Influence of Short-Term Hyperenergetic, High-Fat Feeding on Appetite, Appetite-Related Hormones, and Food Reward in Healthy Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Participant Recruitment
2.2. Participant Screening
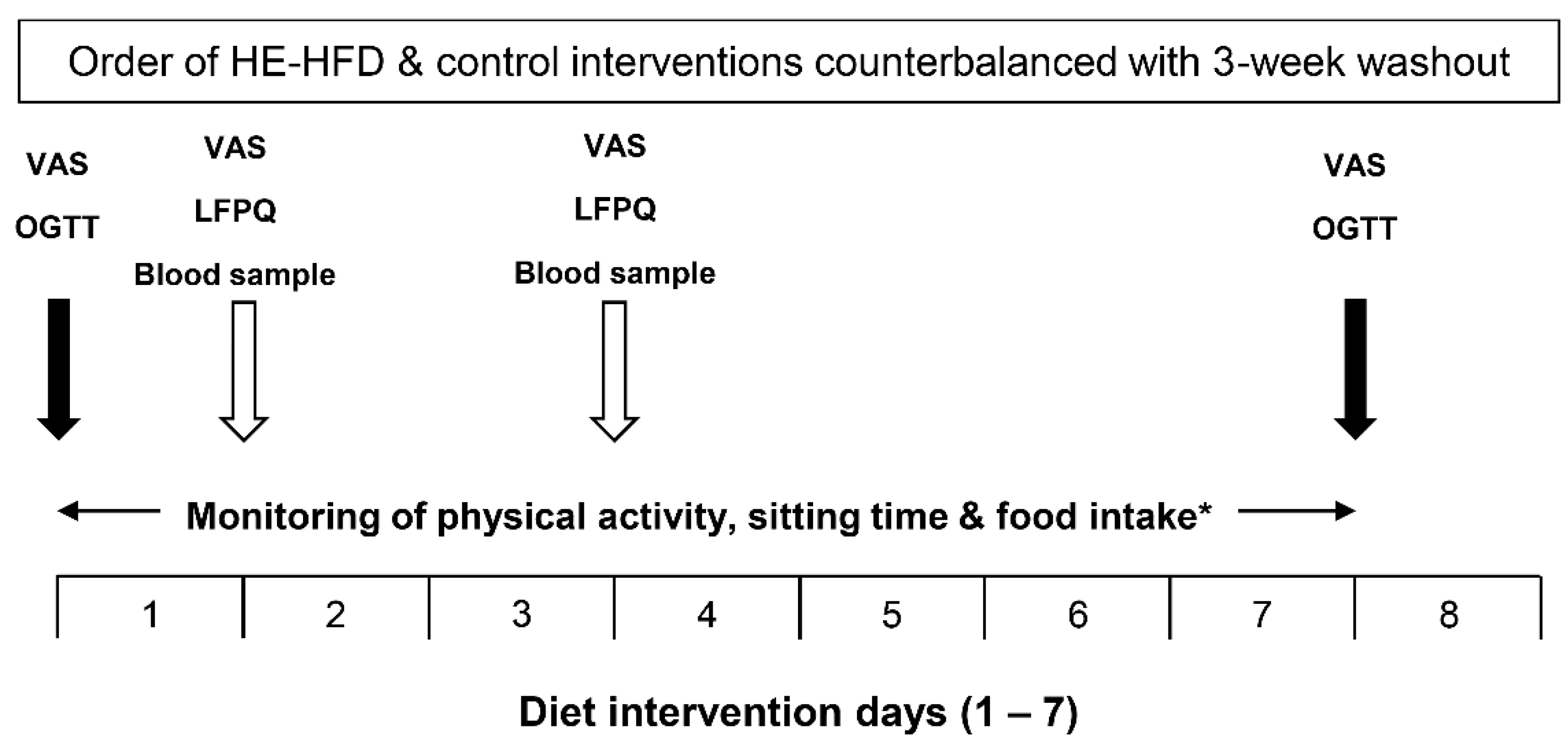
2.3. Study Design and Procedures
2.4. Dietary Interventions
2.5. Leeds Food Preference Questionnaire
2.6. Biochemical Analyses
2.7. Statistical Analyses
3. Results
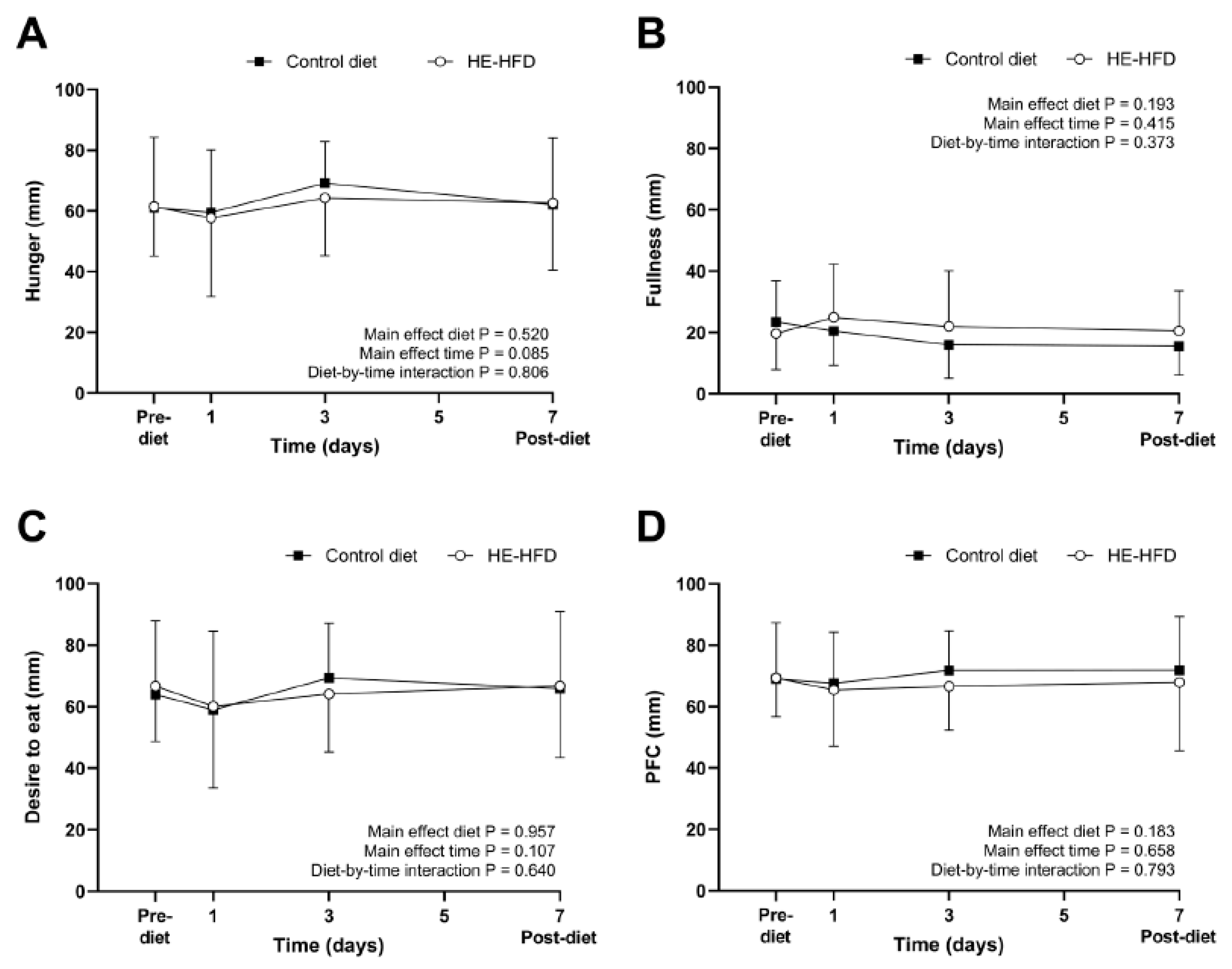
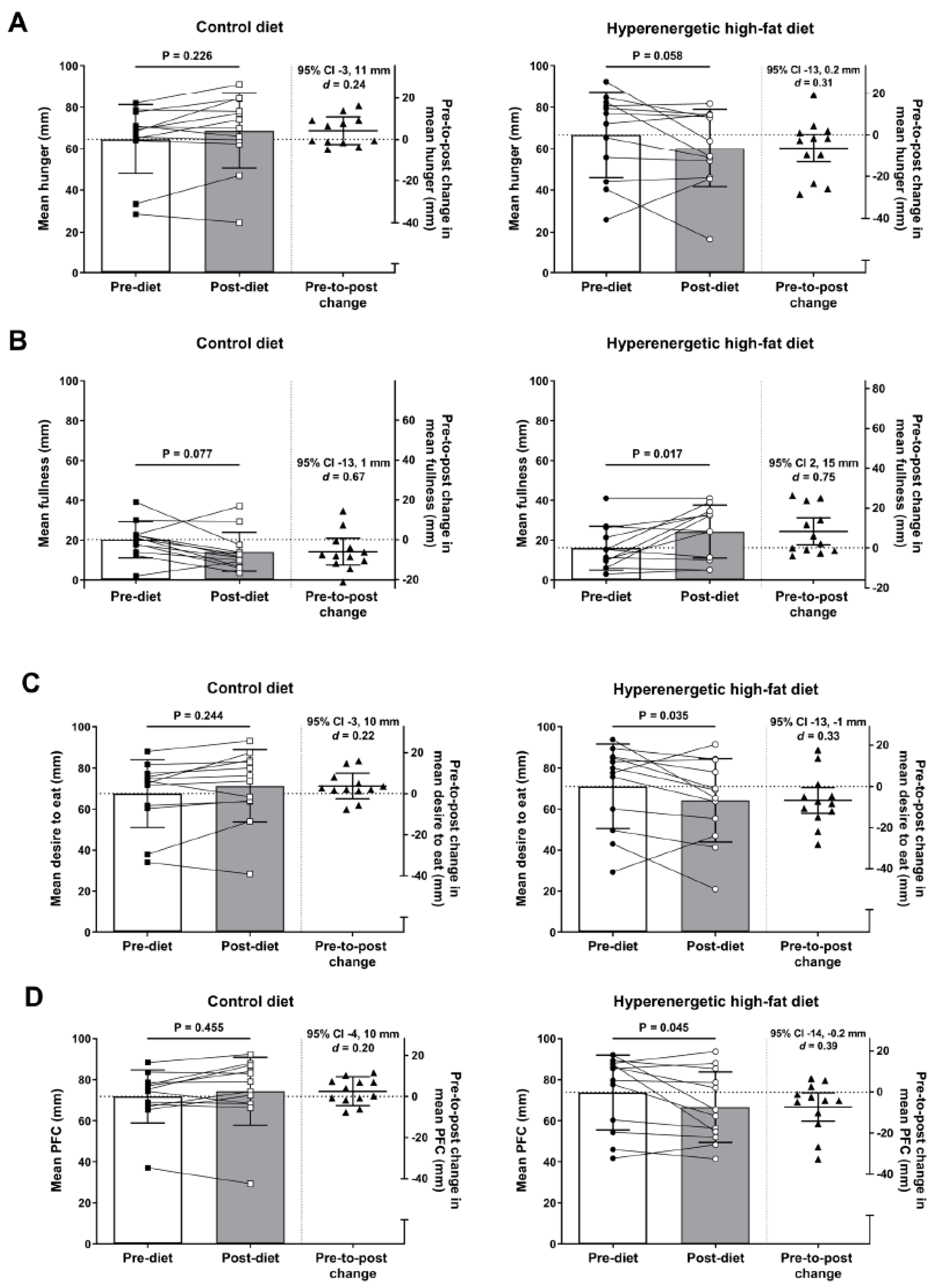
3.1. Ratings of Perceived Appetite
3.2. Appetite-Related Hormones
3.3. Food Preference and Reward
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speakman, J.R.; Levitsky, D.A.; Allison, D.B.; Bray, M.S.; De Castro, J.M.; Clegg, D.J.; Clapham, J.C.; Dulloo, A.G.; Gruer, L.; Haw, S.; et al. Set points, settling points and some alternative models: Theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Model. Mech. 2011, 4, 733–745. [Google Scholar] [CrossRef]
- Ochner, C.N.; Barrios, D.M.; Lee, C.D.; Pi-Sunyer, F.X. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol. Behav. 2013, 120, 106–113. [Google Scholar] [CrossRef]
- Chin-Chance, C.; Polonsky, K.S.; Schoeller, D.A. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J. Clin. Endocrinol. Metab. 2000, 85, 2685–2691. [Google Scholar] [CrossRef]
- Casanova, N.; Beaulieu, K.; Finlayson, G.; Hopkins, M. Metabolic adaptations during negative energy balance and their potential impact on appetite and food intake. Proc. Nutr. Soc. 2019, 78, 279–289. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Ewens, J.; Crystallis, K.; Emmanuel, J.; Batterham, R.L.; Stensel, D.J. Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J. Clin. Endocrinol. Metab. 2011, 96, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P.; Prechtl De Hernandez, C.G.; Beaver, J.D.; Muhammed, K.; Croese, C.; Bell, G.; Durighel, G.; Hughes, E.; Waldman, A.D.; Frost, G.; et al. Fasting biases brain reward systems towards high-calorie foods. Eur. J. Neurosci. 