Author Contributions
Conceptualization, Y.B.N., V.M.A. and M.J.D.; methodology, Y.B.N., and M.J.D.; software, Y.B.N.; validation, M.J.D.; formal analysis, Y.B.N., S.M.B.; data curation, Y.B.N.; writing—original draft preparation, Y.B.N.; writing—review and editing, V.M.A., M.J.D. and S.M.B.; visualization, Y.B.N., M.J.D.; supervision, M.J.D.; project administration, M.J.D.; funding acquisition, M.J.D. All authors have read and agreed to the published version of the manuscript.
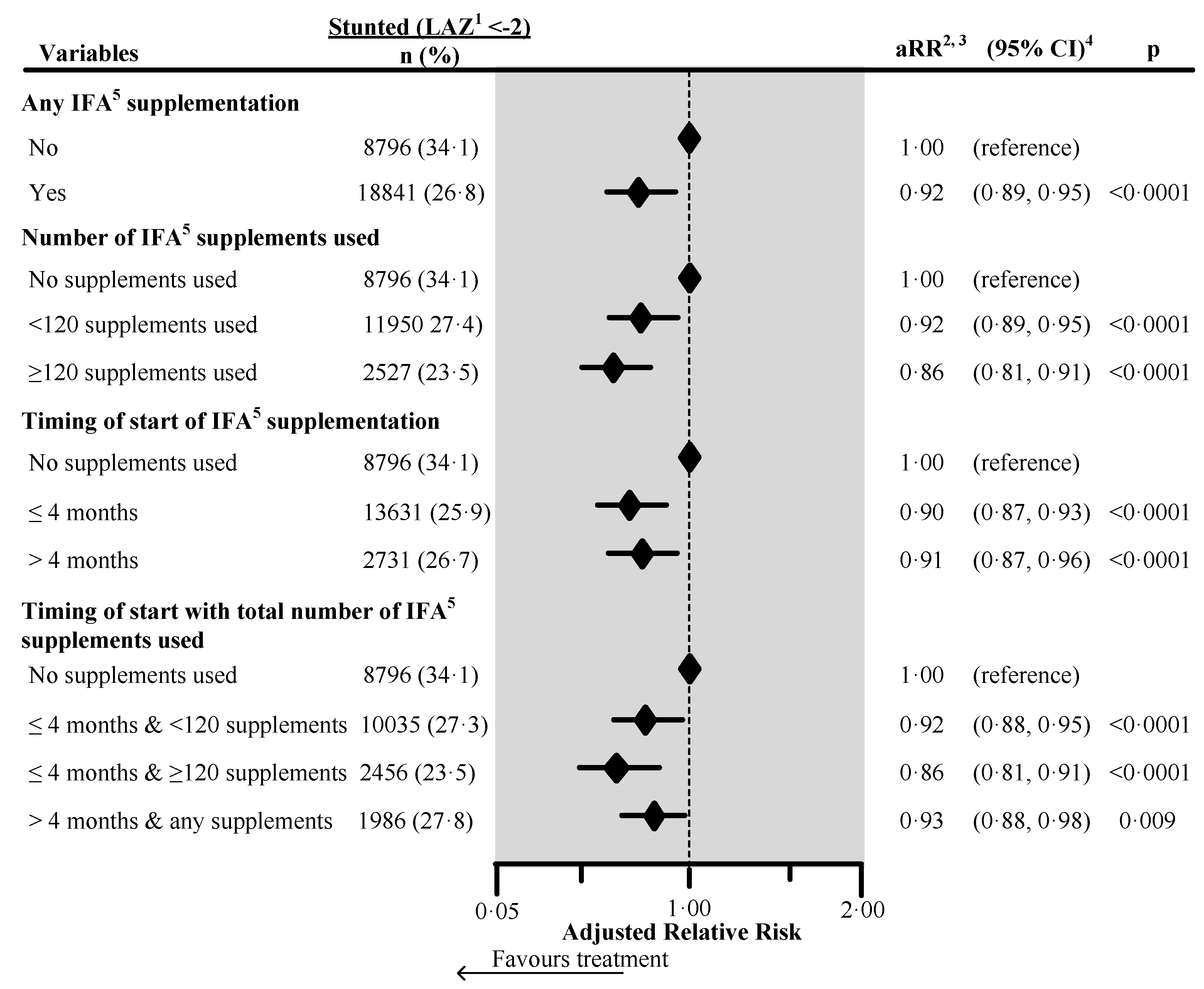
Figure 1.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of supplements, and a combination of the timing of start with the number of supplements used on child stunting in South Asia, using adjusted Poisson regression. The surveys from Bangladesh and Bhutan did not collect information about the number of IFA supplements used during pregnancy. Hence these survey data were not included in the number of IFA supplements analysis (n = 4744). Also, we excluded records of mothers from analysis who reported consumption of >240 IFA supplements during pregnancy (n = 85). We also excluded those records where the number of supplements consumed exceeded the number possible when supplementation started between the fifth and ninth months of pregnancy (n = 183). 1 Length-for-Age Z-score. 2 Adjusted for country, area of residence, maternal marital status, maternal educational status, fuel used for cooking, source of drinking water, sanitation facilities, pooled household wealth index, maternal age at childbirth, sex of child, the timing of initiation of breastfeeding, age of the child, and child had diarrhea during last two weeks before the interview. Also, we adjusted the model for the duration of recall. 3 aRR: Adjusted relative risk. 4 CI: Confidence interval. 5 IFA: Iron/folic acid.
