Granulocytic Myeloid-Derived Suppressor Cells in Breast Milk (BM-MDSC) Correlate with Gestational Age and Postnatal Age and Are Influenced by Infant’s Sex
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Cell Isolation and Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Levels of GR-MDSC in Breast Milk Are Positively Correlated with Gestational Age
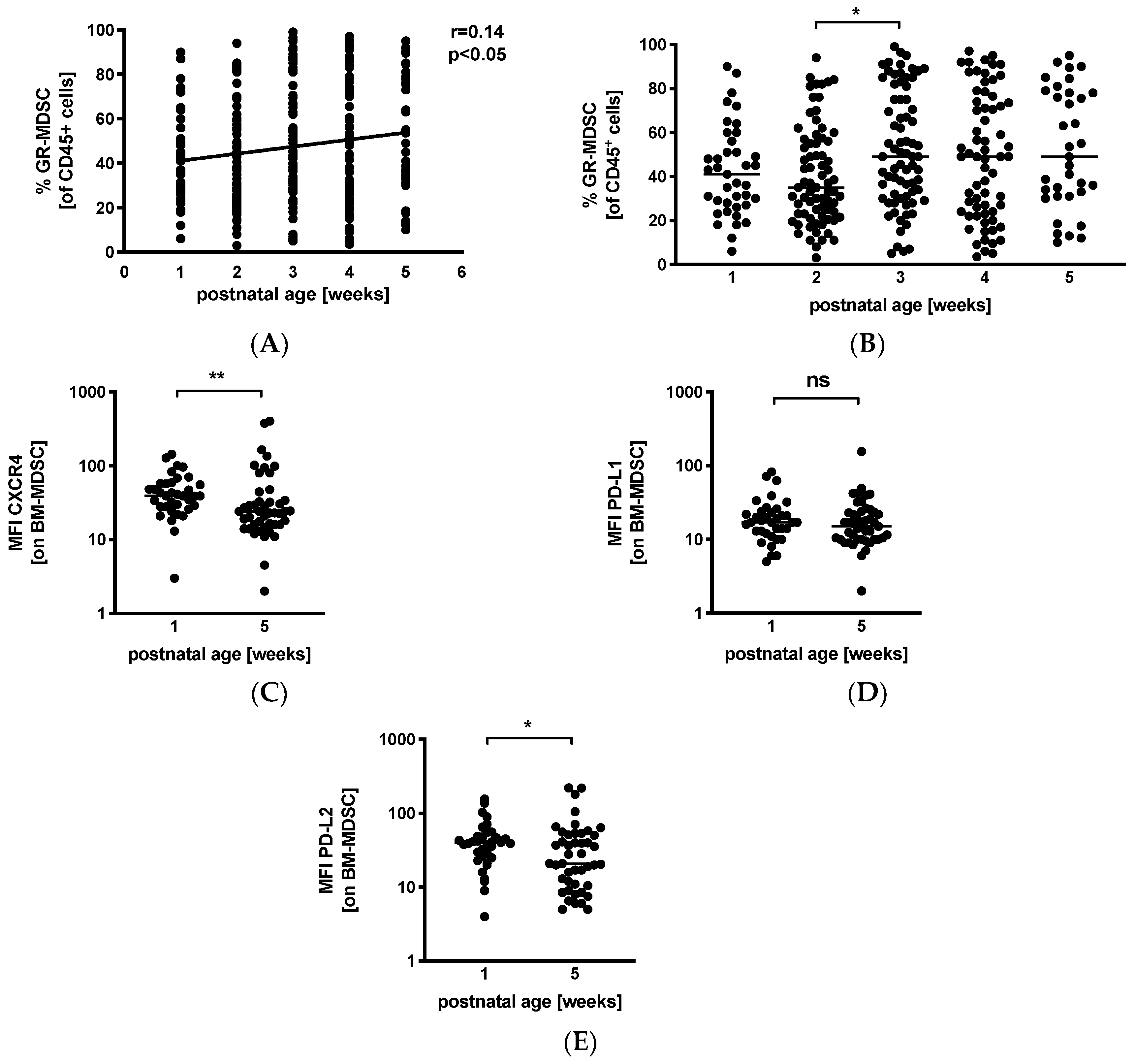
3.3. Levels of GR-MDSC in Breast Milk Increase with Postnatal Age
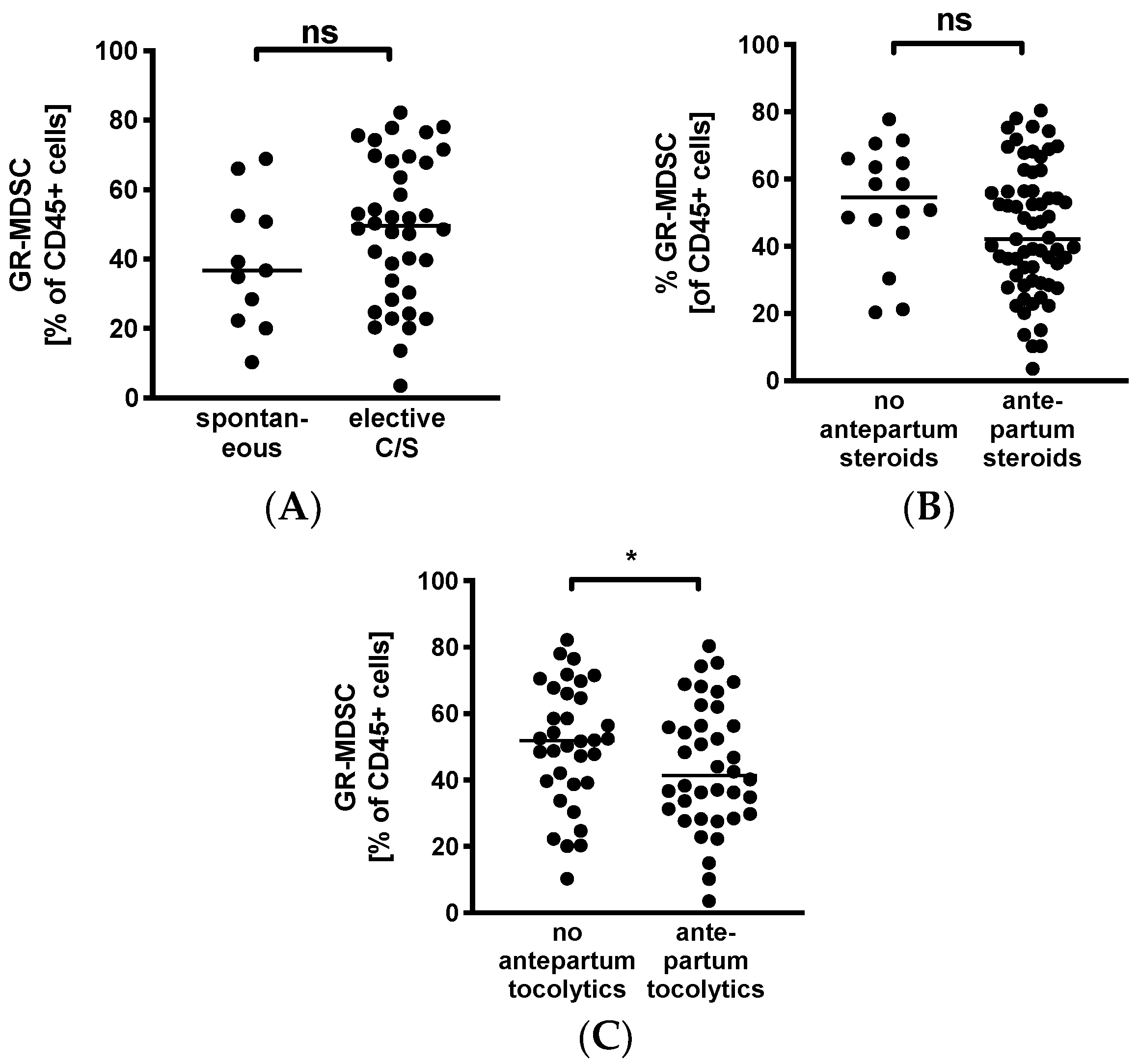
3.4. Decreased Levels of GR-MDSC in Breast Milk from Mothers Receiving Tocolytics
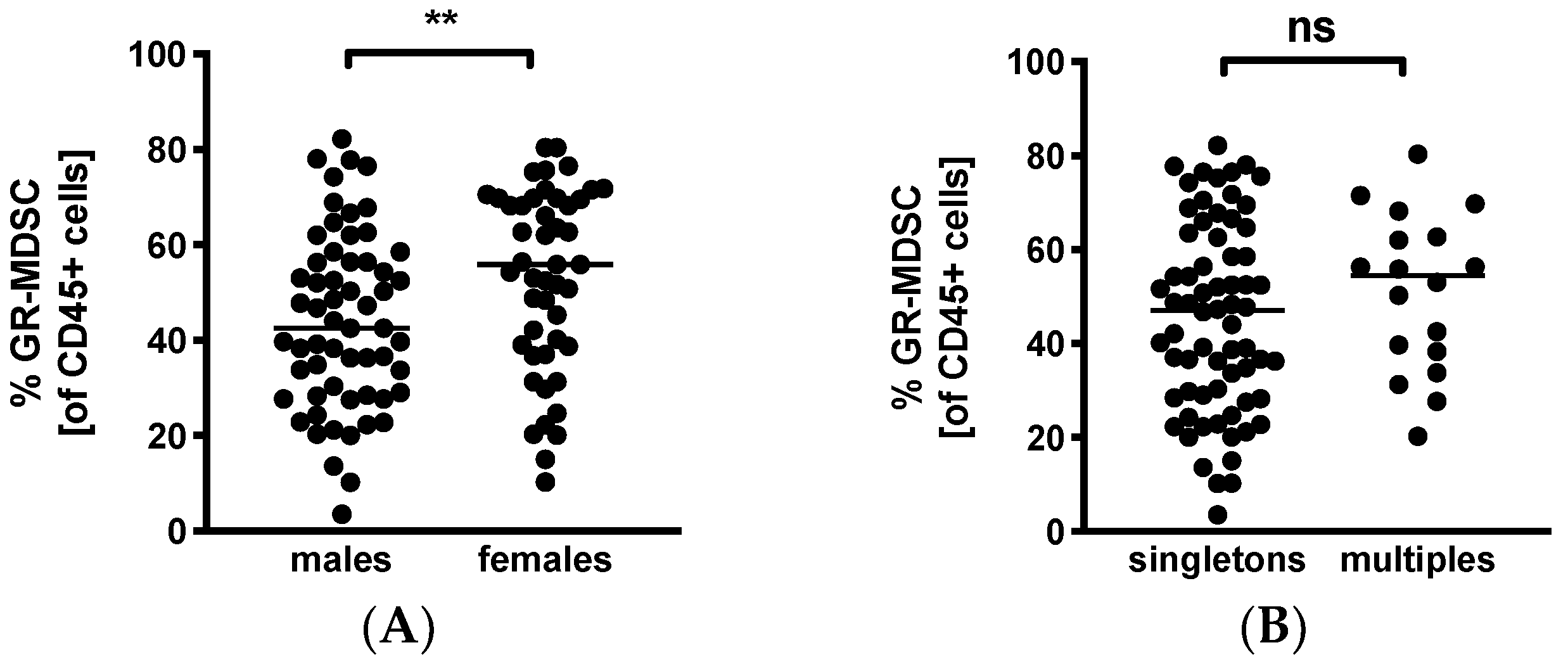
3.5. GR-MDSC Levels in Breast Milk Depend on Baby’s Gender
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stichtenoth, G.; Demmert, M.; Bohnhorst, B.; Stein, A.; Ehlers, S.; Heitmann, F.; Rieger-Fackeldey, E.; Olbertz, D.; Roll, C.; Emeis, M.; et al. Major contributors to hospital mortality in very-low-birth-weight infants: Data of the birth year 2010 cohort of the German Neonatal Network. Klin. Padiatr. 2012, 224, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Carl, M.A.; Ndao, I.M.; Springman, A.C.; Manning, S.D.; Johnson, J.R.; Johnston, B.D.; Burnham, C.A.; Weinstock, E.S.; Weinstock, G.M.; Wylie, T.N.; et al. Sepsis from the gut: The enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin. Infect. Dis. 2014, 58, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.G.; Sim, K.; Randell, P.; Cox, M.J.; McClure, Z.E.; Li, M.S.; Donaldson, H.; Langford, P.R.; Cookson, W.O.; Moffatt, M.F.; et al. Late-Onset Bloodstream Infection and Perturbed Maturation of the Gastrointestinal Microbiota in Premature Infants. PLoS ONE 2015, 10, e0132923. [Google Scholar] [CrossRef] [PubMed]
- Taft, D.H.; Ambalavanan, N.; Schibler, K.R.; Yu, Z.; Newburg, D.S.; Deshmukh, H.; Ward, D.V.; Morrow, A.L. Center Variation in Intestinal Microbiota Prior to Late-Onset Sepsis in Preterm Infants. PLoS ONE 2015, 10, e0130604. [Google Scholar] [CrossRef]
- Wang, Y.; Hoenig, J.D.; Malin, K.J.; Qamar, S.; Petrof, E.O.; Sun, J.; Antonopoulos, D.A.; Chang, E.B.; Claud, E.C. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009, 3, 944–954. [Google Scholar] [CrossRef]
