A Clinical Tool to Predict Low Serum Selenium in Patients with Worsening Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements and Related Definitions
2.3. Statistical Analysis
2.3.1. Baseline Characteristics
2.3.2. Clinical Predictors of Low Se
2.3.3. Prediction Model
2.3.4. Comparing Determinants and Prognosis of Low Se and ID
3. Results
3.1. Patient Characteristics
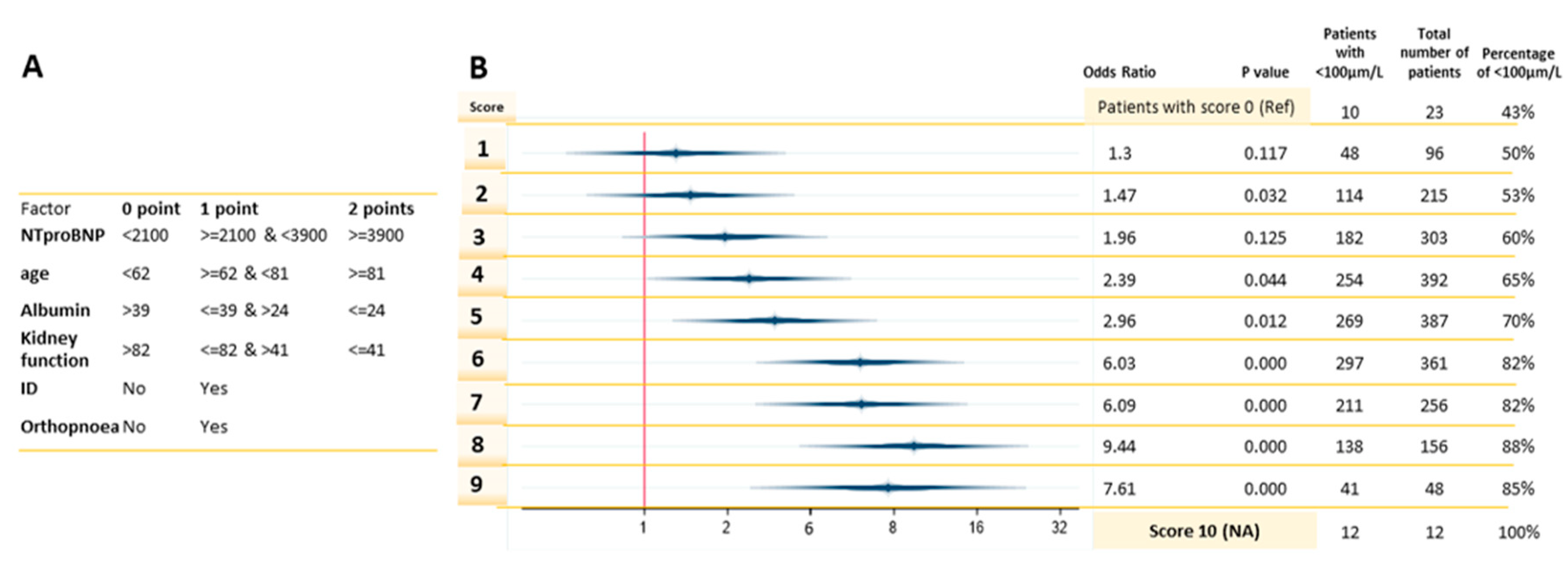
3.2. The Clinical Predictors and Diagnostic Accuracy of the Risk-Model
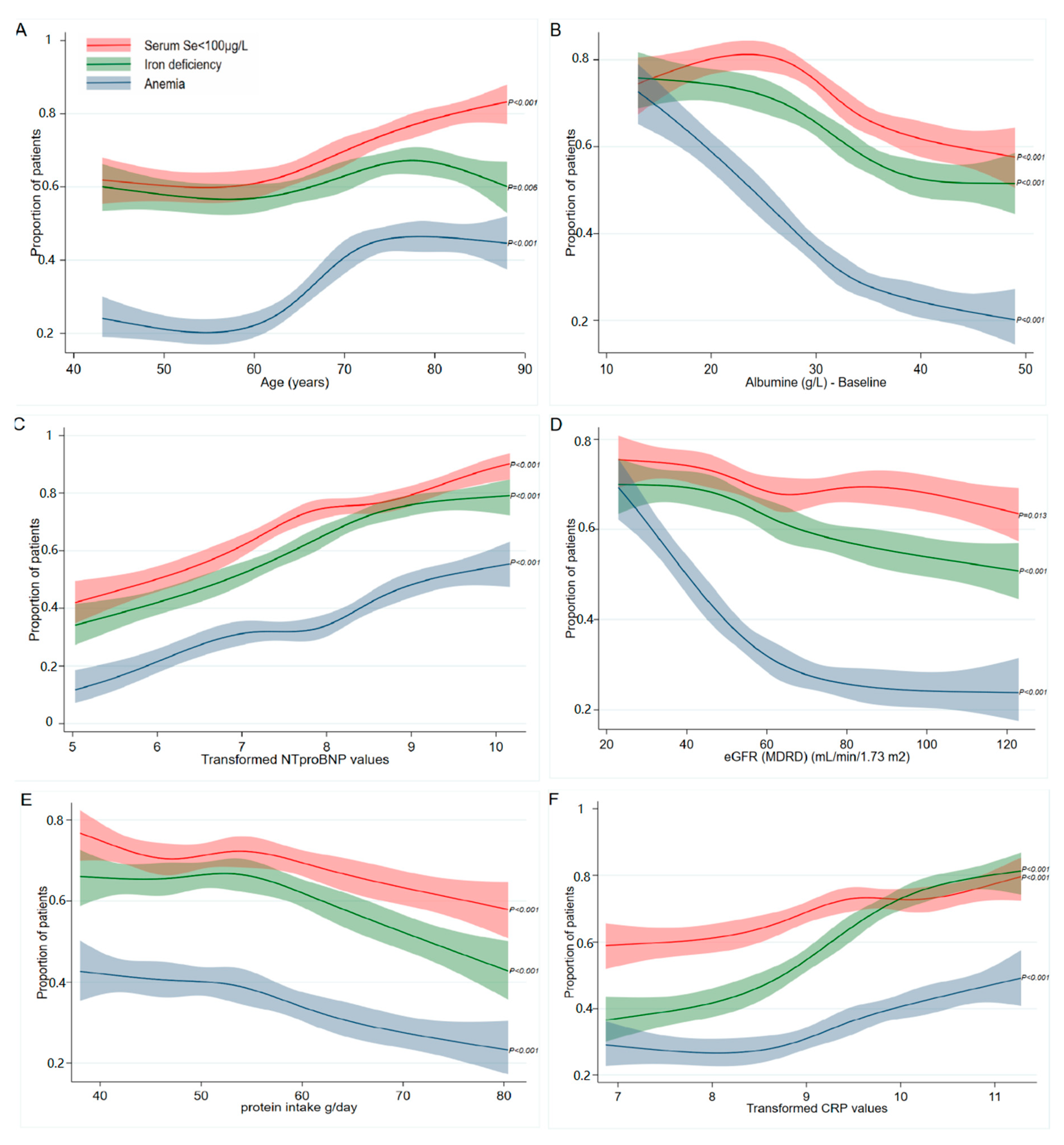
3.3. Prevalence of Se, Iron, and Anemia in Association with the Identified Clinical Predictors
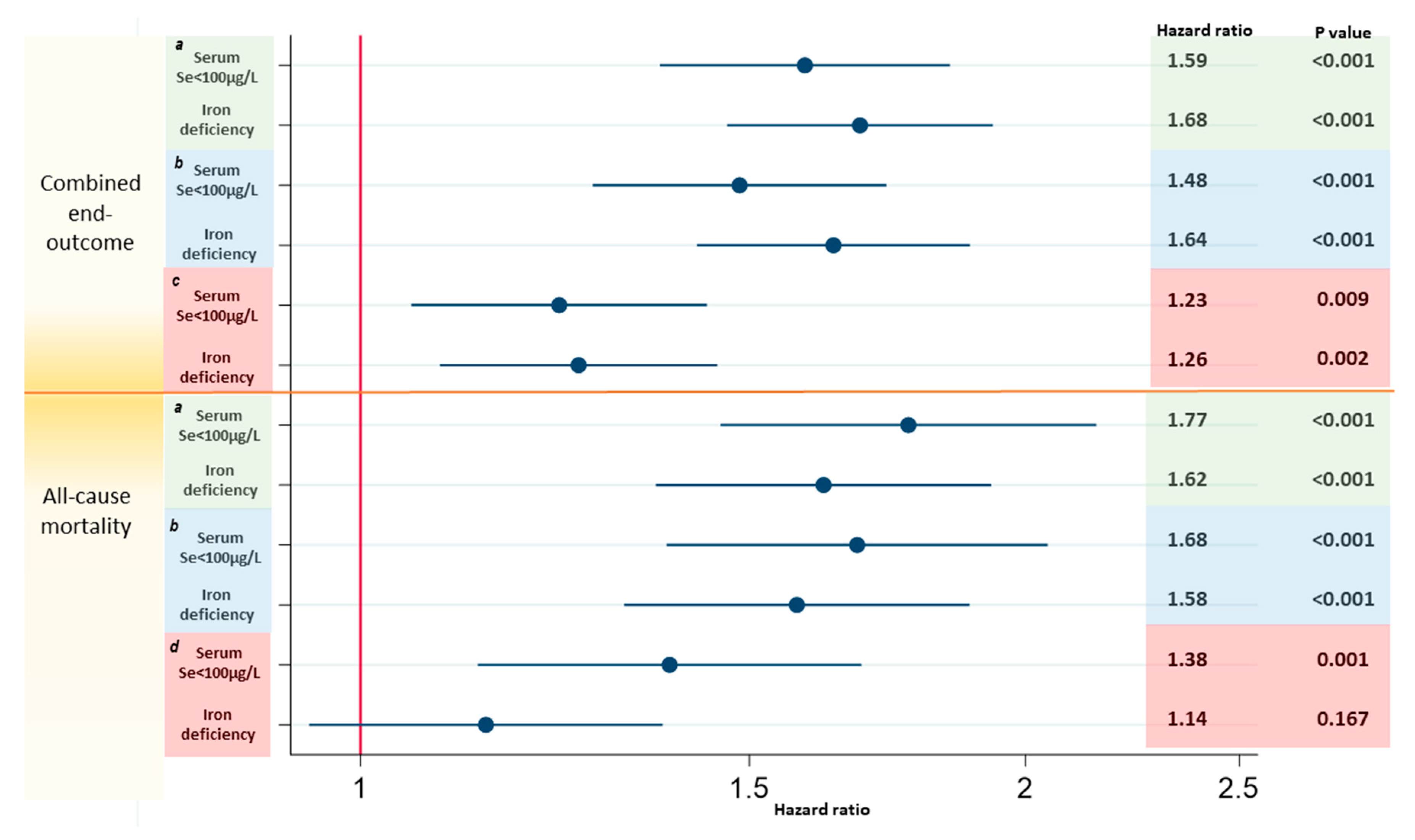
3.4. Associations with Prognosis Comparing Low Serum Se Concentrations and ID
4. Discussion
4.1. Using Clinical Variables to Predict Low Se
4.2. Clinical Predictors of Low Se
4.2.1. Age
4.2.2. NT-proBNP and Orthopnea
4.2.3. Kidney Function and Albumin
4.2.4. Iron Deficiency
4.3. The Prevalence of Low Serum Se Concentrations, ID, and Anemia in Relation to the Determinants
4.4. Low Serum Se Concentrations Associate with Worse Prognosis than ID
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Factor | Percentage | |
|---|---|---|
| Age (years) | 0 | 0.00 |
| Females (%) | 0 | 0.00 |
| Estimated protein intake (g/day) | 167 | 7.17 |
| NYHA functional class | 288 | 12.37 |
| Orthopnoea | 5 | 0.21 |
| Iron deficiency | 13 | 0.55 |
| Atrial fibrillation | 0 | 0.00 |
| CRP (mg/L) | 98 | 4.21 |
| NTproBNP (ng/L) | 58 | 2.49 |
| eGFR (MDRD) (mL/min/1.73 m2) | 7 | 0.30 |
| Albumine (g/L) | 12 | 0.52 |
| Hemoglobin | 200 | 8.59 |
| Beta-blockers - baseline | 0 | 0.00 |
| Aldosteron antagonist | 0 | 0.00 |
| Country | 0 | 0.00 |
| Heart rate (b.p.m) | 6 | 0.99 |
| Factor | Level | Value | Se ≥ 100 µg/L | Se < 100 μg/L | p-Value |
|---|---|---|---|---|---|
| N | 2328 | 695 | 1633 | ||
| Selenium (µg/L) | 89.1 (24.8) | 118.5 (18.2) | 76.6 (14.5) | <0.001 | |
| Demographics | |||||
| Age (years) | 68.8 (12.0) | 66.0 (11.8) | 70.1 (11.9) | <0.001 | |
| Females (%) | 607 (26.1%) | 150 (21.6%) | 457 (28.0%) | 0.001 | |
| BMI (kg/m2) | 27.2 (24.1, 30.6) | 27.7 (24.4, 30.7) | 26.9 (24.0, 30.6) | 0.072 | |
| Estimated protein intake (g/day) | 53.4 (46.5, 62.0) | 54.6 (47.3, 64.6) | 52.9 (46.1, 61.1) | <0.001 | |
| Ischemic etiology | 1057 (46.2%) | 315 (46.2%) | 742 (46.2%) | 0.98 | |
| LVEF (%) | 30.0 (25.0, 36.0) | 30.0 (25.0, 35.0) | 30.0 (25.0, 38.0) | 0.002 | |
| HFrEF (LVEF < 40) | 1691 (81.3%) | 554 (87.1%) | 1137 (78.7%) | <0.001 | |
| NYHA functional class | Class I | 214 (10.5%) | 83 (13.3%) | 131 (9.3%) | <0.001 |