2009, 30, 1625–1635. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Fildes, A.; Charlton, J.; Rudisill, C.; Littlejohns, P.; Prevost, A.T.; Gulliford, M.C. Probability of an obese person attaining normal body weight: Cohort study using electronic health records. Am. J. Public Health 2015, 105, e54–e59. [Google Scholar] [CrossRef]
- Ochner, C.N.; Tsai, A.G.; Kushner, R.F.; Wadden, T.A. Treating obesity seriously: When recommendations for lifestyle change confront biological adaptations. Lancet Diabetes Endocrinol. 2015, 3, 232–234. [Google Scholar] [CrossRef]
- Bray, G.A.; Bouchard, C. The biology of human overfeeding: A systematic review. Obes. Rev. 2020, 21, e13040. [Google Scholar] [CrossRef]
- Deighton, K.; King, A.J.; Matu, J.; Shannon, O.M.; Whiteman, O.; Long, A.; Huby, M.D.; Sekula, M.; Holliday, A. A single day of mixed-macronutrient overfeeding does not elicit compensatory appetite or energy intake responses but exaggerates postprandial lipaemia during the next day in healthy young men. Br. J. Nutr. 2019, 121, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Grunwald, G.K.; Johnson, S.L.; Bessesen, D.H. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite 2004, 43, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Rojas, D.C.; Tregellas, J.R. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 2009, 4, e6310. [Google Scholar] [CrossRef]
- Halliday, T.M.; Rynders, C.A.; Thomas, E.; Bergouignan, A.; Pan, Z.; Kealey, E.H.; Cornier, M.A.; Bessesen, D.H. Appetite-related responses to overfeeding and longitudinal weight change in obesity-prone and obesity-resistant adults. Obesity 2020, 28, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; MacDiarmid, J.I. Passive overconsumption: Fat intake and short-term energy balance. Ann. N. Y. Acad. Sci. 1997, 827, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Münzberg, H.; Morrison, C.D. Blaming the brain for obesity: Integration of hedonic and homeostatic mechanisms. Gastroenterology 2017, 152, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef]
