The Effect of Dietary Supplementation on Aggressive Behaviour in Australian Adult Male Prisoners: A Feasibility and Pilot Study for a Randomised, Double Blind Placebo Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Access to Correctional Centre and Study Design
2.2. Recruitment of Study Participants
2.3. Blood Collection and Analysis
2.4. Dietary Supplements, Compliance, and Potential Confounders
2.5. Outcome Measures
2.6. Statistics
3. Results
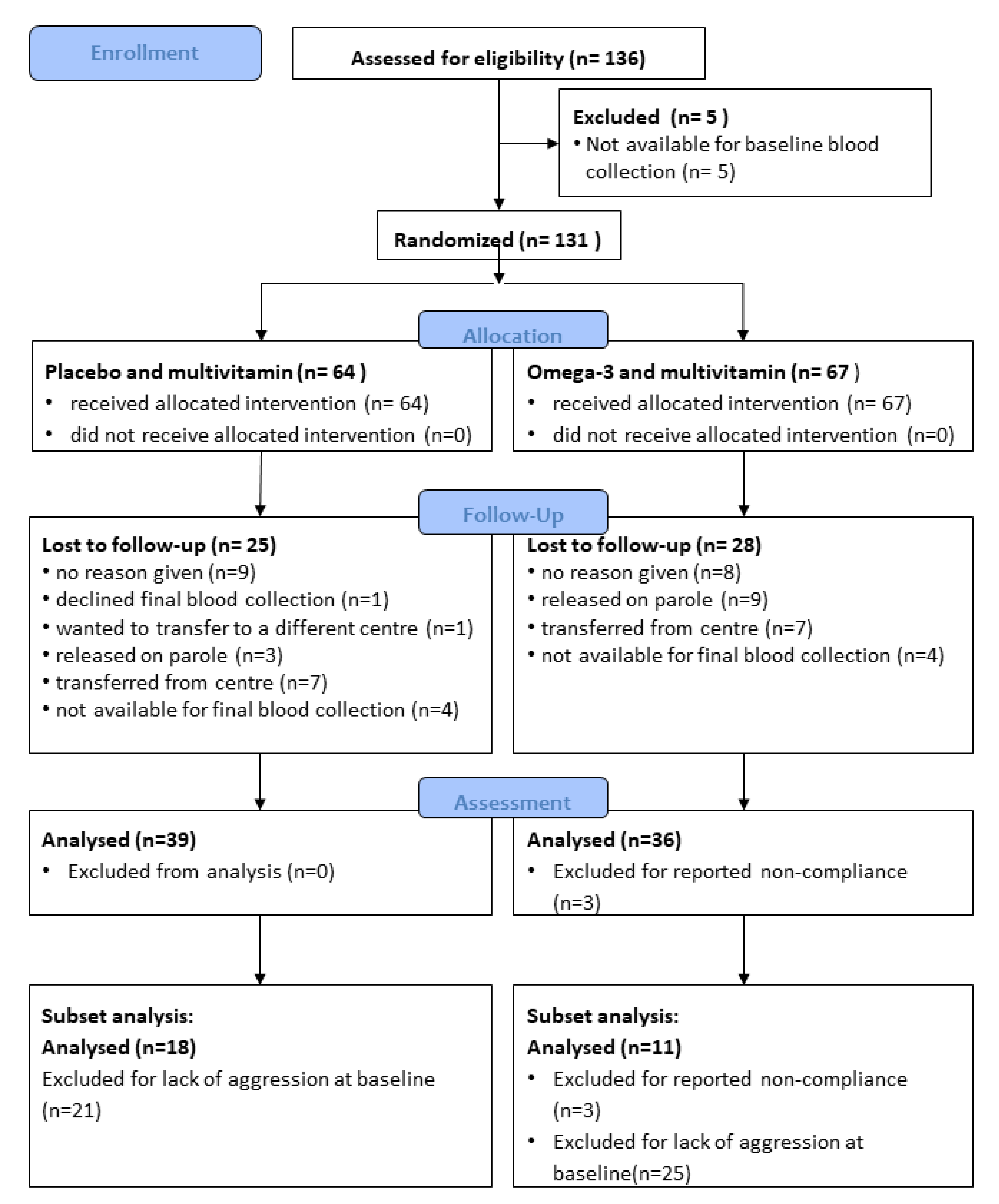
3.1. Recruitment and Retention
3.2. Blood Collection and the Omega-3 Index
3.3. Compliance to Treatment and the Omega-3 Index
3.4. Collection of Outcome Measures
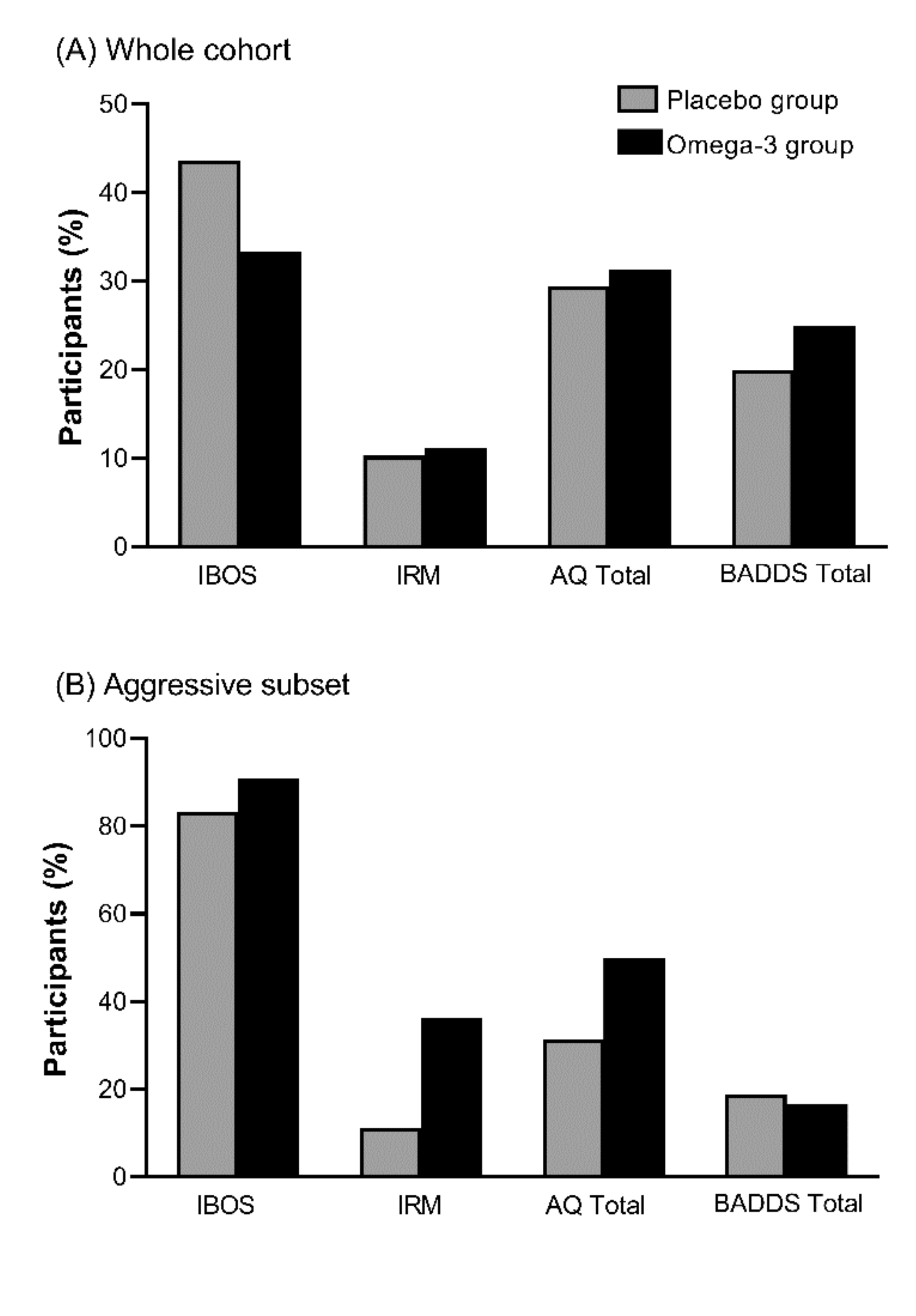
3.5. Changes in Aggression
3.6. Power Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AQ | Aggression Questionnaire |
| BADDS | Brown Attention Deficient Disorder Scale |
| CSNSW | Correctional Services New South Wales |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| IBOS | Inmate Behavioural Observational Scale |
| ICC | Intra-cluster correlation |
| IRM | Institutional records of misconduct |
| ISSFAL | International Society for the Study of Fatty Acids and Lipids |
| n-3 LCPUFA | Omega-3 long chain polyunsaturated fatty acids |
| SCCC | South Coast Correctional Centre |
References
- Benton, D. The impact of diet on anti-social, violent and criminal behaviour. Neurosci. Biobehav. Rev. 2007, 31, 752–774. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Ferguson, T.A.; Blasbalg, T.L. Omega-3 fatty acid deficiencies in neurodevelopment, aggression and autonomic dysregulation: Opportunities for intervention. Int. Rev. Psychiatry 2006, 18, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Gajos, J.M.; Beaver, K.M. The effect of omega-3 fatty acids on aggression: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 147–158. [Google Scholar] [CrossRef]
- Bègue, L.; Zaalberg, A.; Shankland, R.; Duke, A.; Jacquet, J.; Kaliman, P.; Pennel, L.; Chanove, M.; Arvers, P.; Bushman, B.J. Omega-3 supplements reduce self-reported physical aggression in healthy adults. Psychiatry Res. 2018, 261, 307–311. [Google Scholar] [CrossRef]
- Qiao, Y.; Mei, Y.; Han, H.; Liu, F.; Yang, X.M.; Shao, Y.; Xie, B.; Long, B. Effects of Omega-3 in the treatment of violent schizophrenia patients. Schizophr. Res. 2018, 195, 283–285. [Google Scholar] [CrossRef]
