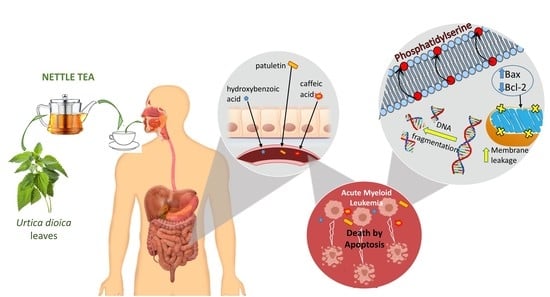
Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of UD Aqueous Extract
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Cell-Cycle Analysis
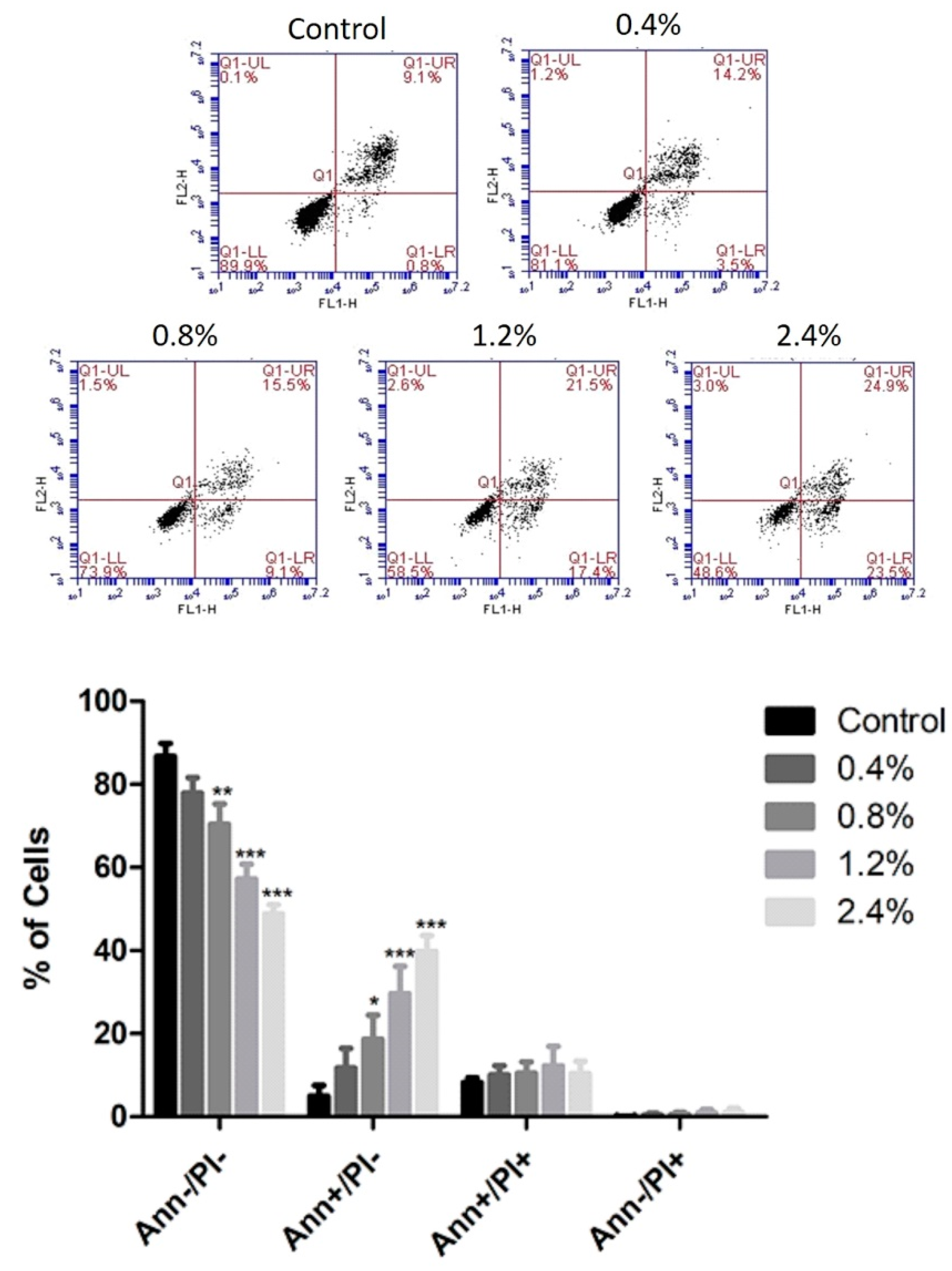
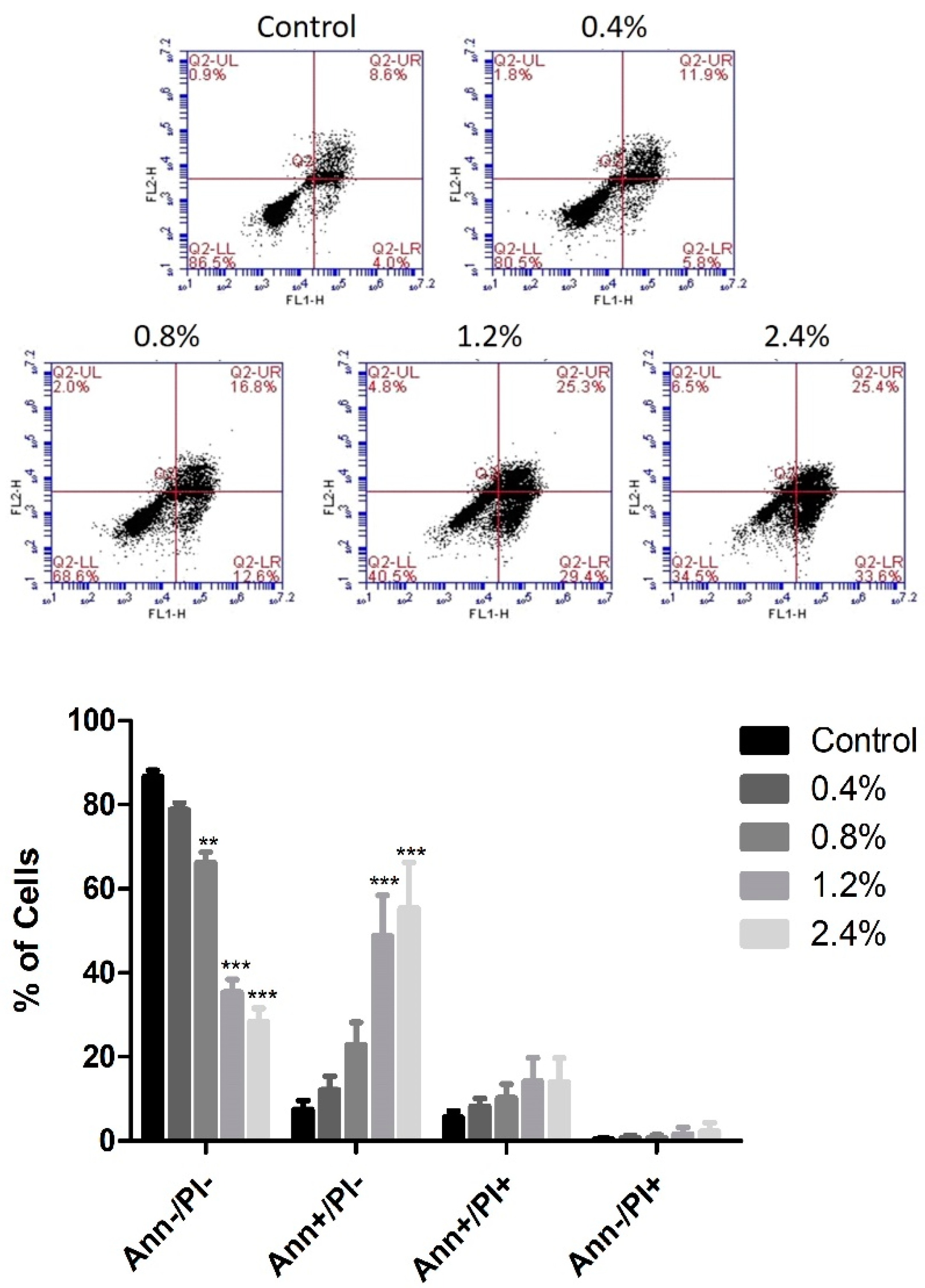
2.5. Apoptosis Detection
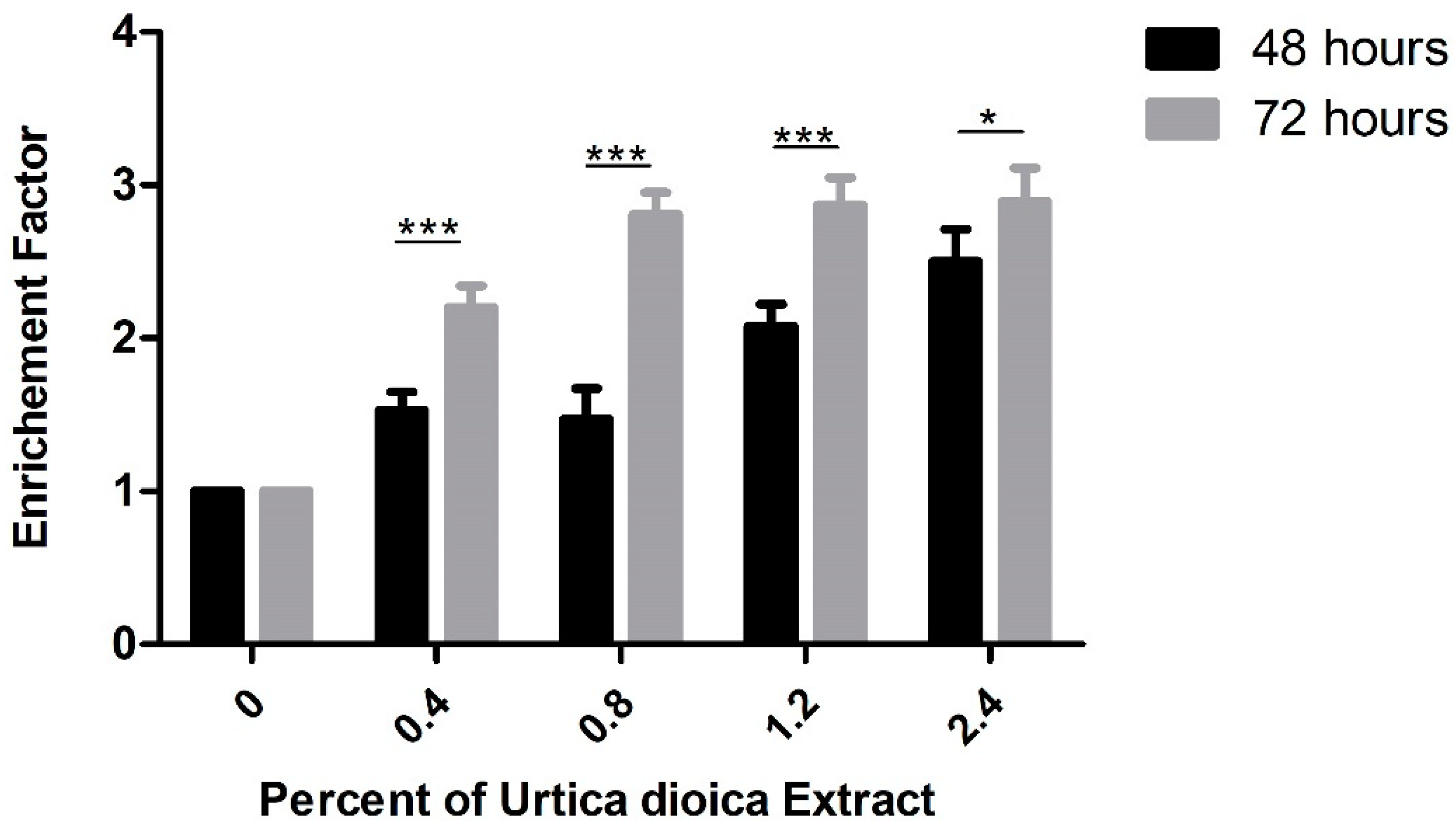
2.6. Assessment of DNA Fragmentation Using Cell-Death ELISA
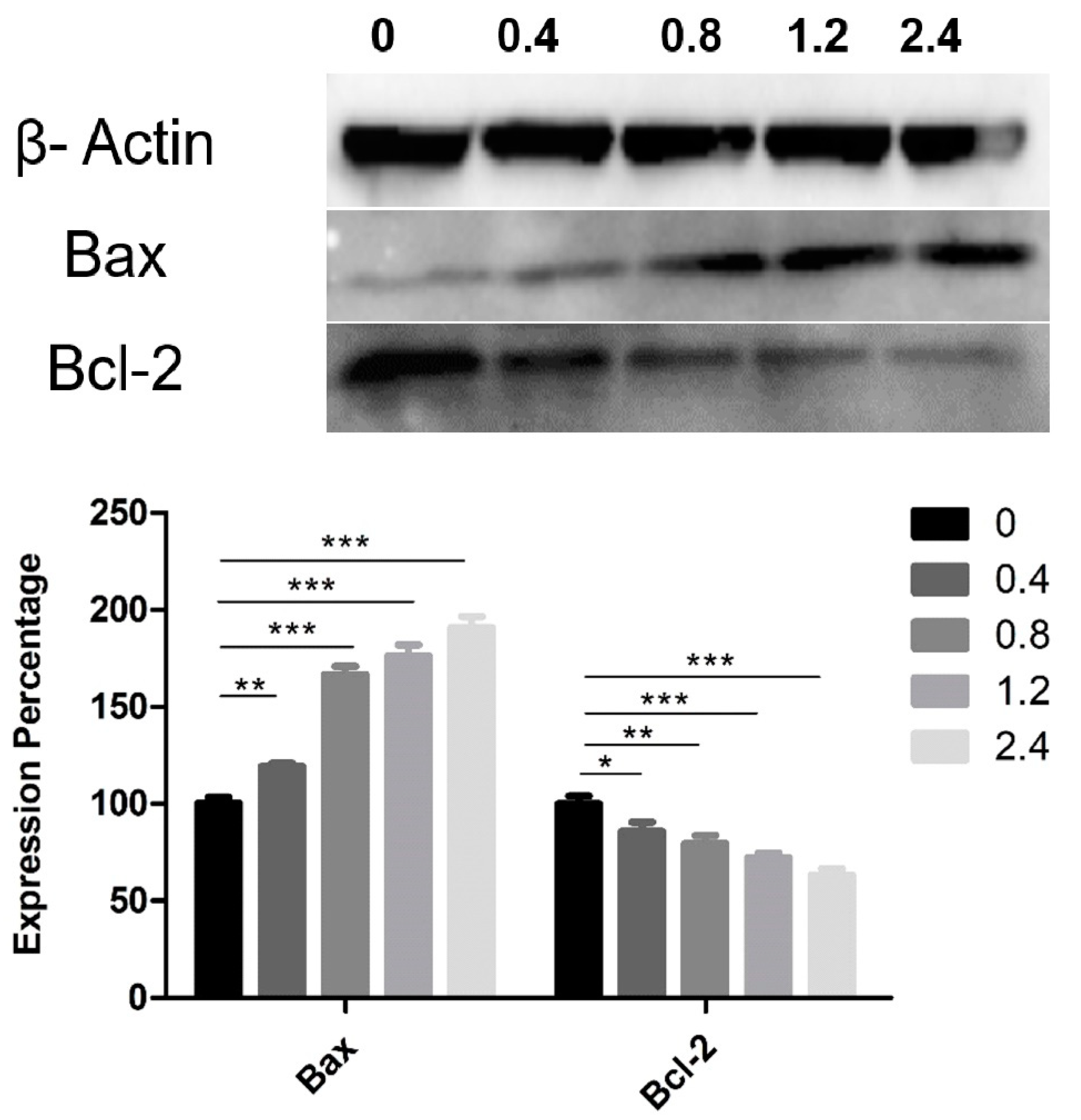
2.7. Western Blot Analysis
2.8. Extraction and Chemical Analysis
2.8.1. Extraction and Ultra-Performance Liquid Chromatography (UPLC) Analysis of Polyphenols, Terpenes, Sesquiterpenes, Fatty Acids, and Flavonoids
2.8.2. LC-TSQ-Endura-MS/MS Analysis
2.9. Statistical Analysis
3. Results
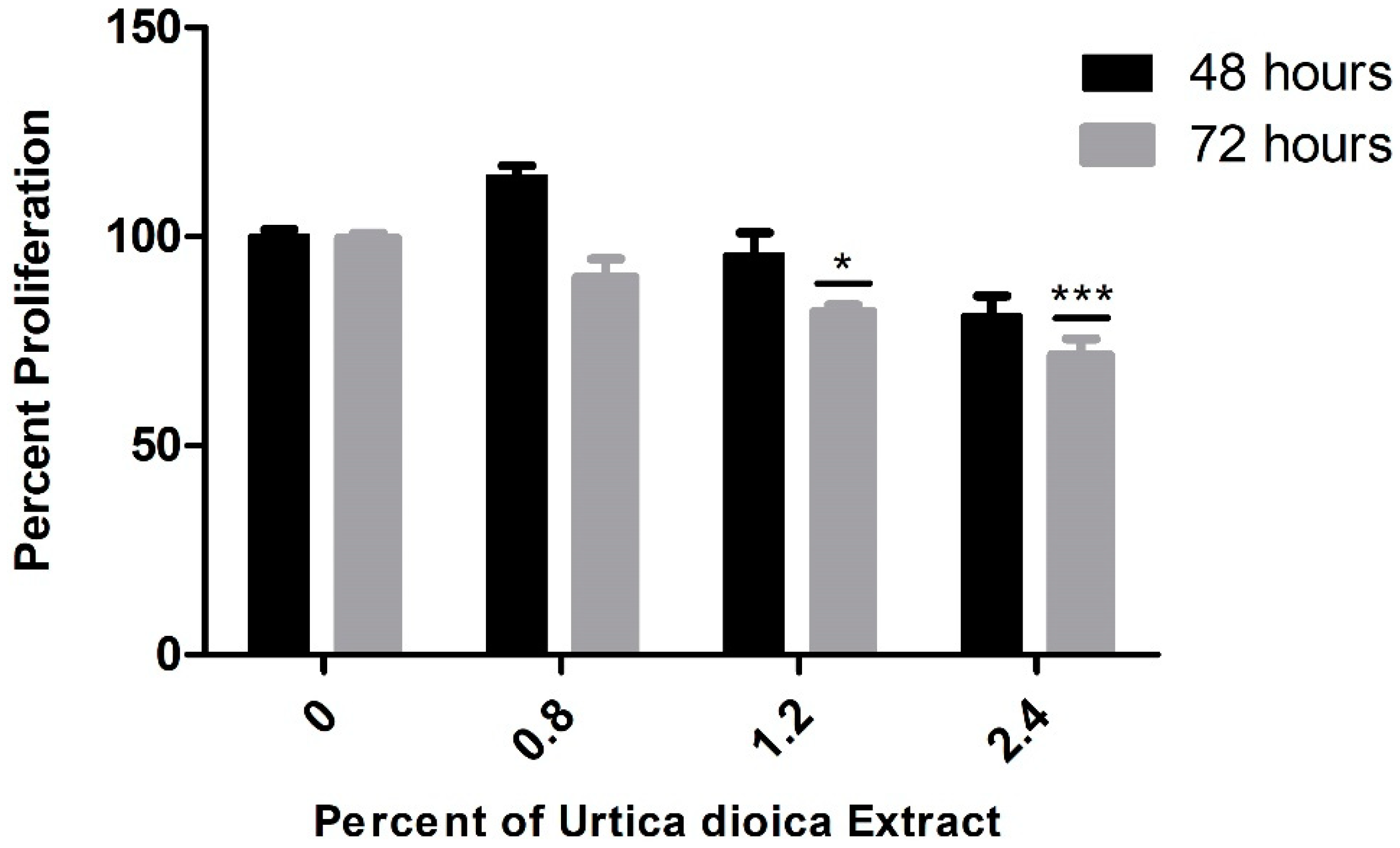
3.1. Cytotoxic Effect of UD Extract on AML and Normal B-Lymphocyte Cells
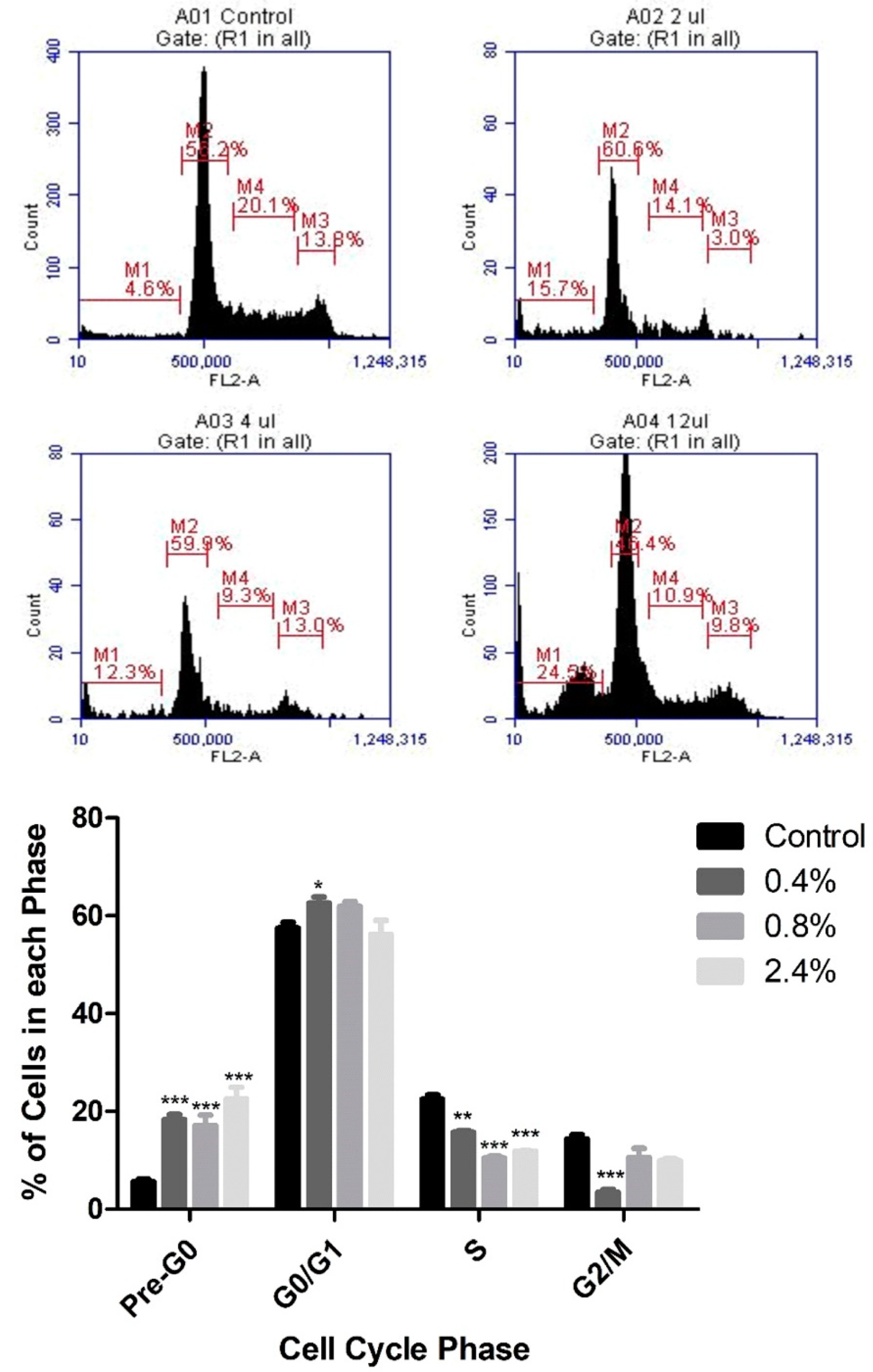
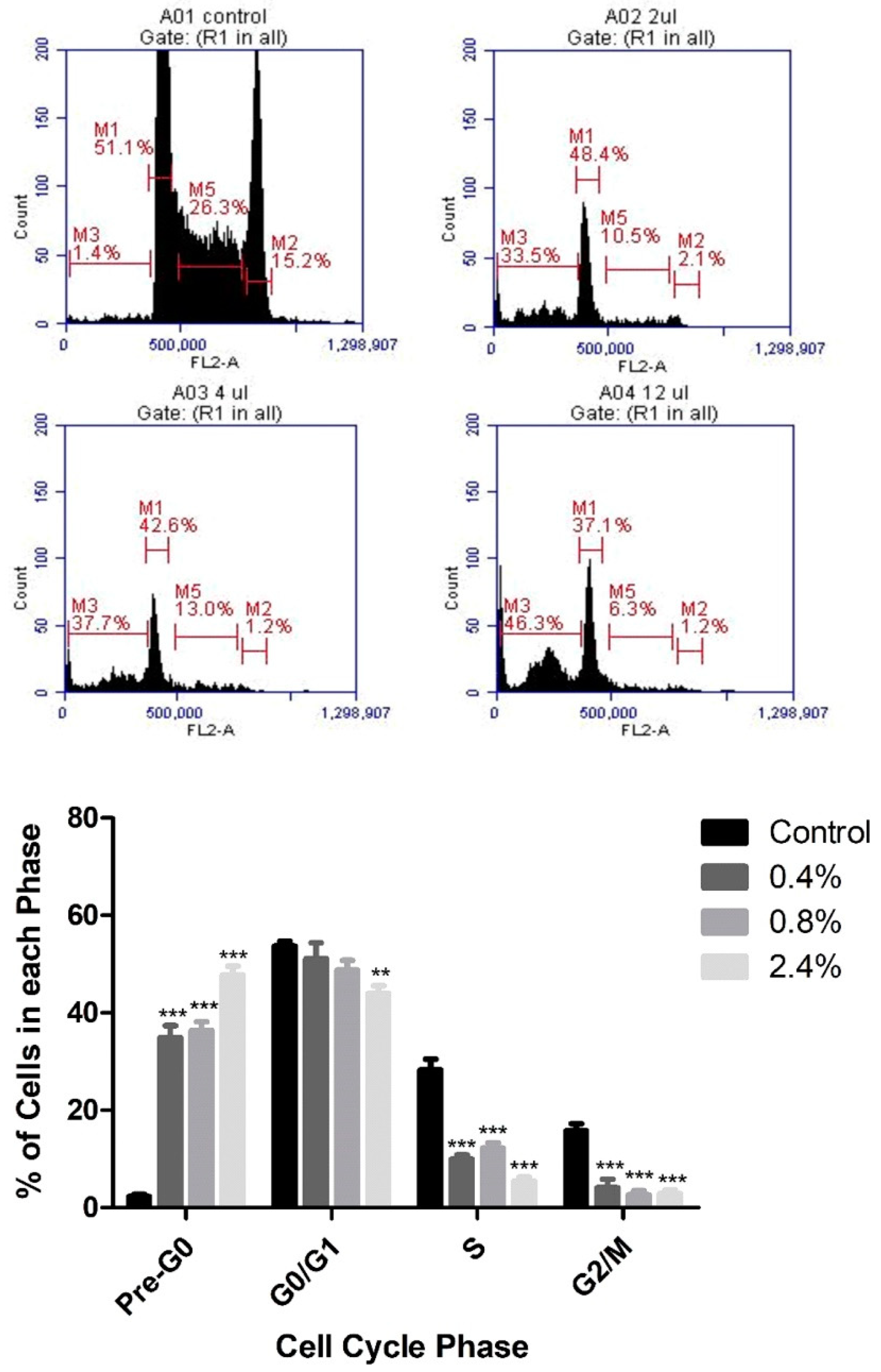
3.2. Effect of UD Extract on Cell-Cycle Progression in U937 Cells