- Berthoud, H.R. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef]
- Hagobian, T.A.; Sharoff, C.G.; Braun, B. Effects of short-term exercise and energy surplus on hormones related to regulation of energy balance. Metab. Clin. Exp. 2008, 57, 393–398. [Google Scholar] [CrossRef][Green Version]
- Brøns, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef]
- Wijers, S.L.J.; Saris, W.H.M.; Van Marken Lichtenbelt, W.D. Individual thermogenic responses to mild cold and overfeeding are closely related. J. Clin. Endocrinol. Metab. 2007, 92, 4299–4305. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Bechtell, J.L.; Bessesen, D.H.; Tregellas, J.R.; Cornier, M.-A. Hormonal and metabolic effects of short-term energy imbalance in obese-prone as compared to obese-resistant individuals. Am. J. Diabetes Obes. Metab. 2014, 1, 201300233. [Google Scholar]
- Kolaczynski, J.W.; Ohannesian, J.P.; Considine, R.V.; Marco, C.C.; Caro, J.F. Response of leptin to short-term and prolonged overfeeding in humans. J. Clin. Endocrinol. Metab. 1996, 81, 4162–4165. [Google Scholar] [CrossRef][Green Version]
- Parry, S.A.; Smith, J.R.; Corbett, T.R.B.; Woods, R.M.; Hulston, C.J. Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. Br. J. Nutr. 2017, 117, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, M.; Di Vetta, V.; Guenat, E.; Battilana, P.; Seematter, G.; Schneiter, P.; Jéquier, E.; Tappy, L. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1413–1418. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Von Kaenel, S.S.; Bessesen, D.H.; Tregellas, J.R. Effects of overfeeding on the neuronal response to visual food cues. Am. J. Clin. Nutr. 2007, 86, 965–971. [Google Scholar] [CrossRef]