- Raine, A.; Ang, R.P.; Choy, O.; Hibbeln, J.R.; Ho, R.M.; Lim, C.G.; Lim-Ashworth, N.S.J.; Ling, S.; Liu, J.C.J.; Ooi, Y.P.; et al. Omega-3 (omega-3) and social skills interventions for reactive aggression and childhood externalizing behavior problems: A randomized, stratified, double-blind, placebo-controlled, factorial trial. Psychol. Med. 2019, 49, 335–344. [Google Scholar] [CrossRef]
- Raine, A.; Cheney, R.A.; Ho, R.; Portnoy, J.; Liu, J.; Soyfer, L.; Hibbeln, J.; Richmond, T.S. Nutritional supplementation to reduce child aggression: A randomized, stratified, single-blind, factorial trial. J. Child Psychol. Psychiatry 2016, 57, 1038–1046. [Google Scholar] [CrossRef]
- Gifford, B. Prison crime and the economics of incarceration. Stanf. Law Rev. 2019, 71, 71–135. [Google Scholar]
- Gesch, C.B.; Hammond, S.M.; Hampson, S.E.; Eves, A.; Crowder, M.J. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners-Randomised, placebo-controlled trial. Br. J. Psychiatry 2002, 181, 22–28. [Google Scholar] [CrossRef]
- Walker, R.E.; Jackson, K.H.; Tintle, N.L.; Shearer, G.C.; Bernasconi, A.; Masson, S.; Latini, R.; Heydari, B.; Kwong, R.Y.; Flock, M.; et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 2019, 110, 1034–1040. [Google Scholar] [CrossRef]
- Zaalberg, A.; Nijman, H.; Bulten, E.; Stroosma, L.; van der Staak, C. Effects of nutritional supplements on aggression, rule-breaking, and psychopathology among young adult prisoners. Aggress. Behav. 2010, 36, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Raine, A.; Leung, C.-C.; Singh, M.; Kaur, J. Omega-3 supplementation in young offenders: A randomized, stratified, double-blind, placebo-controlled, parallel-group trial. J. Exp. Criminol. 2020. [Google Scholar] [CrossRef]
- Sparkes, C.; Sinclair, A.J.; Gibson, R.A.; Else, P.L.; Meyer, B.J. High Variability in Erythrocyte, Plasma and Whole Blood EPA and DHA Levels in Response to Supplementation. Nutrients 2020, 12, 1017. [Google Scholar] [CrossRef]