3.3. Effect of UD Extract on Apoptosis Induction in U937 Cells
3.4. Effect of UD Extract on the Expression of Proteins Involved in Apoptosis Induction
3.5. Chemical Profile of the Aqueous Extract of UD Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Zhao, L.; McGirr, C.; Gonda, T.J. MYB down-regulation enhances sensitivity of U937 myeloid leukemia cells to the histone deacetylase inhibitor LBH589 in vitro and in vivo. Cancer Lett. 2014, 343, 98–106. [Google Scholar] [CrossRef]
- Bartakke, S.P.; Sampagar, A.A.; Bafna, V.S.; Patel, P. Human Leukocyte Antigen-B27: The Genetic Predisposition Leading to Reactive Arthritis during Induction Phase Chemotherapy for Acute Myeloid Leukemia. Indian J. Med. Paediatr. Oncol. 2017, 38, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.C. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer 2011, 2, 95–107. [Google Scholar] [CrossRef]
- Elias, A.; Shebaby, W.N.; Nehme, B.; Faour, W.; Bassil, B.S.; Hakim, J.E.; Iskandar, R.; Dib-Jalbout, N.; Mroueh, M.; Daher, C.; et al. In Vitro and In Vivo Evaluation of the Anticancer and Anti-inflammatory Activities of 2-Himachelen-7-ol isolated from Cedrus libani. Sci. Rep. 2019, 9, 12855. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Jardaly, A.; Abi Raad, S.; Zouein, A.; Rizk, S. Andrographolide potentiates the antitumor effect of topotecan in acute myeloid leukemia cells through an intrinsic apoptotic pathway. Cancer Manag. Res. 2018, 10, 1079–1088. [Google Scholar] [CrossRef]
- Shebaby, W.N.; Mroueh, M.A.; Boukamp, P.; Taleb, R.I.; Bodman-Smith, K.; El-Sibai, M.; Daher, C.F. Wild carrot pentane-based fractions suppress proliferation of human HaCaT keratinocytes and protect against chemically-induced skin cancer. BMC Complement. Altern. Med. 2017, 17, 36. [Google Scholar] [CrossRef]
- Blumenthal, M.; Wagner, H.; Emmelin, N.; Hirano, T. Urtica dioica: Urtica urens (Nettle). Altern. Med. Rev. 2007, 12, 280–284. [Google Scholar]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. Comptes Rendus Chimie 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Janda, K.; Szkyrpan, S.; Gutowska, I.; Wolska, J. Stinging nettle (Urtica dioica L.)