- Hylander, M.A.; Strobino, D.M.; Dhanireddy, R. Human milk feedings and infection among very low birth weight infants. Pediatrics 1998, 102, E38. [Google Scholar] [CrossRef]
- Nino, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016. [CrossRef]
- Schanler, R.J.; Lau, C.; Hurst, N.M.; Smith, E.O. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef]
- Patel, R.M.; Denning, P.W. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr. Res. 2015, 78, 232–238. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Xu, L.; Lochhead, P.; Ko, Y.; Claggett, B.; Leong, R.W.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Arenz, S.; Ruckerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes Relat. Metab. Disord. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70 (Suppl. S2), 26–36. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am. J. Clin. Nutr. 2006, 84, 1043–1054. [Google Scholar] [CrossRef]
- Munblit, D.; Peroni, D.G.; Boix-Amoros, A.; Hsu, P.S.; Van’t Land, B.; Gay, M.C.L.; Kolotilina, A.; Skevaki, C.; Boyle, R.J.; Collado, M.C.; et al. Human Milk and Allergic Diseases: An Unsolved Puzzle. Nutrients 2017, 9, 894. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.R.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.E.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2013, 2, e3. [Google Scholar] [CrossRef]
- Budhwar, S.; Verma, P.; Verma, R.; Rai, S.; Singh, K. The Yin and Yang of Myeloid Derived Suppressor Cells. Front. Immunol. 2018, 9, 2776. [Google Scholar] [CrossRef]
- Brandau, S.; Trellakis, S.; Bruderek, K.; Schmaltz, D.; Steller, G.; Elian, M.; Suttmann, H.; Schenck, M.; Welling, J.; Zabel, P.; et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 2011, 89, 311–317. [Google Scholar] [CrossRef]
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Kostlin, N.; Hofstadter, K.; Ostermeir, A.L.; Spring, B.; Leiber, A.; Haen, S.; Abele, H.; Bauer, P.; Pollheimer, J.; Hartl, D.; et al. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J. Immunol. 2016, 196, 1132–1145. [Google Scholar] [CrossRef]
