of Gluten-free Bread and on the Content of Inulin and Fructooligosaccharides. Food Sci. Technol. Int. 2006, 12, 489–495. [Google Scholar] [CrossRef]
- Fuller, Z.; Louis, P.; Mihajlovski, A.; Rungapamestry, V.; Ratcliffe, B.; Duncan, A.J. Influence of cabbage processing methods and prebiotic manipulationof colonic microflora on glucosinolate breakdown in man. Br. J. Nutr. 2007, 98, 364–372. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: A review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-free diet: Gaps and needs for a healthier diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.D.; Chen, Y. Oat safety for celiac disease patients: Theoretical analysis correlates adverse symptoms in clinical studies to contaminated study oats. Nutr. Res. 2018, 60, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Högberg, L.; Laurin, P.; Fâlth-Magnusson, K.; Grant, C.; Grodzinsky, E.; Jansson, G.; Ascher, H.; Browaldh, L.; Hammersjö, J.Å.; Lindberg, E.; et al. Oats to children with newly diagnosed coeliac disease: A randomised double blind study. Gut 2004, 53, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lundin, K.E.A.; Nilsen, E.M.; Scott, H.G.; Løberg, E.M.; Gjøen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Loøvik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.A.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, O.; Villanen, M.; Korponay-Szabo, I.; Lindfors, K.; Mäki, M.; Kaukinen, K. Oats Do Not Induce Systemic or Mucosal Autoantibody Response in Children With Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 559–565. [Google Scholar] [CrossRef]
- Hollén, E.; Peterson, K.H.; Sundqvist, T.; Grodzinsky, E.; Högberg, L.; Laurin, P.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.E. Coeliac children on a gluten-free diet with or without oats display equal anti-avenin antibody titres. Scand. J. Gastroenterol. 2006, 41, 42–47. [Google Scholar] [CrossRef]
- Hollén, E.; Forslund, T.; Högberg, L.; Laurin, P.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.E.; Sundqvist, T. Urinary nitric oxide during one year of gluten-free diet with or without oats in children with coeliac disease. Scand. J. Gastroenterol. 2006, 41, 1272–1278. [Google Scholar] [CrossRef]
- Troncone, R.; Ivarsson, A.; Szajewska, H.; Mearin, M.L. Review article: Future research on coeliac disease—A position report from the European multistakeholder platform on coeliac disease (CDEUSSA). Aliment. Pharmacol. Ther. 2008, 27, 1030–1043. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).