-botanical characteristics, biochemical composition and health benefits. Pomeranian J. Life Sci. 2015, 61, 191–198. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Singh, R.; Dar, S.A.; Sharma, P. Antibacterial activity and toxicological evaluation of semipurified hexane extract of Urtica dioica leaves. Res. J. Med. Plants 2012, 6, 123–135. [Google Scholar]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica Linn. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar]
- Morgia, G.; Privitera, S. Phytotherapy in benign prostatic hyperplasia. In Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia; Morgia, G., Russo, G.I., Eds.; Academic Press: Waltham, MA, USA, 2018; Chapter 7; pp. 135–175. [Google Scholar] [CrossRef]
- Orcic, D.; Franciskovic, M.; Bekvalac, K.; Svircev, E.; Beara, I.; Lesjak, M.; Mimica-Dukic, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Fattahi, S.; Ardekani, A.M.; Zabihi, E.; Abedian, Z.; Mostafazadeh, A.; Pourbagher, R.; Akhavan-Niaki, H. Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 5317–5323. [Google Scholar] [CrossRef]
- Levy, A.; Sivanesan, D.; Murugan, R.; Jornadal, J. Urtica dioica Induces cytotoxicity in human prostate carcinoma LNCaP Cells: Involvement of oxidative stress, mitochondrial depolarization and apoptosis. Trop. J. Pharm. Res. 2014, 13, 711–717. [Google Scholar] [CrossRef]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Aghapour, M.; Shirjang, S.; Nami, S.; Baradaran, B. The Urtica dioica extract enhances sensitivity of paclitaxel drug to MDA-MB-468 breast cancer cells. Biomed. Pharmacother. 2016, 83, 835–842. [Google Scholar] [CrossRef]
- Weigend, M. Urtica dioica subsp. cypria, with a re-evaluation of the U. dioica group (Urticaceae) in western Asia. Willdenowia 2006, 36, 811–822. [Google Scholar] [CrossRef]
- Nisrine Machaka-Houri. Available online: http://www.nisrinemachaka.com/ (accessed on 24 April 2019).