| Class II | 1077 (52.8%) | 353 (56.6%) | 724 (51.1%) | ||
| Class III | 672 (32.9%) | 174 (27.9%) | 498 (35.2%) | ||
| Class IV | 77 (3.8%) | 14 (2.2%) | 63 (4.4%) | ||
| Previous hospitalization for HF | 720 (30.9%) | 201 (28.9%) | 519 (31.8%) | 0.17 | |
| Heart rate (b.p.m) | 76.0 (66.0, 90.0) | 75.0 (65.0, 86.0) | 77.0 (68.0, 90.0) | <0.001 | |
| Systolic blood pressure (mmHg) | 120.0 (110.0, 138.0) | 120.0 (110.0, 135.0) | 120.0 (110.0, 140.0) | 0.39 | |
| Diastolic blood pressure (mmHg) | 74.9 (13.1) | 75.0 (12.3) | 74.8 (13.5) | 0.71 | |
| Peripheral oedema | 1149 (59.4%) | 255 (44.6%) | 894 (65.6%) | <0.001 | |
| Pulmonary oedema | 507 (32.2%) | 128 (30.7%) | 379 (32.8%) | 0.44 | |
| Elevated JVP | No | 1025 (62.9%) | 393 (75.9%) | 632 (56.8%) | <0.001 |
| Yes | 514 (31.5%) | 102 (19.7%) | 412 (37.1%) | ||
| Uncertain | 91 (5.6%) | 23 (4.4%) | 68 (6.1%) | ||
| Orthopnoea | 802 (34.5%) | 180 (25.9%) | 622 (38.2%) | <0.001 | |
| Hepatomegaly | 331 (14.3%) | 102 (14.7%) | 229 (14.1%) | 0.71 | |
| Completion of 6MWT | 1462 (64.9%) | 499 (73.4%) | 963 (61.2%) | <0.001 | |
| Result 6MWT (m) | 330.0 (180.0, 429.0) | 365.5 (240.0, 449.0) | 310.0 (143.0, 420.0) | <0.001 | |
| KCCQ (overall score) | 49.0 (31.3, 66.7) | 56.3 (38.5, 71.9) | 44.8 (29.2, 63.5) | <0.001 | |
| Smoking (none, past, current) | None | 853 (36.7%) | 235 (33.9%) | 618 (37.9%) | 0.18 |
| Past | 1138 (48.9%) | 354 (51.0%) | 784 (48.1%) | ||
| Current | 334 (14.4%) | 105 (15.1%) | 229 (14.0%) | ||
| Current alcohol use | 644 (27.7%) | 196 (28.3%) | 448 (27.5%) | 0.69 | |
| Medical history | |||||
| Myocardial infarction | 891 (38.3%) | 258 (37.1%) | 633 (38.8%) | 0.46 | |
| CABG | 402 (17.3%) | 133 (19.1%) | 269 (16.5%) | 0.12 | |
| Valvular surgery | 171 (7.3%) | 54 (7.8%) | 117 (7.2%) | 0.61 | |
| PCI | 512 (22.0%) | 162 (23.3%) | 350 (21.4%) | 0.32 | |
| Atrial fibrillation | 1048 (45.0%) | 272 (39.1%) | 776 (47.5%) | <0.001 | |
| Device therapy | 580 (24.9%) | 190 (27.3%) | 390 (23.9%) | 0.078 | |
| Stroke | 214 (9.2%) | 53 (7.6%) | 161 (9.9%) | 0.088 | |
| Peripheral arterial disease | 251 (10.8%) | 75 (10.8%) | 176 (10.8%) | 0.99 | |
| Renal insufficiency (eGFR <60 mL/min) | 1068 (46.0%) | 287 (41.4%) | 781 (48.0%) | 0.003 | |
| Renal disease (history) | 646 (27.7%) | 167 (24.0%) | 479 (29.3%) | 0.009 | |
| Hypertension | 1452 (62.4%) | 428 (61.6%) | 1024 (62.7%) | 0.61 | |
| Diabetes mellitus | 750 (32.2%) | 222 (31.9%) | 528 (32.3%) | 0.85 | |
| COPD | 403 (17.3%) | 109 (15.7%) | 294 (18.0%) | 0.18 | |
| Current malignancy | 87 (3.7%) | 18 (2.6%) | 69 (4.2%) | 0.057 | |
| Laboratory | |||||