- Kostlin, N.; Kugel, H.; Spring, B.; Leiber, A.; Marme, A.; Henes, M.; Rieber, N.; Hartl, D.; Poets, C.F.; Gille, C. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur. J. Immunol. 2014, 44, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Kostlin, N.; Vogelmann, M.; Spring, B.; Schwarz, J.; Feucht, J.; Hartel, C.; Orlikowsky, T.W.; Poets, C.F.; Gille, C. Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology 2017, 152, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Rieber, N.; Gille, C.; Kostlin, N.; Schafer, I.; Spring, B.; Ost, M.; Spieles, H.; Kugel, H.A.; Pfeiffer, M.; Heininger, V.; et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin. Exp. Immunol. 2013, 174, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Scheckenbach, V.; Kugel, H.; Spring, B.; Pagel, J.; Hartel, C.; Pauluschke-Frohlich, J.; Peter, A.; Poets, C.F.; Gille, C.; et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin. Exp. Immunol. 2018, 191, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Sinha, P.; Figley, C.; Long, R.; Park, D.; Carter, D.; Clements, V.K. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J. Leukoc. Biol. 2017, 101, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Kostlin, N.; Schoetensack, C.; Schwarz, J.; Spring, B.; Marme, A.; Goelz, R.; Brodbeck, G.; Poets, C.F.; Gille, C. Granulocytic Myeloid-Derived Suppressor Cells (GR-MDSC) in Breast Milk (BM); GR-MDSC Accumulate in Human BM and Modulate T-Cell and Monocyte Function. Front. Immunol. 2018, 9, 1098. [Google Scholar] [CrossRef]
- Hanson, L.A.; Korotkova, M.; Lundin, S.; Haversen, L.; Silfverdal, S.A.; Mattsby-Baltzer, I.; Strandvik, B.; Telemo, E. The transfer of immunity from mother to child. Ann. N. Y. Acad. Sci. 2003, 987, 199–206. [Google Scholar] [CrossRef]