Figure 1.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of supplements, and a combination of the timing of start with the number of supplements used on child stunting in South Asia, using adjusted Poisson regression. The surveys from Bangladesh and Bhutan did not collect information about the number of IFA supplements used during pregnancy. Hence these survey data were not included in the number of IFA supplements analysis (n = 4744). Also, we excluded records of mothers from analysis who reported consumption of >240 IFA supplements during pregnancy (n = 85). We also excluded those records where the number of supplements consumed exceeded the number possible when supplementation started between the fifth and ninth months of pregnancy (n = 183). 1 Length-for-Age Z-score. 2 Adjusted for country, area of residence, maternal marital status, maternal educational status, fuel used for cooking, source of drinking water, sanitation facilities, pooled household wealth index, maternal age at childbirth, sex of child, the timing of initiation of breastfeeding, age of the child, and child had diarrhea during last two weeks before the interview. Also, we adjusted the model for the duration of recall. 3 aRR: Adjusted relative risk. 4 CI: Confidence interval. 5 IFA: Iron/folic acid.
![Nutrients 12 02632 g001 Nutrients 12 02632 g001]()
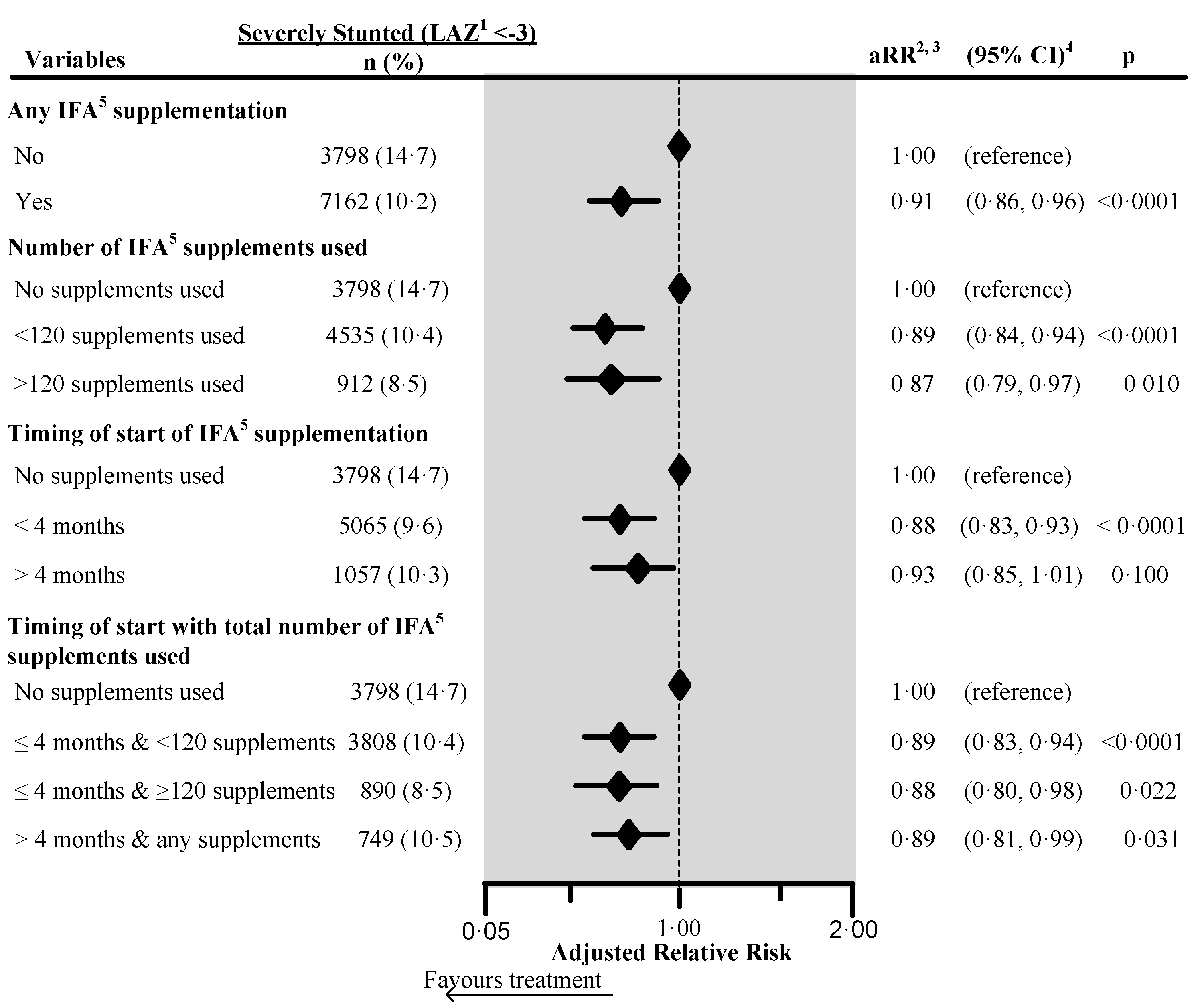
Figure 2.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of supplements, and a combination of the timing of start with the number of supplements used on child severe stunting in South Asia, using adjusted Poisson regression. The surveys from Bangladesh and Bhutan did not collect information about the number of IFA supplements used during pregnancy. Hence these survey data were not included in the number of IFA supplementation analyses (n = 4744). Also, we excluded records of mothers from analysis who reported consumption of >240 IFA supplements during pregnancy (n = 85). We also excluded those records where the number of supplements consumed exceeded the number possible when supplementation started between the fifth and ninth months of pregnancy (n = 183). 1 Length-for-Age Z-score. 2 Adjusted for country, area of residence, maternal marital status, maternal educational status, fuel used for cooking, source of drinking water, sanitation facilities, pooled household wealth index, maternal age at childbirth, sex of child, the timing of initiation of breastfeeding, age of the child, and child had diarrhea during last two weeks before the interview. Also, we adjusted the model for the duration of recall. 3 aRR: Adjusted relative risk. 4 CI: Confidence interval. 5 IFA: Iron/folic acid.