| CRP (mg/L) | 13,142.2 (5818.3, 26416.6) | 11,091.9 (4127.5, 22689.7) | 14,151.5 (6760.5, 27734.3) | <0.001 | |
| Creatinin (µmol/L) | 101.0 (82.2, 129.0) | 100.0 (82.1, 123.8) | 101.4 (82.2, 131.6) | 0.24 | |
| Urea (mmol/L) | 11.3 (7.6, 18.2) | 12.1 (7.8, 19.2) | 11.0 (7.5, 17.8) | 0.049 | |
| Proteinuria (mg/dL) | 5.0 (0.0, 20.0) | 5.0 (0.0, 20.0) | 2.0 (0.0, 20.0) | 0.68 | |
| Sodium (mmol/L) | 139.2 (3.9) | 139.4 (3.5) | 139.1 (4.1) | 0.25 | |
| Potassium (mmol/L) | 4.3 (0.6) | 4.3 (0.5) | 4.3 (0.6) | 0.95 | |
| NTproBNP (ng/L) | 2698.0 (1179.0, 5751.0) | 1628.5 (696.5, 3791.0) | 3224.0 (1483.0, 6635.5) | <0.001 | |
| GDF15, median (IQR) | 2720.0 (1712.0, 4556.0) | 2133.0 (1346.0, 3577.0) | 3021.5 (1915.5, 5224.5) | <0.001 | |
| eGFR (MDRD) (mL/min/1.73 m2) | 65.0 (26.3) | 67.7 (27.5) | 63.9 (25.7) | 0.001 | |
| IL-6 (pg/mL) | 5.2 (2.8, 10.2) | 3.9 (2.1, 7.4) | 5.8 (3.2, 11.4) | <0.001 | |
| Total bilirubin (µmol/L) | 14.0 (9.9, 21.0) | 13.2 (9.1, 18.8) | 14.3 (10.0, 22.2) | 0.001 | |
| Leucocytes (10e9/L) | 8.3 (2.9) | 8.3 (2.7) | 8.3 (2.9) | 0.95 | |
| Troponin I (µg/L) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.006 | |
| Troponin T (µg/L) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.71 | |
| Glucose (mmol/L) | 6.3 (5.4, 7.9) | 6.3 (5.4, 8.0) | 6.2 (5.3, 7.9) | 0.22 | |
| HbA1c (%) | 6.3 (5.7, 7.1) | 6.4 (5.8, 7.3) | 6.2 (5.7, 7.0) | 0.24 | |
| ASAT (U/L) | 25.4 (19.0, 35.0) | 25.0 (19.0, 35.0) | 26.0 (20.0, 35.0) | 0.35 | |
| ALAT (U/L) | 25.0 (17.0, 38.0) | 28.0 (20.0, 42.0) | 23.0 (16.0, 36.0) | <0.001 | |
| γ-GT (U/L) | 55.0 (28.0, 108.5) | 44.0 (26.0, 84.0) | 60.0 (29.0, 118.0) | <0.001 | |
| Alkaline phosphatase (µg/L) | 84.0 (65.0, 118.0) | 77.0 (62.0, 104.0) | 88.0 (67.0, 122.0) | <0.001 | |
| HDL (mmol/L) | 1.1 (0.8, 1.3) | 1.1 (0.9, 1.3) | 1.0 (0.8, 1.3) | 0.065 | |
| LDL (mmol/L) | 2.6 (1.1) | 2.8 (1.1) | 2.5 (1.1) | <0.001 | |
| Total cholesterol (mmol/L) | 4.3 (1.3) | 4.5 (1.4) | 4.2 (1.3) | <0.001 | |
| Triglycerides (mmol/L) | 1.2 (0.9, 1.7) | 1.3 (1.0, 1.8) | 1.2 (0.9, 1.6) | <0.001 | |
| TSH (mU/L) | 1.8 (1.2, 3.0) | 1.8 (1.1, 3.2) | 1.8 (1.2, 3.0) | 0.85 | |
| FT4 (pmol/L) | 16.8 (7.6) | 17.3 (10.6) | 16.5 (5.6) | 0.29 | |
| Albumine (g/L) | 33.0 (27.0, 38.0) | 34.0 (30.0, 40.0) | 32.0 (26.5, 37.0) | <0.001 | |
| Hemoglobin (g/dL) | 13.2 (1.9) | 13.6 (1.8) | 13.0 (1.9) | <0.001 | |
| Anemia | 772 (36.3%) | 176 (28.9%) | 596 (39.2%) | <0.001 | |
| Mean corpuscular volume (fL) | 90.4 (8.6) | 91.0 (7.8) | 90.2 (8.9) | 0.097 | |
| Iron deficiency | 1436 (62.0%) | 357 (51.7%) | 1079 (66.4%) | <0.001 | |