- Oustric, P.; Thivel, D.; Dalton, M.; Beaulieu, K.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Measuring food preference and reward: Application and cross-cultural adaptation of the Leeds Food Preference Questionnaire in human experimental research. Food Qual. Prefer. 2020, 80, 103824. [Google Scholar] [CrossRef]
- Cameron, J.D.; Goldfield, G.S.; Finlayson, G.; Blundell, J.E.; Doucet, É. Fasting for 24 hours heightens reward from food and food-related cues. PLoS ONE 2014, 9, e85970. [Google Scholar] [CrossRef]
- Thivel, D.; Finlayson, G.; Miguet, M.; Pereira, B.; Duclos, M.; Boirie, Y.; Doucet, E.; Blundell, J.E.; Metz, L. Energy depletion by 24-h fast leads to compensatory appetite responses compared with matched energy depletion by exercise in healthy young males. Br. J. Nutr. 2018, 120, 583–592. [Google Scholar] [CrossRef]
- Willis, S.A.; Sargeant, J.A.; Yates, T.; Takamura, T.; Takayama, H.; Gupta, V.; Brittain, E.; Crawford, J.; Parry, S.A.; Thackray, A.E.; et al. Acute hyperenergetic, high-fat feeding increases circulating FGF21, LECT2, and fetuin-A in healthy men. J. Nutr. 2020, 150, 1076–1085. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; King, N.; Blundell, J. The role of implicit wanting in relation to explicit liking and wanting for food: Implications for appetite control. Appetite 2008, 50, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.; Finlayson, G. Psychobiological examination of liking and wanting for fat and sweet taste in trait binge eating females. Physiol. Behav. 2014, 136, 128–134. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a world beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Thomas, E.A.; Bechtell, J.L.; Vestal, B.E.; Johnson, S.L.; Bessesen, D.H.; Tregellas, J.R.; Cornier, M.-A. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite 2013, 65, 96–102. [Google Scholar] [CrossRef]
- Blundell, J.E.; Tremblay, A. Appetite control and energy (fuel) balance. Nutr. Res. Rev. 1995, 8, 225–242. [Google Scholar] [CrossRef]