Figure 2.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of supplements, and a combination of the timing of start with the number of supplements used on child severe stunting in South Asia, using adjusted Poisson regression. The surveys from Bangladesh and Bhutan did not collect information about the number of IFA supplements used during pregnancy. Hence these survey data were not included in the number of IFA supplementation analyses (n = 4744). Also, we excluded records of mothers from analysis who reported consumption of >240 IFA supplements during pregnancy (n = 85). We also excluded those records where the number of supplements consumed exceeded the number possible when supplementation started between the fifth and ninth months of pregnancy (n = 183). 1 Length-for-Age Z-score. 2 Adjusted for country, area of residence, maternal marital status, maternal educational status, fuel used for cooking, source of drinking water, sanitation facilities, pooled household wealth index, maternal age at childbirth, sex of child, the timing of initiation of breastfeeding, age of the child, and child had diarrhea during last two weeks before the interview. Also, we adjusted the model for the duration of recall. 3 aRR: Adjusted relative risk. 4 CI: Confidence interval. 5 IFA: Iron/folic acid.
![Nutrients 12 02632 g002 Nutrients 12 02632 g002]()
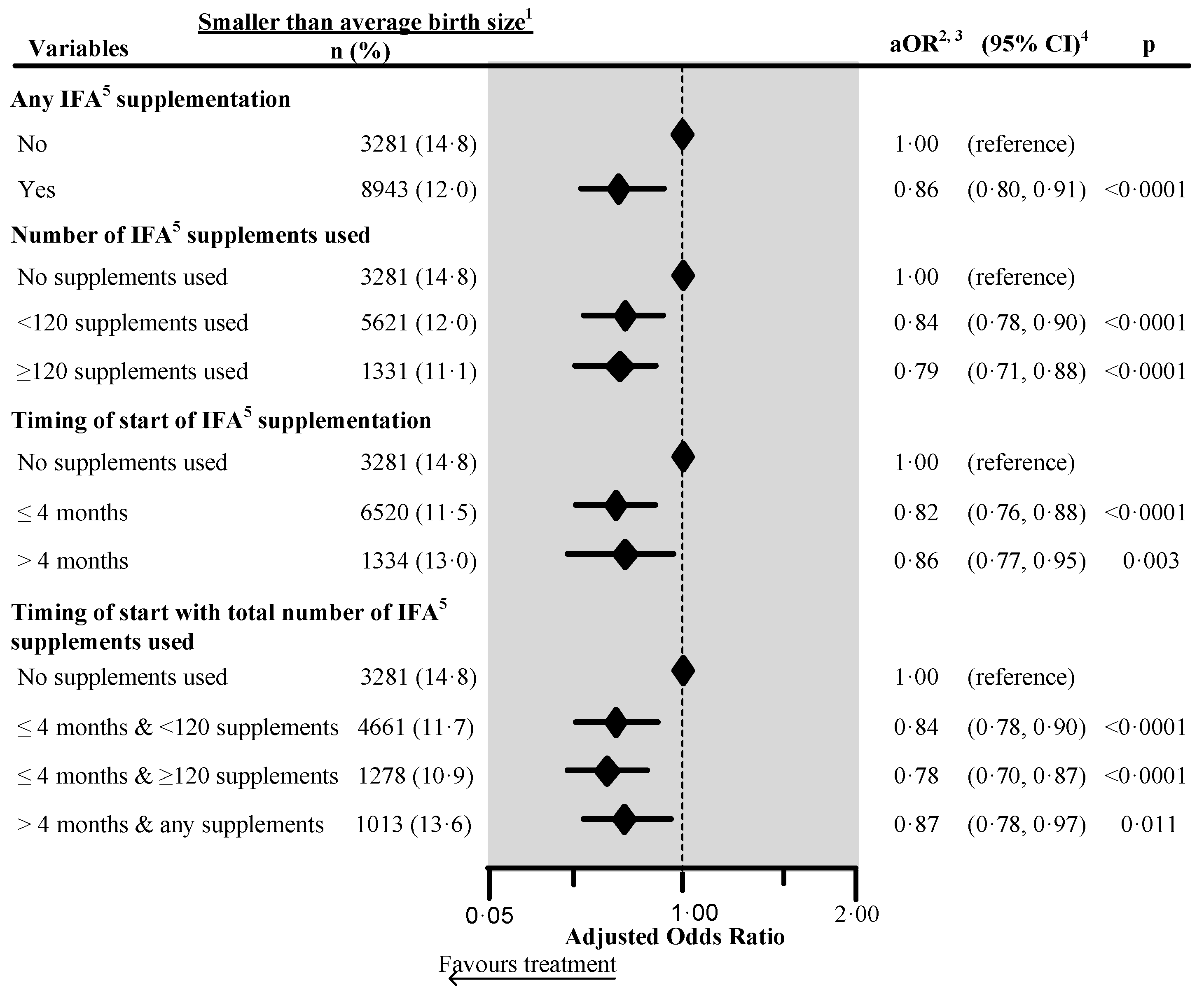
Figure 3.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of the supplements and a combination of the timing of start with the number of supplements used on the maternally perceived birth size in four South Asian countries 6, using adjusted logistic regression. 1 Included smaller than average and smallest categories. 2 Adjusted for country, place of residence, maternal marital status, maternal educational status, maternal working status, paternal educational status, paternal working status, fuel used for cooking, source of drinking water, sanitation facilities, pooled household wealth index, maternal age at childbirth, maternal desire for pregnancy, maternal smoking status, maternal height, birth outcome status, birth interval and rank, and sex of child. Also, we adjusted the model for the duration of recall. 3 aOR: Adjusted odds ratio. 4 CI: Confidence interval. 5 IFA: Iron/folic acid. 6 The surveys from India, Maldives, Nepal, and Pakistan collected information on maternally perceived birth size. We excluded records of mothers from analysis who reported consumption of >240 IFA supplements during pregnancy. We also excluded those records where the number of supplements consumed exceeded the number possible when supplementation started between the fifth and ninth months of pregnancy.