| Iron (mg/dL) | 44.7 (27.9, 72.6) | 55.9 (39.1, 78.2) | 44.7 (27.9, 67.0) | <0.001 | |
| Ferritin (μg/L) | 101.0 (50.0, 191.5) | 126.0 (62.5, 208.0) | 93.0 (46.0, 185.0) | <0.001 | |
| Transferrin (mg/dL) | 206.0 (69.7) | 211.9 (67.9) | 203.5 (70.4) | 0.008 | |
| Transferrin saturation (%) | 17.1 (10.9, 24.9) | 19.9 (13.3, 27.2) | 15.9 (9.9, 23.5) | <0.001 | |
| Hepcidin (nmol/L) | 6.3 (2.2, 16.5) | 7.5 (3.1, 17.3) | 5.7 (1.8, 15.9) | <0.001 | |
| sTfR (mg/L) | 1.8 (1.0) | 1.5 (0.8) | 1.9 (1.1) | <0.001 | |
| Medication | |||||
| Betablocker - baseline | 1941 (83.4%) | 597 (85.9%) | 1344 (82.3%) | 0.033 | |
| ACE/ARB - baseline | 1685 (72.4%) | 522 (75.1%) | 1163 (71.2%) | 0.055 | |
| Antiplatelets | 1208 (51.9%) | 363 (52.2%) | 845 (51.7%) | 0.83 | |
| P2Y12V2 | 359 (15.4%) | 101 (14.5%) | 258 (15.8%) | 0.44 | |
| Diuretics | 2326 (99.9%) | 694 (99.9%) | 1632 (99.9%) | 0.53 | |
| Loop Diuretics | 2317 (99.5%) | 690 (99.3%) | 1627 (99.6%) | 0.26 | |
| Oral anti-diabetics | 469 (62.5%) | 133 (59.9%) | 336 (63.6%) | 0.34 | |
| Insuline use | 301 (40.1%) | 93 (41.9%) | 208 (39.4%) | 0.52 | |
| Proton pump inhibitor | 815 (35.0%) | 237 (34.1%) | 578 (35.4%) | 0.55 | |
| Acenocoumarol | 896 (38.5%) | 258 (37.1%) | 638 (39.1%) | 0.38 | |
| Aldosteron antagonist | 1241 (53.3%) | 398 (57.3%) | 843 (51.6%) | 0.013 | |
| Spironolactone | 855 (36.7%) | 250 (36.0%) | 605 (37.0%) | 0.62 |
| N. of Repetion | |
|---|---|
| Age (years) | 856 |
| NTproBNP (per doubling) | 1000 |
| eGFR (MDRD) (mL/min/1.73 m2) | 838 |
| Albumine (g/L) | 859 |
| Iron deficiency | 553 |
| Orthopnoea | 813 |
| Predictor # | Penalized Odds Ratios |
|---|---|
| NTproBNP | 1.32 |
| Albumin | 0.98 |
| Kidney function (eGFR MDRD) | 1.01 |
| Age | 1.01 |
| Iron deficiency | 1.23 |
| Orthopnoea | 1.38 |
| Country | Number of Included Patients | Mean Selenium | Percentage of Patients < 100 μg/L |
|---|---|---|---|
| the Netherlands | 386 | 86.00 | 76.68 |
| France | 243 | 80.70 | 80.66 |
| Germancy | 87 | 90.69 | 67.82 |
| Serbia | 382 | 91.23 | 66.49 |
| Slovenia | 43 | 66.83 | 97.67 |
| Greece | 285 | 90.87 | 68.42 |
| Italy | 297 | 115.48 | 27.61 |
| Norway | 108 | 79.16 | 86.11 |
| Sweden | 97 | 79.49 | 84.54 |
| Poland | 239 | 79.33 | 87.45 |
| United Kingdom | 161 | 84.42 | 77.64 |
| Factor | 0 Point | 1 Point | 2 Points | Total Number of Patients |
|---|---|---|---|---|
| NTproBNP | 555 | 352 | 685 | 1592 |
| eGFR Kidney Function | 343 | 977 | 307 | 1627 |
| Albumin | 264 | 1050 | 310 | 1624 |
| Age | 374 | 972 | 287 | 1633 |
| Iron deficiency | 545 | 1079 | NA | 1624 |
| Orthopnoea | 1006 | 622 | NA | 1628 |