- Fried, S.K.; Ricci, M.R.; Russell, C.D.; Laferrère, B. Regulation of leptin production in humans. J. Nutr. 2000, 130, 3127S–3131S. [Google Scholar] [CrossRef]
- Ravussin, Y.; Edwin, E.; Gallop, M.; Xu, L.; Bartolomé, A.; Kraakman, M.J.; LeDuc, C.A.; Ferrante, A.W. Evidence for a non-leptin system that defends against weight gain in overfeeding. Cell Metab. 2018, 28, 289–299. [Google Scholar] [CrossRef]
- Flier, J.S.; Maratos-Flier, E. Leptin’s physiologic role: Does the emperor of energy balance have no clothes? Cell Metab. 2017, 26, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol. Behav. 2006, 89, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.P.; Le Roux, C.W. The satiety hormone peptide YY as a regulator of appetite. J. Clin. Pathol. 2008, 61, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992–5995. [Google Scholar] [CrossRef] [PubMed]
- Begg, D.P.; Woods, S.C. The endocrinology of food intake. Nat. Rev. Endocrinol. 2013, 9, 584–597. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Lin, H.C.; Zhao, X.T.; Wang, L.; Wong, H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology 1996, 110, 1491–1495. [Google Scholar] [CrossRef]
- Cahill, F.; Shea, J.L.; Randell, E.; Vasdev, S.; Sun, G. Serum peptide YY in response to short-term overfeeding in young men. Am. J. Clin. Nutr. 2011, 93, 741–747. [Google Scholar] [CrossRef]
- Beaulieu, K.; Oustric, P.; Finlayson, G. The impact of physical activity on food reward: Review and conceptual synthesis of evidence from observational, acute, and chronic exercise training studies. Curr. Obes. Rep. 2020, 9, 63–80. [Google Scholar] [CrossRef]

| Outcome | Control Diet | HE-HFD | Main Effect Diet HE-HFD vs. Control Diet | ||
|---|---|---|---|---|---|
| Mean Difference (95% CI) 1 | p Value | Effect Size (d) | |||
| Ratings of Perceived Appetite | |||||
| Hunger (mm) | 61 (16) | 61 (23) | 0.3 (−9, 10) | 0.939 | 0.02 |
| Fullness (mm) | 24 (13) | 20 (12) | −4 (−14, 6) | 0.428 | 0.31 |
| Desire to eat (mm) | 64 (15) | 67 (21) | 3 (−7, 13) | 0.556 | 0.15 |