- Ammoury, C.; Younes, M.; El Khoury, M.; Hodroj, M.H.; Haykal, T.; Nasr, P.; Sily, M.; Taleb, R.I.; Sarkis, R.; Khalife, R.; et al. The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines. BMC Complement. Altern. Med. 2019, 19, 365. [Google Scholar] [CrossRef]
- Khalife, R.; El-Hayek, S.; Tarras, O.; Hodroj, M.H.; Rizk, S. Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clin. Lymphoma Myeloma Leuk. 2014, 14, S46–S55. [Google Scholar] [CrossRef]
- Kassab, E.; Darwish, M.; Timsah, Z.; Liu, S.; Leppla, S.H.; Frankel, A.E.; Abi-Habib, R.J. Cytotoxicity of anthrax lethal toxin to human acute myeloid leukemia cells is nonapoptotic and dependent on extracellular signal-regulated kinase 1/2 activity. Transl. Oncol. 2013, 6, 25–32. [Google Scholar] [CrossRef]
- Ghanem, P.; Zouein, A.; Mohamad, M.; Hodroj, M.H.; Haykal, T.; Abou Najem, S.; Naim, H.Y.; Rizk, S. The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines. Nutrients 2019, 11, 2808. [Google Scholar] [CrossRef]
- El Khoury, M.; Haykal, T.; Hodroj, M.H.; Najem, S.A.; Sarkis, R.; Taleb, R.I.; Rizk, S. Malva pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells in Vitro. Cancers 2020, 12, 435. [Google Scholar] [CrossRef]
- Idriss, M.; Hodroj, M.H. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules 2020, 10, 577. [Google Scholar] [CrossRef]
- Haykal, T.; Nasr, P.; Hodroj, M.H.; Taleb, R.I.; Sarkis, R.; Moujabber, M.N.E.; Rizk, S. Annona cherimola Seed Extract Activates Extrinsic and Intrinsic Apoptotic Pathways in Leukemic Cells. Toxins 2019, 11, 506. [Google Scholar] [CrossRef]
- Abou Najem, S.; Khawaja, G.; Hodroj, M.H.; Rizk, S. Synergistic Effect of Epigenetic Inhibitors Decitabine and Suberoylanilide Hydroxamic Acid on Colorectal Cancer In vitro. Curr. Mol. Pharmacol. 2019, 12, 281–300. [Google Scholar] [CrossRef]
- Shammas, H.; Kuech, E.-M.; Rizk, S.; Das, A.M.; Naim, H.Y. Different Niemann-Pick C1 Genotypes Generate Protein Phenotypes that Vary in their Intracellular Processing, Trafficking and Localization. Sci. Rep. 2019, 9, 5292. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Gupta, S. Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Mol. Cell. Pharmacol. 2009, 1, 138–147. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future (review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information, PubChem. Available online: https://www.ncbi.nlm.nih.gov/pccompound (accessed on 20 May 2020).