Figure 3.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of the supplements and a combination of the timing of start with the number of supplements used on the maternally perceived birth size in four South Asian countries 6, using adjusted logistic regression. 1 Included smaller than average and smallest categories. 2 Adjusted for country, place of residence, maternal marital status, maternal educational status, maternal working status, paternal educational status, paternal working status, fuel used for cooking, source of drinking water, sanitation facilities, pooled household wealth index, maternal age at childbirth, maternal desire for pregnancy, maternal smoking status, maternal height, birth outcome status, birth interval and rank, and sex of child. Also, we adjusted the model for the duration of recall. 3 aOR: Adjusted odds ratio. 4 CI: Confidence interval. 5 IFA: Iron/folic acid. 6 The surveys from India, Maldives, Nepal, and Pakistan collected information on maternally perceived birth size. We excluded records of mothers from analysis who reported consumption of >240 IFA supplements during pregnancy. We also excluded those records where the number of supplements consumed exceeded the number possible when supplementation started between the fifth and ninth months of pregnancy.
![Nutrients 12 02632 g003 Nutrients 12 02632 g003]()
Table 1.
Country-wise type of survey, year of survey and data source for selected country-specific survey data used in the current study.
Table 1.
Country-wise type of survey, year of survey and data source for selected country-specific survey data used in the current study.
| Country | Type & Year of Survey | Data Source |
|---|
| Afghanistan | National Nutrition Survey (NNS) 2013 | Institutional (UNICEF) |
| Bangladesh | Demographic and Health Survey (DHS) 2007 | Public domain |
| Bhutan | National Nutrition and Anaemia Survey 2015 | Institutional (UNICEF) |
| India | National Family Health Survey (NFHS) 2015/16 | Public domain |
| Maldives | Demographic and Health Survey (DHS) 2010 | Public domain |
| Nepal | Demographic and Health Survey (DHS) 2006 | Public domain |
| Pakistan | Demographic and Health Survey (DHS) 2012/13 | Public domain |
Table 2.
Operational definition and categorisation of the potential confounding variables used in the analysis.
Table 2.