| PFC (mm) | 69 (12) | 69 (18) | 0.3 (−8, 9) | 0.933 | 0.02 |
| Appetite-related hormones | |||||
| Leptin (pg/mL) | 3653 (2114, 6310) | 3956 (2290, 6833) | 8% (−17, 41%) | 0.520 | 0.09 |
| Acylated ghrelin (pg/mL) | 49.8 (44.2, 56.2) | 50.5 (44.8, 56.9) | 1% (−4, 8%) | 0.623 | 0.07 |
| Total PYY (pg/mL) | 122.8 (96.7, 155.8) | 105.8 (83.4, 134.3) | −14% (−31, 7%) | 0.165 | 0.38 |
| Outcome | Pre-Diet | 1 Day | 3 Day | 7 Day | Main Effect Diet HE-HFD vs. Control Diet | Diet-by-Time Interaction p Value | ||
|---|---|---|---|---|---|---|---|---|
| Mean Difference (95% CI) 1 | p Value | Effect Size (d) | ||||||
| Relative Preference Fat Appeal Bias (AU) | ||||||||
| Control diet | 10.1 (7.7) | 10.8 (5.7) | 12.0 (6.1) | 10.5 (6.7) | −2.1 (−4.0, −0.1) | 0.036 | 0.32 | 0.470 |
| HE-HFD | 10.7 (8.3) | 8.0 (8.6) | 8.9 (7.7) | 7.3 (7.6) | ||||
| Relative Preference Sweet Appeal Bias (AU) | ||||||||
| Control diet | 12.8 (13.4) | 10.3 (13.0) | 12.1 (9.7) | 7.9 (12.1) | −0.3 (−2.3, 1.7) | 0.788 | 0.09 | 0.558 |
| HE-HFD | 10.7 (12.8) | 9.4 (15.3) | 9.2 (13.3) | 9.6 (13.4) | ||||
| Implicit Wanting Fat Appeal Bias (AU) | ||||||||
| Control diet | 25.5 (17.7) | 26.8 (13.1) | 28.3 (13.3) | 23.3 (14.5) | −4.9 (−10.5, 0.7) | 0.086 | 0.33 | 0.441 |
| HE-HFD | 27.6 (19.3) | 16.5 (24.3) | 21.4 (18.4) | 19.0 (19.1) | ||||
| Implicit Wanting Sweet Appeal Bias (AU) | ||||||||
| Control diet | 32.7 (40.8) | 27.3 (37.0) | 32.3 (22.7) | 21.0 (30.9) | −0.5 (−6.4, 5.4) | 0.863 | 0.07 | 0.228 |
| HE-HFD | 27.5 (32.4) | 19.9 (39.8) | 26.3 (35.2) | 29.3 (42.1) | ||||
| Explicit Wanting Fat Appeal Bias (mm) | ||||||||
| Control diet | 7.8 (10.7) | 4.6 (8.1) | 6.7 (9.5) | 7.3 (9.5) | −1.9 (−4.8, 1.0) | 0.197 | 0.19 | 0.148 |
| HE-HFD | 7.0 (6.0) | 7.2 (9.0) | 4.8 (12.0) | 0.6 (10.7) | ||||
| Explicit Wanting Sweet Appeal Bias (mm) | ||||||||
| Control diet | 14.6 (14.2) | 14.8 (14.6) | 13.7 (16.6) | 11.0 (18.1) | −0.3 (−3.2, 2.5) | 0.822 | 0.07 | 0.973 |