- de Groot, R.H.M.; Emmett, R.; Meyer, B.J. Non-dietary factors associated with n-3 long-chain PUFA levels in humans-a systematic literature review. Br. J. Nutr. 2019, 121, 793–808. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.H.M.; Meyer, B.J. ISSFAL Official Statement Number 6 The importance of measuring blood omega-3 long chain polyunsaturated fatty acid levels in research. Prostaglandins Leukot. Essent. Fat. Acids 2020, 157, 102029. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Procter, N.; Gordon, A.; Bogomolova, S.; O’Dea, K.; Strachan, J.; Ballestrin, M.; et al. People with schizophrenia and depression have a low omega-3 index. Prostaglandins Leukot. Essent. Fat. Acids 2016, 110, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.; Mitchell, T.W.; McGorry, P.D.; Nelson, B.; Markulev, C.; Yuen, H.P.; Schäfer, M.R.; Berger, M.; Mossaheb, N.; Schlögelhofer, M.; et al. Comparison of erythrocyte omega-3 index, fatty acids and molecular phospholipid species in people at ultra-high risk of developing psychosis and healthy people. Schizophr. Res. 2019. [Google Scholar] [CrossRef]
- Meyer, B.J.; Byrne, M.K.; Collier, C.; Parletta, N.; Crawford, D.; Winberg, P.C.; Webster, D.; Chapman, K.; Thomas, G.; Dally, J.; et al. Baseline omega-3 index correlates with aggressive and attention deficit disorder behaviours in adult prisoners. PLoS ONE 2015, 10, e0120220. [Google Scholar]
- Miles, L.; Simpson, M.; Butler, T.; Wood, L.G.; Knight, L.; Greenberg, D.; Schofield, P. Effect of omega-3 fatty acids on offending behavior in repeat violent offenders: A randomized controlled trial feasibility study. J. Psychiatry Behav. Sci. 2018, 1. [Google Scholar] [CrossRef]
- Auty, K.M.; Cope, A.; Liebling, A. Psychoeducational programs for reducing prison violence: A systematic review. Aggress. Violent Behav. 2017, 33, 126–143. [Google Scholar] [CrossRef]
- Papalia, N.; Spivak, B.; Daffern, M.; Ogloff, J.R.P. A meta-analytic review of the efficacy of psychological treatments for violent offenders in correctional and forensic mental health settings. Clin. Psychol. Sci. Pr. 2019, 26, e12282. [Google Scholar] [CrossRef]
- Buss, A.H.; Perry, M. The Aggression Questionnaire. J. Personal. Soc. Psychol. 1992, 63, 452. [Google Scholar] [CrossRef]