- Trend, S.; de Jong, E.; Lloyd, M.L.; Kok, C.H.; Richmond, P.; Doherty, D.A.; Simmer, K.; Kakulas, F.; Strunk, T.; Currie, A. Leukocyte Populations in Human Preterm and Term Breast Milk Identified by Multicolour Flow Cytometry. PLoS ONE 2015, 10, e0135580. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Delano, M.J.; Kelly-Scumpia, K.M.; Moreno, C.; Scumpia, P.O.; Laface, D.M.; Heyworth, P.G.; Efron, P.A.; Moldawer, L.L. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 2011, 17, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Liu, Y.; Zhong, L.M.; Shi, M.H.; Duan, X.B.; Wu, K.; Yang, Q.; Liu, C.; Wei, J.Y.; Ma, X.R.; et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J. Leukoc. Biol. 2016. [CrossRef] [PubMed]
- Verma, P.; Verma, R.; Nair, R.R.; Budhwar, S.; Khanna, A.; Agrawal, N.R.; Sinha, R.; Birendra, R.; Rajender, S.; Singh, K. Altered crosstalk of estradiol and progesterone with Myeloid-derived suppressor cells and Th1/Th2 cytokines in early miscarriage is associated with early breakdown of maternal-fetal tolerance. Am. J. Reprod. Immunol. 2019, 81, e13081. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Petrova, A. Intrapartum magnesium sulfate exposure attenuates neutrophil function in preterm neonates. Biol. Neonate 2006, 89, 99–103. [Google Scholar] [CrossRef]
- Diepenbruck, I.; Much, C.C.; Krumbholz, A.; Kolster, M.; Thieme, R.; Thieme, D.; Diepenbruck, S.; Solano, M.E.; Arck, P.C.; Tolosa, E. Effect of prenatal steroid treatment on the developing immune system. J. Mol. Med. (Berl.) 2013, 91, 1293–1302. [Google Scholar] [CrossRef]
- Gieras, A.; Gehbauer, C.; Perna-Barrull, D.; Engler, J.B.; Diepenbruck, I.; Glau, L.; Joosse, S.A.; Kersten, N.; Klinge, S.; Mittrucker, H.W.; et al. Prenatal Administration of Betamethasone Causes Changes in the T Cell Receptor Repertoire Influencing Development of Autoimmunity. Front. Immunol. 2017, 8, 1505. [Google Scholar] [CrossRef]
- Kramer, B.W.; Ikegami, M.; Moss, T.J.; Nitsos, I.; Newnham, J.P.; Jobe, A.H. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr. Res. 2004, 55, 764–768. [Google Scholar] [CrossRef][Green Version]
- Schulz, D.; Schlieckau, F.; Fill Malfertheiner, S.; Reuschel, E.; Seelbach-Gobel, B.; Ernst, W. Effect of betamethasone, indomethacin and fenoterol on neonatal and maternal mononuclear cells stimulated with Escherichia coli. Cytokine 2019, 116, 97–105. [Google Scholar] [CrossRef]