Operational definition and categorisation of the potential confounding variables used in the analysis.
| Variables | Definition and Categorisation |
|---|
| Community-level and socio-economic factors | |
| Country | Country of survey (1 = India; 2 = Afghanistan; 3 = Bangladesh; 4 = Bhutan; 5 = Maldives; 6 = Nepal; 7 = Pakistan) |
| Place of residence | Place of residence of the respondent (1 = urban; and 2 = rural) |
| Maternal marital status | Marital status of the mother (1 = currently married, and 2 = formerly married) |
| Maternal educational status | Maternal level of attained education (1 = secondary and above; 2 = completed primary; and 3 = no education) |
| Fuel used for cooking | Fuel used for cooking at home (1 = biomass energy; and 2 = natural gas). |
| Source of water used for drinking | Source of water used for drinking at home was classified based on WHO/UNICEF guidelines (1 = improved; and 2 = unimproved). |
| Sanitation facility | Sanitation referred to the toilet facility at home and was classified based on WHO/UNICEF guidelines (1 = improved; and 2 = unimproved). |
| Pooled household wealth index | A composite index of household amenities using pooled data and a principal component analysis [23] of household assets that was ranked into quintiles |
| Maternal and child characteristics | |
| Maternal age at childbirth | Maternal age at childbirth (1 = 20–24 years; 2 = <20 years; and 3 = ≥25 years). |
| Sex of child | Sex of the child (1 = female; and 2 = male). |
| Age of child | Age of the child in months (as a continuous variable) |
| Initiation of breastfeeding | Timing of initiation of breastfeeding (1 = <1 h; 2 = 1 to 24 h; 3 = >24 h; and 4 = never). |
| Child diarrhea in the last two weeks | The child had diarrhea within two weeks before the interview date (1 = no; and 2 = yes). |
| Perinatal health services variables | |
| Number of ANC visits | Number of antenatal care visits (1 = no ANC visit; 2 = <4 visits; and 3 = ≥4 visits). |
Table 3.
Prevalence of community-level, socio-economic status, maternal and child characteristics and perinatal health services of most recent live-births two years before the interview in pooled data from seven countries in South Asia (N = 96,552).
Table 3.
Prevalence of community-level, socio-economic status, maternal and child characteristics and perinatal health services of most recent live-births two years before the interview in pooled data from seven countries in South Asia (N = 96,552).
| Variables | Unweighted | Weighted † |
|---|
| N | n | % |
|---|
| Community-level and socio-economic status factors | | | |
| Country | | | |
| India | 83,594 | 77,276 | 85.7 |
| Afghanistan | 6343 | 6366 | 7.1 |
| Bangladesh | 2079 | 2058 | 2.3 |
| Bhutan | 562 | 526 | 0.6 |
| Maldives | 992 | 977 | 1.1 |
| Nepal | 1944 | 1841 | 2.0 |
| Pakistan | 1038 | 1170 | 1.3 |
| Place of residence | | | |
| Urban | 22,165 | 24,163 | 26.8 |
| Rural | 74,387 | 66,051 | 73.2 |
| Maternal marital status | | | |
| Currently married | 95,452 | 89,480 | 99.2 |
| Formerly married | 1100 | 733 | 0.8 |
| Maternal educational status | | | |
| Secondary and above | 51,431 | 48,585 | 53.9 |
| Up to primary | 14,032 | 12,903 | 14.3 |
| No education | 31,068 | 28,710 | 31.8 |
| Missing | 21 | 16 | <0.1 |
| Fuel used for cooking | | | |
| Biomass energy | 63,694 | 54,876 | 60.8 |
| Natural gas | 32,849 | 35,324 | 39.2 |
| Missing | 9 | 13 | <0.1 |
| Source of drinking water | | | |
| Improved | 77,924 | 75,321 | 83.5 |
| Unimproved | 18,233 | 14,515 | 16.1 |
| Missing | 395 | 377 | 0.4 |
| Sanitary facility used at home | | | |
| Improved | 48,924 | 43,073 | 47.8 |
| Unimproved | 47,597 | 47,107 | 52.2 |
| Missing | 31 | 34 | <0.1 |
| Household wealth index | | | |
| Quintile 1 (Wealthiest) | 12,423 | 13,005 | 14.4 |
| Quintile 2 | 18,692 | 19,540 | 21.7 |
| Quintile 3 (Middle) | 18,502 | 17,222 | 19.1 |
| Quintile 4 | 20,572 | 16,712 | 18.5 |
| Quintile 5 (Poorest) | 25,945 | 23,331 | 25.9 |
| Missing | 418 | 405 | 0.4 |
| Maternal and child characteristics | | | |
| Maternal age at childbirth | | | |
| 20–24 years | 46,627 | 43,590 | 48.3 |
| <20 years | 31,610 | 30,879 | 34.2 |
| >24 years | 18,315 | 15,745 | 17.5 |
| Sex of Child | | | |
| Male | 50,347 | 47,093 | 52.2 |
| Female | 46,205 | 43,120 | 47.8 |
| Time to initiate breastfeeding after birth | | | |
| Never breastfed | 2834 | 2586 | 2.9 |
| Within 1 h | 65,016 | 59,355 | 65.8 |
| 1 h to 1 day | 16,260 | 15,375 | 17.0 |
| More than 1 day | 12,418 | 12,878 | 14.3 |
| Missing | 24 | 19 | <0.1 |
| Age of child (in months) | | | |
| Mean (SD *) | 11.6 (0.02) | 11.7 (0.03) | |
| The child had diarrhea in the last two weeks | | | |
| No | 81,288 | 75,486 | 83.7 |
| Yes | 15,226 | 14,683 | 16.3 |
| Missing | 38 | 44 | <0.1 |
| Perinatal health services variable | | | |
| Number of ANC visits | | | |
| No ANC visit | 18,858 | 16,873 | 18.7 |
| 1–3 ANC visits | 34,551 | 30,101 | 33.4 |
| 4 or more ANC visits | 43,094 | 43,173 | 47.9 |
| Missing | 49 | 66 | <0.1 |
Table 4.