- Samuels, N.; Morag, O.; Maimon, Y. Use of herbal medicine for cancer treatment-related toxicities. Harefuah 2015, 154, 43–46. [Google Scholar] [PubMed]
- Yin, S.-Y.; Wei, W.-C.; Jian, F.-Y.; Yang, N.-S. Therapeutic applications of herbal medicines for cancer patients. Evid. Based Complement. Alternat. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef]
- Telo, S.; Halifeoglu, I.; Ozercan, I.H. Effects of Stinging Nettle (Urtica Dioica, L.) on Antioxidant Enzyme Activities in Rat Model of Mammary Gland Cancer. Iran. J. Pharm. Res. 2017, 16, 164–170. [Google Scholar]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Daher, C.F.; Baroody, K.G.; Baroody, G.M. Effect of Urtica dioica extract intake upon blood lipid profile in the rats. Fitoterapia 2006, 77, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mansoori, B.; Baradaran, P.C.; Khaze, V.; Aghapour, M.; Farhadi, M.; Baradaran, B. Urtica dioica Extract Inhibits Proliferation and Induces Apoptosis and Related Gene Expression of Breast Cancer Cells In Vitro and In Vivo. Clin. Breast Cancer 2017, 17, 463–470. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansoori, B.; Aghapour, M.; Baradaran, P.C.; Shajari, N.; Davudian, S.; Salehi, S.; Baradaran, B. The Herbal Medicine Utrica dioica Inhibits Proliferation of Colorectal Cancer Cell Line by Inducing Apoptosis and Arrest at the G2/M Phase. J. Gastrointest. Cancer 2016, 47, 187–195. [Google Scholar] [CrossRef]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytometry A 2007, 71, 125–131. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Della Corte, C.M.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica L. inhibits proliferation and enhances cisplatin cytotoxicity in NSCLC cells via Endoplasmic Reticulum-stress mediated apoptosis. Sci. Rep. 2019, 9, 4986. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Ghadami, E.; Asouri, M.; Motevalizadeh Ardekanid, A.; Akhavan-Niaki, H. Urtica dioica inhibits cell growth and induces apoptosis by targeting Ornithine decarboxylase and Adenosine deaminase as key regulatory enzymes in adenosine and polyamines homeostasis in human breast cancer cell lines. Cell Mol. Biol. 2018, 64, 97–102. [Google Scholar] [CrossRef]
- Keshavarz, S.; Ardekani, M.R.S.; Safavi, M.; Chahardouli, B.; Nadali, F. In vitro cytotoxic effect of Urtica dioica extracts on acute myelogenous leukemia cell line (kg-1). Arch. Med. Lab. Sci. 2016, 2. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Hashemzadeh, S.; Shirjang, S.; Baradaran, A.; Asadi, M.; Doustvandi, M.A.; Baradaran, B. Urtica dioica extract suppresses miR-21 and metastasis-related genes in breast cancer. Biomed. Pharmacother. 2017, 93, 95–102. [Google Scholar] [CrossRef]
- Zhu, Q.; Jin, L.; Casero, R.A.; Davidson, N.E.; Huang, Y. Role of ornithine decarboxylase in regulation of estrogen receptor alpha expression and growth in human breast cancer cells. Breast Cancer Res. Treat. 2012, 136, 57–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, W.; Lv, C.; Wang, J.; Gao, Q.; Zhu, H.; Wen, H. Patuletin induces apoptosis of human breast cancer SK-BR-3 cell line via inhibiting fatty acid synthase gene expression and activity. Oncol. Lett. 2017, 14, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Sansininea, J.J.; Sánchez-Sánchez, L.; López-Muñoz, H.; Escobar, M.L.; Flores-Guzmán, F.; Tavera-Hernández, R.; Jiménez-Estrada, M. Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules 2018, 23, 2579. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Chang, W.C.; Hsieh, C.H.; Hsiao, M.W.; Lin, W.C.; Hung, Y.C.; Ye, J.C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Sourani, Z.M.; Pourgheysari, B.P.; Beshkar, P.M.; Shirzad, H.P.; Shirzad, M.M. Gallic Acid Inhibits Proliferation and Induces Apoptosis in Lymphoblastic Leukemia Cell Line (C121). Iran. J. Med. Sci. 2016, 41, 525–530. [Google Scholar]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nakazato, T.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Segawa, K.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: Implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004, 64, 1071–1078. [Google Scholar] [CrossRef] [PubMed]

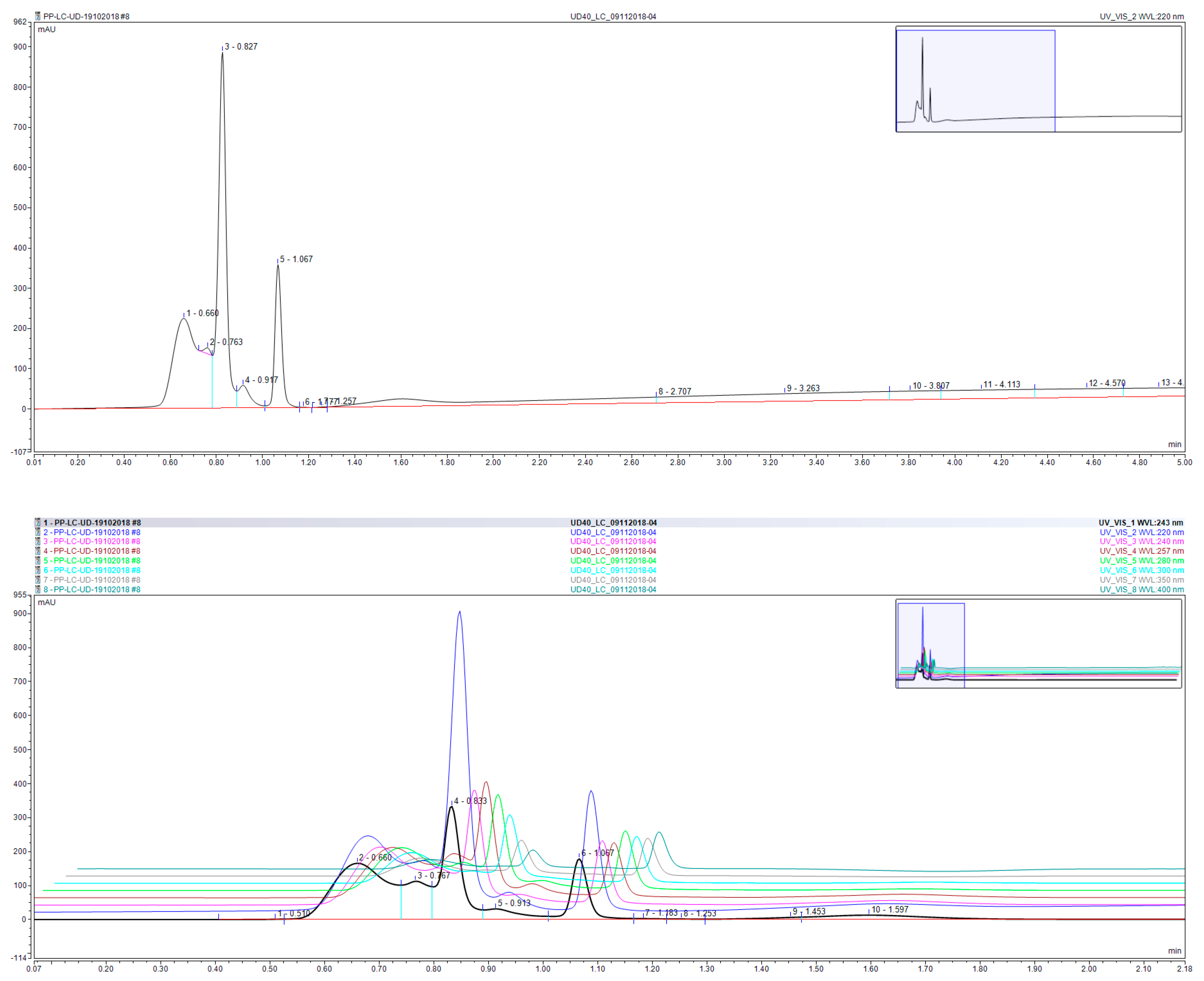
| Compound | Compound | ||