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Cascino, T.M.; Hummel, S.L. Nutrient deficiencies in heart failure: A micro problem with macro effects? J. Am. Heart Assoc. 2018, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Keshan Disease, Selenium Deficiency, and the Selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Abe, S.; Kiko, T.; Kimishima, Y.; Sato, Y.; Watanabe, S.; Kanno, Y.; Miyata-Tatsumi, M.; Misaka, T.; Sato, T.; et al. Association of Serum Zinc Level with Prognosis in Patients With Heart Failure. J. Card. Fail. 2018, 24, 375–383. [Google Scholar] [CrossRef] [PubMed]
- McKeag, N.A.; McKinley, M.C.; Harbinson, M.T.; McGinty, A.; Neville, C.E.; Woodside, J.V.; McKeown, P.P. Dietary Micronutrient Intake and Micronutrient Status in Patients With Chronic Stable Heart Failure. J. Cardiovasc. Nurs. 2017, 32, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lennie, T.A.; Andreae, C.; Rayens, M.K.; Song, E.K.; Dunbar, S.B.; Pressler, S.J.; Heo, S.; Kim, J.S.; Moser, D.K. Micronutrient deficiency independently predicts time to event in patients with heart failure. J. Am. Heart Assoc. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; Van Der Wal, H.H.; Klip, I.T.; Anker, S.D.; Cleland, J.; Dickstein, K.; Van Veldhuisen, D.J.; Voors, A.A.; Van Der Meer, P. Differences in Clinical Profile and Outcomes of Low Iron Storage vs Defective Iron Utilization in Patients with Heart Failure: Results from the DEFINE-HF and BIOSTAT-CHF Studies. JAMA Cardiol. 2019, 4, 696–701. [Google Scholar] [CrossRef]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef]
- van der Wal, H.H.; Grote Beverborg, N.; Dickstein, K.; Anker, S.D.; Lang, C.C.; Ng, L.L.; van Veldhuisen, D.J.; Voors, A.A.; van der Meer, P. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur. Heart J. 2019, 40, 3616–3625. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Van Veldhuisen, D.J.; Ponikowski, P.; Van Der Meer, P.; Metra, M.; Böhm, M.; Doletsky, A.; Voors, A.A.; MacDougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency. Circulation 2017, 136, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur. Heart J. 2014, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Kirwan, B.-A.; Anker, S.D.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Haboubi, T.; Keren, A.; Khintibidze, I.; et al. Rationale and design of the AFFIRM-AHF trial: A randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure. Eur. J. Heart Fail. 2019, 21, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, T.; Li, Q.; Li, D. Prevention of Keshan Disease by Selenium Supplementation: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2018, 186, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Bomer, N.; Grote Beverborg, N.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; IJmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2019, 1–9. [Google Scholar] [CrossRef]