Prevalence of study exposure and outcome variables of the most recent live-births two years before the interview, in South Asia, 2005–2016.
Table 4.
Prevalence of study exposure and outcome variables of the most recent live-births two years before the interview, in South Asia, 2005–2016.
| Variable | Unweighted | Weighted |
|---|
| N | n | % | 95% CI |
|---|
| Exposure variables | | | | |
| IFA used | | | | |
| No | 25,928 | 23,892 | 26.5 | (26.0, 27.0) |
| Yes | 70,586 | 66,277 | 73.5 | (73.0, 74.0) |
| Missing | 38 | 44 | <0.1 | (<0.0, <0.1) |
| Number of IFA used during Pregnancy | | | | |
| No IFA | 25,928 | 23,892 | 26.5 | (26.0, 27.0) |
| <120 IFA | 43,810 | 38,673 | 42.9 | (42.3, 43.4) |
| ≥120 IFA | 10,809 | 11,695 | 13.0 | (12.5, 13.4) |
| Missing | 16,005 | 15,956 | 17.8 | (17.3, 18.1) |
| Timing of initiation of IFA supplements | | | | |
| No IFA used | 25,928 | 23,892 | 26.5 | (26.0, 27.0) |
| >4 months of pregnancy | 52,891 | 49,240 | 54.6 | (54.0, 55.2) |
| ≤4 months of pregnancy | 10,276 | 10,117 | 11.2 | (10.9, 11.6) |
| Missing | 7457 | 6964 | 7.7 | (7.4, 8.0) |
| Timing of initiation and number of IFA used | | | | |
| No IFA | 25,928 | 23,892 | 26.5 | (26.0, 27.0) |
| >4 mo of pregnancy and any IFA | 36,936 | 32,144 | 35.6 | (35.1, 36.2) |
| ≤4 mo of pregnancy and <120 IFA | 10,496 | 11,382 | 12.6 | (12.2, 13.1) |
| ≤4 mo of pregnancy and ≥120 IFA | 7187 | 6843 | 7.6 | (7.3, 7.9) |
| Missing | 16,005 | 15,956 | 17.7 | (17.3, 18.1) |
| Outcome variables | | | | |
| Stunting (LAZ < −2) | | | | |
| Normal | 68,794 | 64,259 | 71.1 | (70.8, 71.7) |
| Stunting | 27,758 | 25,954 | 28.9 | (28.3, 29.2) |
| Severe stunting (LAZ < −3) | | | | |
| Normal | 85,557 | 80,092 | 88.8 | (88.5, 89.1) |
| Severe stunting | 10,995 | 10,121 | 11.2 | (10.9, 11.5) |
| LAZ (as a continuous variable) | |
| Mean (SD) | −1.10 (0.01) | −1.10 (0.01) |
| Maternal perceived birth size 1 | | | | |
| Average or larger than average | 75,313 | 70,521 | 87.6 | (87.3, 88.0) |
| Smaller than average | 10,790 | 9969 | 12.4 | (12.0, 12.7) |
Table 5.
Factors associated with stunting (LAZ < −2) in South Asian children <2 years old (2005–2016): Results of adjusted Poisson regression 1.
Table 5.
