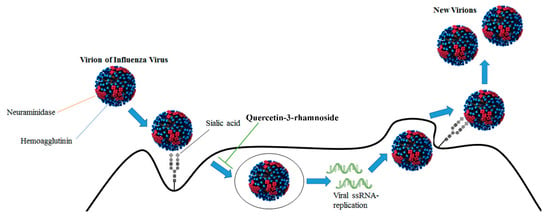
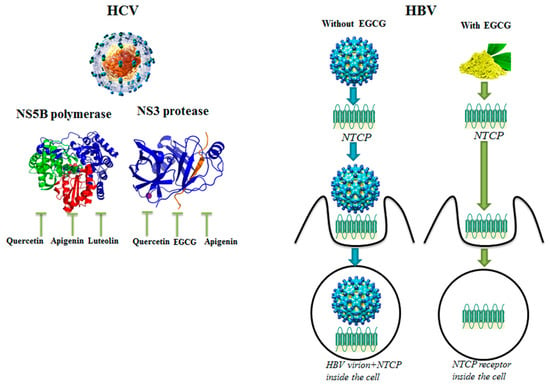
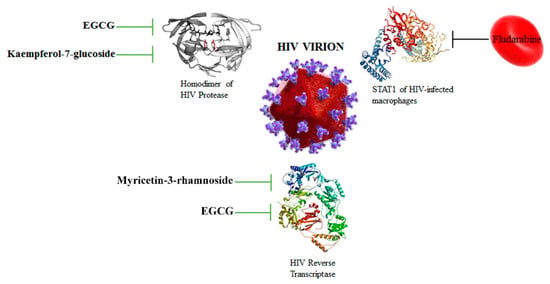
Abstract
This review summarizes the latest advancements in phytochemicals as functional antiviral agents. We focused on flavonoids, like apigenin, vitexin, quercetin, rutin and naringenin, which have shown a wide range of biological effects including antiviral activities. The molecular mechanisms of their antiviral effects mainly consist in the inhibition of viral neuraminidase, proteases and DNA/RNA polymerases, as well as in the modification of various viral proteins. Mixtures of different flavonoids or combination of flavonoids with antiviral synthetic drugs provide an enhancement of their antiviral effects. Recent strategies in drug delivery significantly contribute to overcoming the low bioavailability of flavonoids. Frequent viral infections worldwide have led to the need for new effective antiviral agents, which can be identified among the various phytochemicals. In this light, screening the antiviral activities of a cocktail of flavonoids would be advantageous in order to prevent viral infections and improve current antiviral therapies.
1. Introduction
In the past two decades, studies conducted in our laboratory focused on the antioxidants present in vegetable foods and on their capacity to reduce the adverse physiological effects of the oxygen free radicals [1,2]. The research was also aimed at the technologies of food transformation in order to preserve, as much as possible, the antioxidants in the final products [3]. Antioxidants are mainly represented in nature by the liposoluble vitamins E and A, β-carotene, hydro-soluble vitamin C and a wide range of amphipathic molecules, broadly termed phenolic compounds [1]. These compounds are divided into several sub-classes, including phenolic acids and analogues, stilbenes, flavonoids and their analogues. Flavonoids possess important health protective effects, including anti-inflammatory, anticancer and antiviral properties [4,5,6,7,8,9,10]. There are in nature more than 6000 flavonoids, which have been structurally identified and divided in classes: flavones (e.g., apigenin), flavanols (e.g., quercetin), flavins (e.g., epigallocatechin-3-gallate), isoflavones (e.g., genistein) and anthocyanidins (e.g., cyanidin). Flavonoids occur in their free or conjugated form or are often esterified with one or two sugars with O-glycosidic or C-glycosidic bonds [11]. In the past decade we purified and studied the biological effects of a group of the flavonoid C-glycosides derived from apigenin, namely vitexin, vitexin-2-O-rhamnoside and vitexin-2-O-xyloside (Figure 1).