- Spertus, J.; Peterson, E.; Conard, M.W.; Heidenreich, P.A.; Krumholz, H.M.; Jones, P.; McCullough, P.A.; Pina, I.; Tooley, J.; Weintraub, W.S.; et al. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am. Heart J. 2005, 150, 707–715. [Google Scholar] [CrossRef]
- Spertus, J.A.; Jones, P.G. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 469–476. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Orangi, J.; Sorenson, C.M.; Sheibani, N. Functional role of inorganic trace elements in angiogenesis-Part I: N, Fe, Se, P, Au, and Ca. Crit. Rev. Oncol. Hematol. 2015, 96, 129–142. [Google Scholar] [CrossRef]
- Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; ter Maaten, J.M.; Ng, L.; et al. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: Rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur. J. Heart Fail. 2016, 18, 716–726. [Google Scholar] [CrossRef]
- Voors, A.A.; Ouwerkerk, W.; Zannad, F.; van Veldhuisen, D.J.; Samani, N.J.; Ponikowski, P.; Ng, L.L.; Metra, M.; ter Maaten, J.M.; Lang, C.C.; et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur. J. Heart Fail. 2017, 19, 627–634. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; Klip, I.T.; Meijers, W.C.; Voors, A.A.; Vegter, E.L.; van der Wal, H.H.; Swinkels, D.W.; van Pelt, J.; Mulder, A.B.; Bulstra, S.K.; et al. Definition of Iron Deficiency Based on the Gold Standard of Bone Marrow Iron Staining in Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004519. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Prentice-Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software. ACM Sigkdd Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. First Trimester Microelements and Their Relationships with Pregnancy Outcomes and Complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Bertrais, S.; Roussel, A.M.; Arnault, N.; Ruffieux, D.; Favier, A.; Berthelin, S.; Estaquio, C.; Galan, P.; Czernichow, S.; et al. Serum selenium determinants in French adults: The SU.VI.M.AX study. Br. J. Nutr. 2006, 95, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Selenium status and cardiovascular diseases: Meta-analysis of prospective observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 162–169. [Google Scholar] [CrossRef]
- Rayman, M.P. Dietary selenium: Time to act. BMJ 1997, 314, 387–388. [Google Scholar] [CrossRef]