- Ronchetti, S.; Ricci, E.; Migliorati, G.; Gentili, M.; Riccardi, C. How Glucocorticoids Affect the Neutrophil Life. Int. J. Mol. Sci. 2018, 19, 4090. [Google Scholar] [CrossRef]
- Naeye, R.L.; Burt, L.S.; Wright, D.L.; Blanc, W.A.; Tatter, D. Neonatal mortality, the male disadvantage. Pediatrics 1971, 48, 902–906. [Google Scholar]
- Stevenson, D.K.; Verter, J.; Fanaroff, A.A.; Oh, W.; Ehrenkranz, R.A.; Shankaran, S.; Donovan, E.F.; Wright, L.L.; Lemons, J.A.; Tyson, J.E.; et al. Sex differences in outcomes of very low birthweight infants: The newborn male disadvantage. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 83, F182–F185. [Google Scholar] [CrossRef] [PubMed]
- Galante, L.; Milan, A.M.; Reynolds, C.M.; Cameron-Smith, D.; Vickers, M.H.; Pundir, S. Sex-Specific Human Milk Composition: The Role of Infant Sex in Determining Early Life Nutrition. Nutrients 2018, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Valizadeh, E.; Hosseini, N.; Khatibshahidi, S.; Raeisi, S. The Role of Infant Sex on Human Milk Composition. Breastfeed. Med. 2020, 15, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tonon, K.M.; de Morais, M.B.; Abrão, A.C.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef]
- Baldeon, M.E.; Zertuche, F.; Flores, N.; Fornasini, M. Free Amino Acid Content in Human Milk is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation. Nutrients 2019, 11, 2239. [Google Scholar] [CrossRef]

| All (n = 105) | GA < 28 0/7 WOG (n = 45) | GA 28 0/7–31 6/7 WOG (n = 41) | GA > 32 0/7 WOG (n = 19) | |
|---|---|---|---|---|
| GA (mean) | 29 | 26 3/7 | 29 6/7 | 33 2/7 |
| Birth weight (g) (mean) | 1239 | 889 | 1327 | 1877 |
| Gender male [%] | 56.2 | 48.9 | 65.9 | 52.6 |
| SGA (%) | 6.7 | 2.2 | 12.2 | 5.3 |
| Antenatal steroids (%) | 77.1 | 88.9 | 75.6 | 52.6 |
| Tocolytics (%) | 46.7 | 60 | 36.6 | 36.8 |
| MgSO4 (%) | 32.1 | 46.7 | 22 | 21 |
| Birth mode | ||||
| Spontaneous (%) | 11.4 | 8.9 | 12.2 | 15.7 |
| Elective C/S (%) | 41 | 31.1 | 51,2 | 42.1 |
| Emergency C/S (%) | 47.6 | 60 | 36.6 | 42.1 |