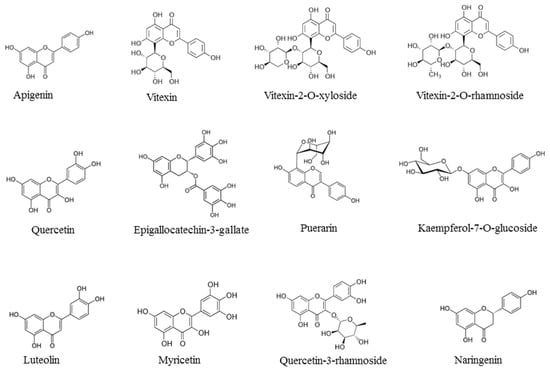
Figure 1.
Chemical formulae of representative flavonoids with antiviral activities: apigenin, vitexin, vitexin-2-O-xyloside, vitexin-2-O-rhamnoside, quercetin, epigallocatechin-3-gallate, puerarin, kaempferol-7-O-glucoside, luteolin, myricetin, quercetin-3-rhamnoside, naringenin.
The interest in the antiviral activity of natural flavonoids has increased in the last decade because of the frequency of viral infections, particularly influenza infections, which affect several million patients annually [12]. While vaccination is the primary strategy for influenza prevention, there are scenarios for which vaccination is not possible and antiviral molecules represent an important sanitary presidium. Synthetic antiviral drugs often show limited efficacy and serious adverse effects [13], whereas herbal extracts, known for their antiviral properties with no or mild side effects, may be a viable alternative for treating various viral diseases [14]. Viruses consist of nucleic acid (DNA or RNA) enclosed in a protein structure, called capsid, which can be surrounded by a lipid membrane named the envelope. The infective unit of the virus is called the virion and this parasite can replicate only inside host cells, hijacking the molecular machinery and controlling DNA replication, RNA transcription and the protein translation processes. Viruses attack the host cells through adsorption to receptors specific for each type of target cells, penetrating through the cell membrane, then the genetic material of the virus is liberated to replicate its own genome, produce new viral proteins and obtain new virions [15]. In 2017, the antiviral effects of various phytochemicals were reviewed by Kapoor et al. [15], taking into consideration many categories of compounds, ranging from flavonoids to saponins and lignans. In the same year, another group published a review focused specifically on the antiviral effects of the six classes of flavonoids [16]. In 2020, an interesting review regarding the methods for delivery of phytochemicals to increase their bioavailability in human tissues has been published [17].
In this review, we first summarize flavonoids along with their class and plant sources, with particular attention to apigenin, vitexin and its derivatives (Table 1). We then discuss the antiviral action mechanisms of flavonoids, their combinations with conventional antiviral drugs in multi-target cocktails, and the delivery strategies used to increase their bioavailability and antiviral efficacy.

Table 1.
Classification and sources of the flavonoids described in this Review.
6. Conclusions and Future Perspectives
Overall, our review shows that many flavonoids exhibit antiviral activity and could offer a promising alternative for prevention of and therapy for viral infections. Flavonoids are present in many vegetables and the first protection for the immune system resides in the ability to seek foods rich in bioactive nutrients. Education programs for a healthy diet should be implemented during the outbreaks of viral infections [94]. In fact, a diet rich in vegetables activates the AhR in the gut for maintenance of microbiota homeostasis, which in turn regulates the immune system. In the critical periods of viral infections, oral dietary supplementation with nutraceutical preparations based on combinations of flavonoids can be useful in order to inhibit different steps of the viral infective cycle. Molecular mechanisms underlying the antiviral effects of flavonoids, herein described, mainly focus on the inhibition of viral enzyme activities: neuraminidases, DNA/RNA polymerases and proteases. Therefore, a cocktail of flavonoids, selected for their efficacy in the inhibition of different viral enzymes, could be associated with elevated immune response and offer a promising option for antiviral therapies. This option acquires great importance considering that the viral genome frequently mutates, due to the lack of proof-reading activity of most of the viral polymerases. These mutations could hamper the efficacy of antiviral synthetic drugs. On the contrary, antiviral flavonoids, as well as the combination of synthetic antiviral drugs with flavonoids, would enhance therapeutic strategies by targeting the multiple signaling pathways involved in the viral infections [95]. The active concentration of the flavonoids should be investigated, considering the pharmacokinetic studies available in the literature and the synergistic effects of the specific flavonoid combinations [15,16,17].