- Pettersen, C.; Nunes, K.L.; Cortoni, F. The Factor Structure of the Aggression Questionnaire with Violent Offenders. Int. J. Offender Ther. Comp. Criminol. 2018, 62, 1888–1905. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Cortie, C.; Meyer, B.J. The Inmate Behaviour Observation Scale (IBOS): A measure of prisoner aggression and violence based on inmate case notes. 2020; unpublished manuscript. [Google Scholar]
- Ginsberg, Y.; Hirvikoski, T.; Lindefors, N. Attention deficit hyperactivity disorder (ADHD) among longer-term prison inmates is a prevalent, persistent and disabling disorder. BMC Psychiatry 2010, 10, 112. [Google Scholar] [CrossRef]
- Barkley, R.A.; Murphy, K.R.; Fischer, M. ADHD in Adults: What the Science Says; Gilford Press: New York, NY, USA, 2008. [Google Scholar]
- Field, C.; Archer, V.; Bowman, J. Twenty Years in Prison: Reflections on Conducting Research in Correctional Environments. Prison. J. 2019, 99, 135–149. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Gillian, A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Br. Med. J. 2016, 355. [Google Scholar] [CrossRef]
- Jouris, K.B.; McDaniel, J.L.; Weiss, E.P. The Effect of Omega-3 Fatty Acid Supplementation on the Inflammatory Response to eccentric strength exercise. J. Sports Sci. Med. 2011, 10, 432–438. [Google Scholar]
- Swierk, M.; Williams, P.G.; Wilcox, J.; Russell, K.G.; Meyer, B.J. Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake. Nutrition 2011, 27, 641–646. [Google Scholar] [CrossRef][Green Version]
- Lepage, G.; Roy, C. Direct transesterificaton of all classes of lipids in one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar]
- Brown, T.E. Brown Attention-Deficit Disorder Scales (Brown ADD Scales): For Adolescents and Adults: Manual; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistics notes: The odds ratio. BMJ 2000, 320, 1468. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.; Skulas-Ray, A.; Harris, W.; Etherton, T.; Fleming, J.; Kris-Etherton, P. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose-response randomized controlled trial. J. Am. Hear. Assoc. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Benton, D. A double-blind trial of the effect of docosahexaenoic acid and vitamin and mineral supplementation on aggression, impulsivity, and stress. Hum. Psychopharmacol. Clin. Exp. 2013, 28, 238–247. [Google Scholar] [CrossRef] [PubMed]

| Fatty Acid | Placebo mol% | Omega-3 mol% |