| Multiple pregnancies (%) | 35.1 | 51.1 | 24.4 | 21.1 |
| BPD (%) | 10.5 | 22.2 | 2.4 | 0 |
| NEC (%) | 2.9 | 2.2 | 4.9 | 0 |
| Sepsis total (%) | 11.4 | 17.8 | 9.8 | 0 |
| EOS (%) | 3.8 | 2.4 | 7.3 | 0 |
| LOS (%) | 7.6 | 15.6 | 2.4 | 0 |
| All (n = 86) | GA < 28 0/7 WOG (n = 34) | GA 28 0/7–31 6/7 WOG (n = 34) | GA > 32 0/7 WOG (n = 18) | |
|---|---|---|---|---|
| GA (mean) | 29 1/7 | 26 3/7 | 29 5/7 | 33 2/7 |
| Antenatal steroids (%) | 75.6 | 87.9 | 75 | 82.4 |
| Tocolytics (%) | 45.3 | 57.6 | 38.9 | 35.3 |
| MgSO4 (%) | 29.1 | 41.2 | 23.5 | 16.7 |
| Birth mode | ||||
| Spontaneous (%) | 12.8 | 12.1 | 11.1 | 17.6 |
| Elective C/S (%) | 44.2 | 36.4 | 52.8 | 41.2 |
| Emergency C/S (%) | 41.7 | 51.5 | 33.3 | 41.2 |
| Multiple pregnancies (%) | 20.9 | 33.3 | 13.9 | 11.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köstlin-Gille, N.; Flaig, L.-A.; Ginzel, M.; Arand, J.; Poets, C.F.; Gille, C. Granulocytic Myeloid-Derived Suppressor Cells in Breast Milk (BM-MDSC) Correlate with Gestational Age and Postnatal Age and Are Influenced by Infant’s Sex. Nutrients 2020, 12, 2571. https://doi.org/10.3390/nu12092571
Köstlin-Gille N, Flaig L-A, Ginzel M, Arand J, Poets CF, Gille C. Granulocytic Myeloid-Derived Suppressor Cells in Breast Milk (BM-MDSC) Correlate with Gestational Age and Postnatal Age and Are Influenced by Infant’s Sex. Nutrients. 2020; 12(9):2571. https://doi.org/10.3390/nu12092571
Chicago/Turabian StyleKöstlin-Gille, Natascha, Lara-Antonia Flaig, Marco Ginzel, Jörg Arand, Christian F. Poets, and Christian Gille. 2020. "Granulocytic Myeloid-Derived Suppressor Cells in Breast Milk (BM-MDSC) Correlate with Gestational Age and Postnatal Age and Are Influenced by Infant’s Sex" Nutrients 12, no. 9: 2571. https://doi.org/10.3390/nu12092571
APA StyleKöstlin-Gille, N., Flaig, L.-A., Ginzel, M., Arand, J., Poets, C. F., & Gille, C. (2020). Granulocytic Myeloid-Derived Suppressor Cells in Breast Milk (BM-MDSC) Correlate with Gestational Age and Postnatal Age and Are Influenced by Infant’s Sex. Nutrients, 12(9), 2571. https://doi.org/10.3390/nu12092571