The scarce intestinal absorption and bioavailability of flavonoids, when given through food or in pills, may be enhanced by the use of new drug delivery strategies [96]. In fact, since flavonoids have some drawbacks after oral administration such as low stability, bioavailability and bio-efficacy, researchers are developing biocompatible nanomaterial synthesis as novel delivery systems (including nanospheres, nano-capsules, micro and nano-emulsions, micelles, solid lipid nanoparticles and capsules), for overcoming the delivery challenges of flavonoids in the biomedical sector. Phytochemical-nanomaterial complexes can represent innovative drug delivery strategies (alongside those already known) for new antiviral therapies against the seven Baltimore virus classes [97].
Interestingly, three patents regarding the antiviral effects of flavonoids (US 7,998,937; EP1245230; US 6,399,654) have been already assigned to the Korea Research Institute of Bioscience and Biotechnology and Advanced Life Sciences Inc. [35].
However, an important step that must be achieved is to obtain the authorization of novel food including flavonoids with antiviral properties, following regulation EU 2015/2283 [98]. Nowadays, this authorization has been given only to hydroxytyrosol [89] and few other nutrients. If the antiviral efficacy and safety of flavonoids and their mixtures can be clearly demonstrated in vivo, it would be possible to obtain European Food Safety Authority (EFSA) authorization of novel foods, in order to provide new natural tools for preventing and facing outbreaks of viral infections. Indeed, when flavonoids are administered through nano-sized delivery systems, they show increased stability and bioavailability with an enhanced and prolonged activity. However, the in vivo behavior and the antiviral actions of these nano-delivery systems are still under experimental evaluation.
Author Contributions
Writing—original draft preparation: P.N., E.S.S.; writing—review and editing P.N.; M.M.; E.S.S.; A.A.; supervision: P.N.; M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Urbino Carlo Bo. APC: funded by University of Urbino Carlo Bo.
Acknowledgments
The authors want to thank University of Urbino Carlo Bo for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish this manuscript.
Abbreviations
| AhR | Aryl Hydrocarbon Receptor |
| DENV-2 | Dengue Virus-2 |
| EC50 | 50% Effective Concentration |
| EFSA | European Food Safety Authority |
| EGCG | Epigallocatechin-3-gallate |
| HAV | Human Hepatitis A Virus |
| HBV | Human Hepatitis B Virus |
| HCV | Human Hepatitis C Virus |
| HDV | Human Hepatitis D virus |
| HIV | Human Immunodeficiency Virus |
| HSP70 | Heat Shock Protein 70 |
| HSV-1 | Herpes Simplex Virus type-1 |
| IC50 | 50% Inhibitory Concentration |
| IFN-β | Interferon-β |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IRES | Internal Ribosome Entry Site |
| IRF3 | Interferon regulatory factor 3 |
| IRF7 | Interferon regulatory factor 7 |
| MDCK | Madin-Darby Canine Kidney |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric Oxide |
| NOX4 | NADPH Oxidase 4 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NS1 | Non Structural protein 1 |
| NS2 | Non Structural protein 2 |
| NS3 | Non Structural protein 3 |
| NS5A | Non Structural protein 5A |
| NS5B | Non Structural protein 5B |
| NTCP | sodium taurocholate cotransporting polypeptide |
| P protein | RSV phosphoprotein |
| PA | Polymerase Acidic subunit |
| PB1 | Protein Binding 1 subunit |
| PB2 | Protein Binding 2 subunit |
| PLA | Poly (d,l-Lactide) |