|---|---|---|
| SFA | ||
| 14:0 myristic acid | 0.1 | 3.0 |
| 16:0 palmitic acid | 5.7 | 20.4 |
| 18:0 stearic acid | 2.7 | 5.9 |
| MUFA | ||
| 16:1 palmitoleic acid | 0.1 | 5.4 |
| 18:1n-9 oleic acid | 81.5 | 13.0 |
| 18:1n-7 vaccenic acid | 0.0 | 2.3 |
| PUFA n-6 | ||
| 18:2n-6 linoleic acid | 7.7 | 1.3 |
| 20:4n-6 arachidonic acid | 0.0 | 1.8 |
| 22:5n-6 n-6 docosapentaenoic acid | 0.0 | 2.0 |
| PUFA n-3 | ||
| 18:3n-3 alpha-linolenic acid | 0.3 | 0.5 |
| 20:5n-3 eicosapentaenoic acid | 0.0 | 5.1 |
| 22:5n-3 n-3 docosapentaenoic acid | 0.0 | 1.0 |
| 22:6n-3 docosahexaenoic acid | 0.0 | 26.9 |
| Other fatty acids | 1.6 | 2.7 |
| ∑ SFA | 9.5 | 32.3 |
| ∑ MUFA | 82.0 | 23.1 |
| ∑ PUFA | 8.0 | 40.5 |
| ∑ n-6 PUFA | 7.7 | 6.0 |
| ∑ n-3 PUFA | 0.3 | 34.5 |
| Ingredient | Amount |
|---|---|
| Vitamins | |
| Vitamin A | 750 IU |
| Vitamin B1 | 50 mg |
| Vitamin B2 | 20 mg |
| Vitamin B3 | 10 mg |
| Vitamin B3 | 200 mg |
| Vitamin B5 | 100 mg |
| Vitamin B6 | 50 mg |
| Vitamin B7/Vitamin H | 20 mcg |
| Vitamin B9 | 150 mcg |
| Vitamin B12 | 100 mcg |
| Vitamin C | 50 mg |
| Vitamin D3 | 100 IU |
| Vitamin E | 25 IU |
| Minerals | |
| Calcium | 10 mg |
| Cobalt | 25 mcg |
| Copper | 6 mcg |
| Lithium | 140 mcg |
| Magnesium | 7.6 mg |
| Manganese | 93 mcg |
| Potassium | 2 mg |
| Potassium | 13.6 mg |
| Zinc (as gluconate) | 1.3 mg |
| Zinc (as sulphate) | 7.6 mg |
| Other | |
| Amino-benzoic acid | 20 mg |
| Betacarotene | 3 mg |
| Betaine hydrochloride | 10 mg |
| Choline bi-tartrate | 50 mg |
| Inositol | 25 mg |
| L-Glutamine | 50 mg |
| Lysine hydrochloride | 10 mg |
| Scutellaria lateriflora (Equiv. dry herb) | 100 mg |
| Valeriana officinalis (Equiv. dry root) | 100 mg |
| Placebo Group (n = 64) | Omega-3 Group (n = 67) | ||
|---|---|---|---|
| Age | |||
| Mean ± SD | 33.3 ± 10.3 | 33.7 ± 12.6 | p = 0.852 |
| Range | 18–70 | 19–80 | |
| Ethnicity–number (%) | |||
| African | 1 (1.6) | 0 (0) | X2 = 0.888 |
| Arabic | 6 (9.4) | 5 (7.5) | |
| Asian | 6 (9.4) | 6 (9.0) | |
| Australian Aboriginal | 7 (11) | 7 (10) | |
| Caucasian | 36 (56) | 38 (57) | |
| Hispanic | 3 (4.7) | 2 (3.0) | |
| Polynesian | 5 (7.8) | 8 (12) | |
| Not stated | 0 (0) | 1 (1.5) | |
| Education–number (%) | |||
| Primary school | 3 (4.7) | 3 (4.5) | X2 = 0.508 |