| HE-HFD | 13.6 (18.4) | 13.8 (22.0) | 11.4 (17.0) | 11.2 (14.7) | ||||
| Explicit Liking Fat Appeal Bias (mm) | ||||||||
| Control diet | 8.9 (12.3) | 10.2 (8.1) | 8.8 (10.3) | 9.5 (8.8) | −3.1 (−6.3, 0.1) | 0.055 | 0.31 | 0.842 |
| HE-HFD | 8.2 (7.2) | 5.8 (9.6) | 6.1 (12.2) | 5.4 (10.8) | ||||
| Explicit Liking Sweet Appeal Bias (mm) | ||||||||
| Control diet | 18.9 (21.0) | 18.3 (19.0) | 19.1 (20.1) | 15.5 (22.3) | −3.5 (−8.3, 1.3) | 0.150 | 0.21 | 0.934 |
| HE-HFD | 13.8 (17.5) | 15.0 (20.0) | 11.6 (15.4) | 14.5 (16.6) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thackray, A.E.; Willis, S.A.; Clayton, D.J.; Broom, D.R.; Finlayson, G.; Goltz, F.R.; Sargeant, J.A.; Woods, R.M.; Stensel, D.J.; King, J.A. Influence of Short-Term Hyperenergetic, High-Fat Feeding on Appetite, Appetite-Related Hormones, and Food Reward in Healthy Men. Nutrients 2020, 12, 2635. https://doi.org/10.3390/nu12092635
Thackray AE, Willis SA, Clayton DJ, Broom DR, Finlayson G, Goltz FR, Sargeant JA, Woods RM, Stensel DJ, King JA. Influence of Short-Term Hyperenergetic, High-Fat Feeding on Appetite, Appetite-Related Hormones, and Food Reward in Healthy Men. Nutrients. 2020; 12(9):2635. https://doi.org/10.3390/nu12092635
Chicago/Turabian StyleThackray, Alice E., Scott A. Willis, David J. Clayton, David R. Broom, Graham Finlayson, Fernanda R. Goltz, Jack A. Sargeant, Rachel M. Woods, David J. Stensel, and James A. King. 2020. "Influence of Short-Term Hyperenergetic, High-Fat Feeding on Appetite, Appetite-Related Hormones, and Food Reward in Healthy Men" Nutrients 12, no. 9: 2635. https://doi.org/10.3390/nu12092635
APA StyleThackray, A. E., Willis, S. A., Clayton, D. J., Broom, D. R., Finlayson, G., Goltz, F. R., Sargeant, J. A., Woods, R. M., Stensel, D. J., & King, J. A. (2020). Influence of Short-Term Hyperenergetic, High-Fat Feeding on Appetite, Appetite-Related Hormones, and Food Reward in Healthy Men. Nutrients, 12(9), 2635. https://doi.org/10.3390/nu12092635