- Nève, J. New Approaches to Assess Selenium Status and Requirement. Nutr. Rev. 2000, 58, 363–369. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F.; Butler, J.A.; Whanger, P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br. J. Nutr. 1993, 69, 577–588. [Google Scholar] [CrossRef]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Rimm, E.; Siscovick, D.; Spiegelman, D.; Steven Morris, J.; Mozaffarian, D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr. Res. Pract. 2011, 5, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef]
- Polsinelli, V.B.; Sinha, A.; Shah, S.J. Visceral Congestion in Heart Failure: Right Ventricular Dysfunction, Splanchnic Hemodynamics, and the Intestinal Microenvironment. Curr. Heart Fail. Rep. 2017, 14, 519–528. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef]
- Yosuke, F.; Fujishima, Y.; Ohsawa, M.; Itai, K.; Kato, K.; Tanno, K.; Turin, T.C.; Onoda, T.; Endo, S.; Okayama, A.; et al. Serum Selenium Levels in Hemodialysis Patients Are Significantly Lower than Those in Healthy Controls. Blood Purif. 2011, 32, 43–47. [Google Scholar] [CrossRef]
- De Oña, C.G.; Martínez-Morillo, E.; González, E.G.; Argüelles, P.V.; Merayo, C.F.; Menéndez, F.V. Variation of trace element concentrations in patients undergoing hemodialysis in the north of Spain. Scand. J. Clin. Lab. Investig. 2016, 76, 492–499. [Google Scholar] [CrossRef]
- Lai, H.; Nie, T.; Zhang, Y.; Chen, Y.; Tao, J.; Lin, T.; Ge, T.; Li, F.; Li, H. Selenium Deficiency-Induced Damage and Altered Expression of Mitochondrial Biogenesis Markers in the Kidneys of Mice. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef]
- Ghashut, R.A.; McMillan, D.C.; Kinsella, J.; Vasilaki, A.T.; Talwar, D.; Duncan, A. The effect of the systemic inflammatory response on plasma zinc and selenium adjusted for albumin. Clin. Nutr. 2016, 35, 381–387. [Google Scholar] [CrossRef]
- Bates, C.J.; Thane, C.W.; Prentice, A.; Delves, H.T. Selenium status and its correlates in a British National Diet and Nutrition Survey: People aged 65 years and over. J. Trace Elem. Med. Biol. 2002, 16, 1–8. [Google Scholar] [CrossRef]
- Khalili, M.; Chamani, M.; Amanlou, H.; Nikkhah, A.; Sadeghi, A.A. Effects of different sources of selenium supplementation on antioxidant indices, biochemical parameters, thyroid hormones and se status in transition cows. Acta Sci. Anim. Sci. 2019, 41, 1–7. [Google Scholar] [CrossRef]
- Gürgöze, M.K.; Ölçücü, A.; Aygün, A.D.; Taşkin, E.; Kiliç, M. Serum and hair levels of zinc, selenium, iron, and copper in children with iron-deficiency anemia. Biol. Trace Elem. Res. 2006, 111, 23–29. [Google Scholar] [CrossRef]