Factors associated with stunting (LAZ < −2) in South Asian children <2 years old (2005–2016): Results of adjusted Poisson regression 1.
| Factors | aRR 2 | 95% CI 3 | p |
|---|
| Community-Level and Socio-Economic Status Factors |
|---|
| Country | | | |
| India | 1.00 | (reference) | |
| Afghanistan | 1.75 | (1.56, 1.96) | <0.0001 |
| Bangladesh | 0.84 | (0.77, 0.92) | <0.0001 |
| Bhutan | 0.88 | (0.62, 1.27) | 0.510 |
| Maldives | 0.89 | (0.76, 1.04) | 0.150 |
| Nepal | 0.85 | (0.77, 0.94) | 0.002 |
| Pakistan | 1.11 | (0.98, 1.24) | 0.086 |
| Fuel used for cooking | | | |
| Biomass energy | 1.00 | (reference) | |
| Natural gas | 0.87 | (0.83, 0.90) | <0.00010 |
| Maternal educational status | | | |
| Secondary and above | 1.00 | (reference) | |
| Up to primary | 1.20 | (1.15, 1.25) | <0.0001 |
| No education | 1.33 | (1.28, 1.38) | <0.0001 |
| Source of drinking water | | | |
| Improved | 1.00 | (reference) | |
| Unimproved | 0.95 | (0.91, 0.99) | 0.017 |
| Sanitary facility | | | |
| Improved | 1.00 | (reference) | |
| Unimproved | 1.11 | (1.06, 1.15) | <0.0001 |
| Household wealth index | | | |
| Quintile 1 (Wealthiest) | 1.00 | (reference) | |
| Quintile 2 | 1.16 | (1.09, 1.25) | <0.0001 |
| Quintile 3 (Middle) | 1.20 | (1.12, 1.28) | <0.0001 |
| Quintile 4 | 1.27 | (1.18, 1.36) | <0.0001 |
| Quintile 5 (Poorest) | 1.30 | (1.20, 1.39) | <0.0001 |
| Maternal and child characteristics | | | |
| Maternal age at childbirth | | | |
| 20–24 years | 1.00 | (reference) | |
| <20 years | 1.02 | (0.99, 1.05) | 0.110 |
| >24 years | 0.92 | (0.88, 0.96) | <0.0001 |
| Sex of child | | | |
| Female | 1.00 | (reference) | |
| Male | 1.09 | (1.06, 1.12) | <0.0001 |
| Age of child (in months) | 1.09 | (1.08, 1.09) | <0.0001 |
| The child had diarrhea in the last two weeks | | | |
| No | 1.00 | (reference) | |
| Yes | 1.08 | (1.07, 1.08) | <0.0001 |
| Perinatal health services variable | | | |
| Number of ANC visits | | | |
| No ANC visit | 1.00 | (reference) | |
| 1–3 ANC visits | 0.93 | (0.90, 0.96) | <0.0001 |
| 4 or more ANC visits | 0.84 | (0.81, 0.88) | <0.0001 |
Table 6.
Factors associated with severe stunting (LAZ < −3) in South Asian children < 2 years old (2005–2016): Results of adjusted Poisson regression 1.
Table 6.
Factors associated with severe stunting (LAZ < −3) in South Asian children < 2 years old (2005–2016): Results of adjusted Poisson regression 1.
| Factors | aRR 2 | 95% CI 3 | p |
|---|
| Community-Level and Socio-Economic Status Factors |
|---|
| Country | | | |
| India | 1.00 | (reference) | |
| Afghanistan | 2.28 | (1.88, 2.76) | <0.0001 |
| Bangladesh | 0.55 | (0.44, 0.69) | <0.0001 |
| Bhutan | 0.91 | (0.52, 1.59) | 0.734 |
| Maldives | 0.68 | (0.48, 0.95) | 0.024 |
| Nepal | 0.64 | (0.53, 0.78) | <0.0001 |
| Pakistan | 1.25 | (1.00, 1.55) | 0.05 |
| Fuel used for cooking | | | |
| Biomass energy | 1.00 | (reference) | |
| Natural gas | 0.83 | (0.77, 0.89) | <0.0001 |
| Maternal educational status | | | |
| Secondary and above | 1.00 | (reference) | |
| Up to primary | 1.25 | (1.15, 1.35) | <0.0001 |
| No education | 1.50 | (1.41, 1.59) | <0.0001 |
| Source of drinking water | | | |
| Improved | 1.00 | (reference) | |
| Unimproved | 0.90 | (0.83, 0.97) | 0.005 |
| Household wealth index | | | |
| Quintile 1 (Wealthiest) | 1.00 | (reference) | |
| Quintile 2 | 1.21 | (1.07, 1.36) | 0.002 |
| Quintile 3 (Middle) | 1.32 | (1.17, 1.48) | <0.0001 |
| Quintile 4 | 1.47 | (1.31, 1.64) | <0.0001 |
| Quintile 5 (Poorest) | 1.70 | (1.52, 1.91) | <0.0001 |
| Maternal and child characteristics | | | |
| Maternal age at childbirth | | | |
| 20–24 years | 1.00 | (reference) | |
| <20 years | 1.01 | (1.00, 1.19) | 0.043 |
| >24 years | 1.09 | (0.90, 1.14) | 0.874 |
| Sex of child | | | |
| Female | 1.00 | (reference) | |
| Male | 1.17 | (1.12, 1.23) | <0.0001 |
| Age in child (in months) | 1.09 | (1.09, 1.10) | <0.0001 |
| Perinatal health services variable | | | |
| Number of ANC visits | | | |
| No ANC visit | 1.00 | (reference) | |
| 1–3 ANC visits | 0.88 | (0.83, 0.93) | <0.0001 |
| ≥4 ANC visits | 0.77 | (0.72, 0.83) | <0.0001 |
Table 7.