| Lower high school | 32 (50) | 36 (54) | |
| Upper high school | 14 (22) | 9 (13) | |
| Tertiary | 4 (6.2) | 4 (6.0) | |
| Unknown | 11 (17) | 15 (22) | |
| Omega-3 Index | p = 0.067 | ||
| Median (IQR) | 4.91 (4.25, 6.68) | 4.59 (4.08, 5.55) | |
| Range | 2.44–10.02 | 2.27–10.3 | |
| Identified as aggressive by measure–number (%) | |||
| IRM | 4 (6.2) | 9 (13) | X2 = 0.169 |
| IBOS | 27 (42) | 23 (34) | X2 = 0.355 |
| AQ Total | 20 (31) | 27 (40) | X2 = 0.280 |
| Likely to have ADD–number (%) | |||
| BADDS | 17 (27) | 20 (30) | X2 = 0.856 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortie, C.H.; Byrne, M.K.; Collier, C.; Parletta, N.; Crawford, D.; Winberg, P.C.; Webster, D.; Chapman, K.; Thomas, G.; Dally, J.; et al. The Effect of Dietary Supplementation on Aggressive Behaviour in Australian Adult Male Prisoners: A Feasibility and Pilot Study for a Randomised, Double Blind Placebo Controlled Trial. Nutrients 2020, 12, 2617. https://doi.org/10.3390/nu12092617
Cortie CH, Byrne MK, Collier C, Parletta N, Crawford D, Winberg PC, Webster D, Chapman K, Thomas G, Dally J, et al. The Effect of Dietary Supplementation on Aggressive Behaviour in Australian Adult Male Prisoners: A Feasibility and Pilot Study for a Randomised, Double Blind Placebo Controlled Trial. Nutrients. 2020; 12(9):2617. https://doi.org/10.3390/nu12092617
Chicago/Turabian StyleCortie, Colin H., Mitchell K. Byrne, Carole Collier, Natalie Parletta, Donna Crawford, Pia C. Winberg, David Webster, Karen Chapman, Gayle Thomas, Jean Dally, and et al. 2020. "The Effect of Dietary Supplementation on Aggressive Behaviour in Australian Adult Male Prisoners: A Feasibility and Pilot Study for a Randomised, Double Blind Placebo Controlled Trial" Nutrients 12, no. 9: 2617. https://doi.org/10.3390/nu12092617
APA StyleCortie, C. H., Byrne, M. K., Collier, C., Parletta, N., Crawford, D., Winberg, P. C., Webster, D., Chapman, K., Thomas, G., Dally, J., Batterham, M., Martin, A. M., Grant, L., & Meyer, B. J. (2020). The Effect of Dietary Supplementation on Aggressive Behaviour in Australian Adult Male Prisoners: A Feasibility and Pilot Study for a Randomised, Double Blind Placebo Controlled Trial. Nutrients, 12(9), 2617. https://doi.org/10.3390/nu12092617