| Q-CNLC | Quercetin-loaded Cationic Nanostructure Lipid Carrier |
| RBCs | Red Blood Cells |
| RdDp | RNA-dependent DNA polymerase |
| RdRp | RNA-dependent RNA polymerase |
| ROS | Radical Oxygen Species |
| RRV | Rhesus rotavirus |
| RSV | Respiratory Syncytial Virus |
| SMDDS | Self-Micro-emulsifying Drug Delivery System |
| SNEDDS | Self-Nanoemulsifying Drug Delivery System |
| STAT1 | Signal transducer and activator of transcription 1 |
| SU.VI.MAX | Supplementation en Vitamines et Mineraux Antioxydants |
| TLR3 | Toll-Like Receptor 3 |
| TLR4 | Toll-Like Receptor 4 |
| TLR7 | Toll-Like Receptor 7 |
| TNF-α | Tumor Necrosis Factor-α |
References
- Ninfali, P.; Bacchiocca, M. Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. J. Agric. Food Chem. 2003, 51, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Aluigi, G.; Bacchiocca, M.; Magnani, M. Antioxidant capacity of Extra-Virgin Olive Oils. JOACS 2001, 78, 243–247. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Kang, G.H.; Cho, S.G. Effect of flavonoids on human health: Old subjects but new challenges. Recent Pat. Biotechnol. 2007, 1, 139–150. [Google Scholar] [CrossRef]
- Papi, A.; Farabegoli, F.; Iori, R.; Orlandi, M.; De Nicola, G.R.; Bagatta, M.; Angelino, D.; Gennari, L.; Ninfali, P. Vitexin 2-O-xyloside, raphasatin and (-) eepigallocatechin-3-gallate synergistically affect cell growth and apoptosis of colon cancer cells. Food Chem. 2013, 138, 1521–1530. [Google Scholar] [CrossRef]
- Farabegoli, F.; Scarpa, E.S.; Frai, A.; Serafini, G.; Papi, A.; Spisni, E.; Antonini, E.; Benedetti, S.; Ninfali, P. Betalains increase vitexin-2-O-xyloside cytotoxicity in Caco-2 cancer cells. Food Chem. 2017, 218, 356–364. [Google Scholar] [CrossRef]
- Scarpa, E.S.; Antonini, E.; Palma, F.; Mari, M.; Ninfali, P. Antiproliferative activity of vitexin-2-O-xyloside and avenanthramides on CaCo-2 and HepG2 cancer cells occurs through apoptosis induction and reduction of pro-survival mechanisms. Eur. J. Nutr. 2018, 57, 1381–1395. [Google Scholar] [CrossRef]
- Scarpa, E.S.; Mari, M.; Antonini, E.; Palma, F.; Ninfali, P. Natural and synthetic avenathramides activate caspases 2,8,3 and downregulate hTERT, MDR1 and COX-2 genes in CaCo-2 and Hep3B cancer cells. Food Funct. 2018, 9, 2913–2921. [Google Scholar] [CrossRef]
- Antonini, E.; Iori, R.; Ninfali, P.; Scarpa, E.S. A Combination of Moringin and Avenanthramide 2f Inhibits the Proliferation of Hep3B Liver Cancer Cells Inducing Intrinsic and Extrinsic Apoptosis. Nutr. Cancer 2018, 70, 1159–1165. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.-S. C-Glycosyl Flavonoids from Beta vulgaris Cicla and Betalains from Beta vulgais rubra: Antioxidant, Anticancer, Antiinflammatory Activities—A Review. Phytother. Res. 2017, 31, 871–884. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Antonelli, A.; Biagiotti, E.; Di Gioacchino, A.M.; Piccoli, G.; Stocchi, V.; Brandi, G. Characterization and biological activity of the main flaonoids from Swiss Chard (Beta vulgaris subspecies cycla). Phytomedicine 2007, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A. Oseltamivir resistance-disabling our influenza defenses. N. Engl. J. Med. 2005, 22, 2633–2666. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A. Twenty years of research into medicinal plants: Results and perspectives. Phytochem. Rev. 2002, 1, 275–285. [Google Scholar] [CrossRef]