Factors associated with length-for-age Z score (LAZ) in South Asian children <2 years old (2005–2016): Results of adjusted linear regression 1.
Table 7.
Factors associated with length-for-age Z score (LAZ) in South Asian children <2 years old (2005–2016): Results of adjusted linear regression 1.
| Factors | Coefficient | 95% CI 2 | p |
|---|
| Community-Level and Socio-Economic Status Factors |
|---|
| Country | | | |
| India | 1.00 | (reference) | |
| Afghanistan | −0.25 | (−0.40, −0.10) | 0.001 |
| Bangladesh | −0.03 | (−0.10, 0.04) | 0.375 |
| Bhutan | 0.60 | (0.32, 0.89) | <0.0001 |
| Maldives | −0.11 | (−0.23, 0.01) | 0.051 |
| Nepal | −0.02 | (−0.11, 0.07) | 0.615 |
| Pakistan | −0.18 | (−0.29, −0,06) | 0.002 |
| Fuel used for cooking | | | |
| Biomass energy | 1.00 | (reference) | |
| Natural gas | 0.18 | (0.14, 0.22) | <0.0001 |
| Maternal educational status | | | |
| Secondary and above | 1.00 | (reference) | |
| Up to primary | −0.19 | (−0.23, −0.15) | <0.0001 |
| No education | −0.31 | (−0.34, −0.27) | <0.0001 |
| Household wealth index | | | |
| Quintile 1 (Wealthiest) | 1.00 | (reference) | |
| Quintile 2 | −0.14 | (−0.19, −0.08) | <0.0001 |
| Quintile 3 (Middle) | −0.19 | (−0.25, −0.13) | <0.0001 |
| Quintile 4 | −0.27 | (−0.33, −0.20) | <0.0001 |
| Quintile 5 (Poorest) | −0.31 | (−0.38, −0.25) | <0.0001 |
| Source of drinking water | | | | |
| Improved | 1.00 | (reference) | |
| Unimproved | 0.05 | (0.05, 0.0) | 0.031 |
| Sanitary facility | | | |
| Improved | 1.00 | (reference) | |
| Unimproved | −0.08 | (−0.12, −0.04) | <0.0001 |
| Maternal and child characteristics | | | |
| Maternal age at childbirth | | | |
| 20–24 years | 1.00 | (reference) | |
| <20 years | −0.04 | (−0.08, −0.01) | 0.004 |
| >24 years | 0.13 | (0.08, 0.17) | <0.0001 |
| Sex of child | | | |
| Female | 1.00 | (reference) | |
| Male | −0.13 | (−0.16, −0.10) | <0.0001 |
| Age in child (in months) | −0.10 | (−0.10, −0.10) | <0.0001 |
| The child had diarrhea in the last two weeks | | | |
| No | 1.00 | (reference) | |
| Yes | −0.06 | (−0.10, −0.02) | 0.002 |
| Perinatal health services variable | | | |
| Number of ANC visits | | | |
| No ANC visit | 1.00 | (reference) | |
| 1–3 ANC visits | 0.04 | (−0.01, 0.08) | 0.080 |
| 4 or more ANC visits | 0.15 | (0.10, 0.19) | <0.0001 |
Table 8.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of supplements and a combination of the timing of start with the number of supplements used on the length-for-age Z score in south Asian children <2 years old, adjusted linear regression 1.
Table 8.
Effect of any iron/folic acid (IFA) supplementation, the number of supplements used, the timing of the start of supplements and a combination of the timing of start with the number of supplements used on the length-for-age Z score in south Asian children <2 years old, adjusted linear regression 1.
| Variables | Coefficients | 95% CI 2 | p |
|---|
| IFA 3 supplements used | | | |
| No | − | (reference) | |
| Yes | 0.10 | (0.07, 0.13) | <0.0001 |
| Number of IFA3 supplements used during pregnancy 4 | | | |
| No IFA | − | (reference) | |
| <120 IFA | 0.08 | (0.05, 0.12) | <0.0001 |
| >120 IFA | 0.15 | (0.10, 0.21) | <0.0001 |
| Timing of initiation of IFA 3 supplements | | | | |
| No IFA used | − | (reference) | |
| <4 months | 0.11 | (0.08, 0.15) | <0.0001 |
| >4 months | 0.08 | (0.03, 0.13) | 0.002 |
| Timing of initiation and number of IFA 3 supplements used 4 | | |
| No IFA | − | (reference) | |
| <4 months and <120 IFA | 0.09 | (0.05, 0.13) | <0.0001 |
| <4 months and ≥120 IFA | 0.15 | (0.10, 0.21) | <0.0001 |
| >4 months and any IFA | 0.07 | (0.01, 0.12) | 0.019 |