|---|---|---|---|
| 1 | alpha-Bisabolol | 26 | Neochlorogenic acid |
| 2 | Chamazulene | 27 | Protocatechuic acid |
| 3 | Methyl angelate | 28 | Caffeic acid 2-glucoside |
| 4 | Angelic acid | 29 | Homovanillic acid 1-glucoside |
| 5 | Isobutyl angelate | 30 | Caffeic acid 3-glucoside |
| 6 | alpha-Farnesene | 31 | p-Hydroxybenzoic acid |
| 7 | alpha-Pinene | 32 | Chlorogenic acid |
| 8 | Nobilin | 33 | 4-O-beta-D-Glucosyl-4-coumaric acid |
| 9 | 3-Epinobilin | 34 | m-Hydroxybenzoic acid |
| 10 | Bisabolol oxide A | 35 | Cryptochlorogenic acid |
| 11 | Bisabolol oxide B | 36 | Homovanillic acid |
| 12 | Azulene | 37 | Caffeic acid |
| 13 | 4-Hydroxycoumarine | 38 | 4-p-Coumaroylqunic acid |
| 14 | 6-Hydroxycoumarine | 39 | Vanillic acid |
| 15 | 7-Hydroxycoumarine | 40 | p-Coumaric acid |
| 16 | Luteolin | 41 | Ferulic acid |
| 17 | Patuletin | 42 | Rutin |
| 18 | Herniarin | 43 | Hesperetin |
| 19 | Apigenine-7-O-glucoside | 44 | Kaempferol-3-O-rutinoside |
| 20 | Apigenin-8-C-glucoside | 45 | Kaempferol-3-O-glucoside |
| 21 | alpha-Bisabolol, acetate | 46 | Populnetin |
| 22 | Gallic acid | 47 | Quercetin |
| 23 | Vanillic acid 4-β-D-glucoside | 48 | Naringenin |
| 24 | Syringic acid | 49 | Apigenin |
| 25 | Caffeic acid hexoside | 50 | Rosmarinic acid |
| # | Compound | Ionization | Exact Mass | m/z | Signal Intensity | Ions | CE |
|---|---|---|---|---|---|---|---|
| 1 | 3-Epinobilin | pos ESI | 346.178 | 347.186 | e2 | 247.133, 83.042 | 20 |
| 2 | Bisabolol oxide B | pos ESI | 238.193 | 239.200 | e3 | 221.192, 81.071 | 20 |
| 3 | 7-Hydroxycoumarine | pos ESI | 162.032 | 163.039 | e2 | 119.049, 145.029 | 40 |
| 4 | Luteolin | pos ESI | 286.048 | 287.056 | e2 | 153.019, 109.029, 213.055, 269.045 | 40 |
| 5 | Patuletin | pos ESI | 332.053 | 333.061 | e4 | 109.029, 137.024, 183.029, 315.051 (10 EV) | 30 |
| 6 | Apigenin-8C-glucoside | pos ESI | 432.106 | 433.113 | e2 | 415.101, 397.124, 367.103 (10 EV) | 5 |
| 7 | Gallic acid | pos ESI | 170.022 | 171.029 | e3 | 153.019, 125.023 | 20 |
| 8 | Vanillic acid-4-β-D-glucoside | pos ESI | 330.290 | 331.295 | e2 | 169.003 | 20 |
| 9 | p-Hydroxybenzoic acid | pos ESI | 138.032 | 139.039 | e4 | 121.029, 95.050 | 20 |
| 10 | Chlorogenic acid | pos ESI | 354.095 | 355.103 | e3 | 163.039, 337.092, 193. 071, 175.061 | 20 |
| 11 | m-Hydroxybenzoic acid | pos ESI | 138.032 | 139.039 | e4 | 93.034 | 20 |
| 12 | Homovanillic acid | pos ESI | 182.058 | 183.066 | e3 | 137.060, 165.055 | 20 |
| 13 | Caffeic acid | pos ESI | 180.042 | 181.044 | e4 | 135.045, 163.039 | 15 |
| 14 | 4-O-beta-D-Glucosyl-4-coumaric acid | pos ESI | 164.047 | 165.055 | e2 | 91.054, 147.045, 119.049 (30 EV) | 30 |
| 15 | Rutin | pos ESI | 610.153 | 611.161 | e1 | 303.050 | 40 |
| 16 | Kaempferol-3-O-rutinoside | neg ESI | 594.158 | 593.151 | e3 | 285.012, 257.121, 284.206 | 35 |
| 17 | Kaempferol-3-O-glucoside | neg ESI | 448.101 | 447.093 | e2 | 284.024, 255.029, 227.034 | 35 |
| 18 | Rosmarinic acid | neg ESI | 360.085 | 359.077 | e1 | 161.024, 359.077, 197.045, 135.071 | 40 |
| 19 | Populnetin | neg ESI | 286.048 | 285.042 | e2 | 164.999, 255.029, 227.036, 117.035 | 20 |
| 20 | Quercetin | neg ESI | 302.043 | 301.037 | e2 | 151.001, 178.996, 271.025 | 20 |
| 21 | Apigenin | neg ESI | 270.053 | 269.052 | e1 | 117.038, 151.0080 | 30 |
| 22 | Hesperetin | neg ESI | 302.079 | 301.072 | e1 | 136.017, 151.004, 164.012, 285.040 | 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodroj, M.H.; Al Bast, N.a.H.; Taleb, R.I.; Borjac, J.; Rizk, S. Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis. Nutrients 2020, 12, 2629. https://doi.org/10.3390/nu12092629
Hodroj MH, Al Bast NaH, Taleb RI, Borjac J, Rizk S. Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis. Nutrients. 2020; 12(9):2629. https://doi.org/10.3390/nu12092629
Chicago/Turabian StyleHodroj, Mohammad Hassan, Nour al Hoda Al Bast, Robin I. Taleb, Jamilah Borjac, and Sandra Rizk. 2020. "Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis" Nutrients 12, no. 9: 2629. https://doi.org/10.3390/nu12092629
APA StyleHodroj, M. H., Al Bast, N. a. H., Taleb, R. I., Borjac, J., & Rizk, S. (2020). Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis. Nutrients, 12(9), 2629. https://doi.org/10.3390/nu12092629