- Van Nhien, N.; Khan, N.C.; Yabutani, T.; Ninh, N.X.; Kassu, A.; Huong, B.T.M.; Do, T.T.; Motonaka, J.; Ota, F. Serum levels of trace elements and iron-deficiency anemia in adult Vietnamese. Biol. Trace Elem. Res. 2006, 111, 1–9. [Google Scholar] [CrossRef]
| Factor | Odds Ratio | Standard Error | z | p Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| eGFR (Kidney Function) | 1.01 | 0.00 | 2.68 | 0.007 | 1.00 | 1.01 |
| Iron Deficiency | 1.36 | 0.14 | 3.02 | 0.002 | 1.11 | 1.66 |
| NTproBNP | 1.30 | 0.04 | 8.71 | <0.001 | 1.23 | 1.39 |
| Albumin | 0.97 | 0.01 | −4.55 | <0.001 | 0.96 | 0.99 |
| Orthopnoea | 1.43 | 0.15 | 3.31 | 0.001 | 1.16 | 1.77 |
| Age | 1.02 | 0.00 | 4.63 | <0.001 | 1.01 | 1.03 |
| Score | ROC # | Sensitivity | Specificity | NPV ‡ | PPV † | NNT * |
|---|---|---|---|---|---|---|
| 1 | 0.51 | 99% | 2% | 57 | 70 | 1.42 |
| 2 | 0.53 | 96% | 9% | 51 | 71 | 1.40 |
| 3 | 0.57 | 89% | 24% | 49 | 73 | 1.36 |
| 4 | 0.60 | 77% | 42% | 44 | 76 | 1.32 |
| 5 | 0.62 | 61% | 62% | 41 | 79 | 1.26 |
| 6 | 0.62 | 44% | 80% | 38 | 84 | 1.19 |
| 7 | 0.57 | 25% | 89% | 34 | 85 | 1.18 |
| 8 | 0.54 | 12% | 96% | 32 | 88 | 1.14 |
| 9 | 0.51 | 3% | 99% | 30 | 88 | 1.13 |
| 10 | 0.50 | 1% | 100% | 30 | 100 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mubarak, A.A.; Grote Beverborg, N.; Anker, S.D.; Samani, N.J.; Dickstein, K.; Filippatos, G.; van Veldhuisen, D.J.; Voors, A.A.; Bomer, N.; van der Meer, P. A Clinical Tool to Predict Low Serum Selenium in Patients with Worsening Heart Failure. Nutrients 2020, 12, 2541. https://doi.org/10.3390/nu12092541
Al-Mubarak AA, Grote Beverborg N, Anker SD, Samani NJ, Dickstein K, Filippatos G, van Veldhuisen DJ, Voors AA, Bomer N, van der Meer P. A Clinical Tool to Predict Low Serum Selenium in Patients with Worsening Heart Failure. Nutrients. 2020; 12(9):2541. https://doi.org/10.3390/nu12092541
Chicago/Turabian StyleAl-Mubarak, Ali A., Niels Grote Beverborg, Stefan D. Anker, Nilesh J. Samani, Kenneth Dickstein, Gerasimos Filippatos, Dirk Jan van Veldhuisen, Adriaan A. Voors, Nils Bomer, and Peter van der Meer. 2020. "A Clinical Tool to Predict Low Serum Selenium in Patients with Worsening Heart Failure" Nutrients 12, no. 9: 2541. https://doi.org/10.3390/nu12092541
APA StyleAl-Mubarak, A. A., Grote Beverborg, N., Anker, S. D., Samani, N. J., Dickstein, K., Filippatos, G., van Veldhuisen, D. J., Voors, A. A., Bomer, N., & van der Meer, P. (2020). A Clinical Tool to Predict Low Serum Selenium in Patients with Worsening Heart Failure. Nutrients, 12(9), 2541. https://doi.org/10.3390/nu12092541