- Ni, L.; Zhou, L.; Zhou, M.; Zhao, J.; Wang, D.W. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front. Med. 2020, 14, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Sharma, B.; Kanwar, S.S. Antiviral Phytochemicals: An Overview. Biochem. Physiol. 2017, 6, 2–8. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effects of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Simoes, L.R.; Maciel, G.M.; Brandao, G.C.; Kroon, E.G.; Castilho, R.O.; Oliveira, A.B. Antiviral activity of Disticella elongata (Vahl) Urb. (Bignoniaceae), a potentially useful source of anti-dengue drugs from the state of Minas Gerais, Brazil. Lett. Appl. Microbiol. 2011, 53, 602–607. [Google Scholar] [CrossRef]
- Manvar, D.; Mishra, M.; Kumar, S.; Pandey, V.N. Identification and evaluation of anti hepatitis C virus phytochemicals from Eclipta alba. J. Ethnopharmacol. 2012, 144, 545–554. [Google Scholar] [CrossRef]
- Knipping, K.; Garssen, J.; van’t Land, B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol. J. 2012, 9, 137–144. [Google Scholar] [CrossRef]
- Ding, F.; Liu, J.; Du, R.; Yu, Q.; Gong, L.; Jiang, H.; Rong, R. Qualitative and Quantitative Analysis for the Chemical Constituents of Tetrastigma hemsleyanum Diels et Gilg Using Ultra-High Performance Liquid Chromatography/Hybrid Quadrupole—Orbitrap Mass Spectrometry and Preliminary Screening for Anti-Influenza Virus Components. Evid. Based Complement. Alternat. Med. 2019, 2019, 9414926. [Google Scholar] [PubMed]
- Ji, S.; Li, R.; Wang, Q.; Miao, W.J.; Li, Z.W.; Si, L.L.; Qiao, X.; Yu, S.W.; Zhou, D.M.; Ye, M. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol. 2015, 176, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.T.; Mumtaz, A.; Naseem, R.; Ali, A.; Fatima, T.; Jabbar, T.; Ahmad, Z.; Ashfaq, U.A. Molecular Docking Based Screening of Plant Flavonoids as Dengue NS1 inhibitors. Bioinformation 2014, 10, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef]
- Mirza, M.U.; Ghori, N.U.; Ikram, N.; Adil, A.R.; Manzoor, S. Pharmacoinformatics approach for investigation of alternative potential hepatitis C virus nonstructural protein 5B inhibitors. Drug Des. Devel. Ther. 2015, 9, 1825–1841. [Google Scholar] [CrossRef]
- Huang, H.C.; Tao, M.H.; Hung, T.M.; Chen, J.C.; Lin, Z.J.; Huang, C. (-)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir. Res. 2014, 111, 100–111. [Google Scholar] [CrossRef]
- Kehinde, I.; Ramharack, P.; Nlooto, M.; Gordon, M. The pharmacokinetic properties of HIV-1 protease inhibitors: A computational perspective on herbal phytochemicals. Heliyon 2019, 5, e02565. [Google Scholar] [CrossRef]
- Rehman, S.; Ashfaq, U.A.; Ijaz, B.; Riazuddin, S. Anti-hepatitis C virus activity and synergistic effect of Nymphaea alba extracts and bioactive constituents in liver infected cells. Microb. Pathog. 2018, 121, 198–209. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Haid, S.; Novodomskà, A.; Gentzsch, J.; Grethe, C.; Geuenich, S.; Bankwitz, D.; Chhatwal, P.; Jannack, B.; Hennebelle, T.; Bailleul, F.; et al. A Plant-Derived Flavonoid Inhibits Entry of All HCV Genotypes Into Human Hepatocytes. Gastroenterology 2012, 143, 213–222. [Google Scholar] [CrossRef]
- Ortega, J.T.; Suarez, A.I.; Serrano, M.L.; Baptista, J.; Pujol, F.H.; Rangel, H.R. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res. Ther. 2017, 14, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Wu, X.; Li, M.M.; Li, G.Q.; Yang, Y.T.; Luo, H.J.; Huang, W.H.; Chung, H.Y.; Ye, W.C.; Wang, G.C.; et al. Antiviral activity of polymethoxylated flavones from Guangchenpi, the edible and medicinal pericarps of Citrus reticulata “Chachi”. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Loe, M.W.; Lee, R.C.; Chu, J.J. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019, 167, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Wu, J.; Shen, Q. Characterization and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. Int. J. Pharm. 2012, 427, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phytochemicals as Antiviral Agents: Recent Updates. In Plant-Derived Bioactives, 1st ed.; Swany, M.K., Ed.; Springer Nature: Singapore, 2020; Volume 12, pp. 279–295. [Google Scholar]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012, 19, 81–88. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Shi, D.; Chen, M.; Liu, L.; Wang, Q.; Liu, S.; Wang, L.; Wang, R. Anti-influenza A virus mechanism of three representative compounds from Flos Trollii via TLRs signaling pathways. J. Ethnopharmacol. 2020, 253, 112634. [Google Scholar] [CrossRef]
- Ahmed-Belkacem, A.; Guichou, J.F.; Brillet, R.; Ahnou, N.; Hernandez, E.; Pallier, C.; Pawlotsky, J.M. Inhibition of RNA binding to hepatitis C virus RNA-dependent RNA polymerase: A new mechanism for antiviral intervention. Nucleic Acid Res. 2014, 42, 9399–9409. [Google Scholar] [CrossRef]
- Rehman, S.; Ijaz, B.; Fatima, N.; Muhammad, S.A.; Riazuddin, S. Therapeutic potential of Taraxacum officinale against HCV NS5B polymerase: In-vitro and In silico study. Biomed. Pharmacother. 2016, 83, 881–891. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Arumugaswani, V.; Raychauduri, S.; Yeh, G.K.; Maloney, E.M.; Wang, J.; Dasgupta, A.; French, S.W. Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology 2012, 433, 346–355. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina speciosa Extract, Fractions, and the Major Compound. Chem. Biodivers. 2020, 17, e1900511. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hattori, T.; Kodama, E.N. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antivir. Chem. Chemother. 2011, 4, 239–243. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 5, 5452–5459. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, J.H.; Kwon, D.H. Quercetin-3-rhamnoside exerts antiinfluenza A virus activity in mice. Phytother. Res. 2012, 26, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Virology Blog: About Viruses and Viral Disease. Available online: https://www.virology.ws/2014/12/10/how-influenza-virus-infection-might-lead-to-gastrointestinal-symptoms/ (accessed on 1 June 2020).
- Glanz, V.Y.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Inhibition of sialidase activity as a therapeutic approach. Drug Des. Dev. Ther. 2018, 12, 3431–3437. [Google Scholar] [CrossRef]
- Iwai, Y.; Murakami, K.; Gomi, Y.; Hashimoto, T.; Asakawa, Y.; Okuno, Y.; Ishikawa, T.; Hatakeyama, D.; Echigo, N.; Kuzuhara, T. Anti-influenza activity of marchantins, macrocyclic bisbibenxyls contained in liverworts. PLoS ONE 2011, 6, e19825. [Google Scholar] [CrossRef]
- Hepatitis C Virus. Available online: https://en.wikipedia.org/wiki/Hepatitis_C_virus (accessed on 1 June 2020).
- HBV-GLUE: A Sequence Data Resource for Hepatitis B Virus. Available online: http://hbv-glue.cvr.gla.ac.uk/#/home (accessed on 1 June 2020).
- Corriere Nazionale: I 5 Virus Che Spaventano La Scienza. Available online: https://www.corrierenazionale.it/2020/02/09/i-5-virus-che-spaventano-la-scienza/ (accessed on 1 June 2020).
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell. Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Iriti, M.; Setzer, W.N.; Sharifi-Rad, M.; Roointan, A.; Salehi, B. Antiviral activity of Veronica Persica Poir. on herpes virus infection. Cell. Mol. Biol. 2018, 64, 11–17. [Google Scholar] [CrossRef]
- Lembo, D.; Cavalli, R. Nanoparticulate delivery systems for antiviral drugs. Antivir. Chem. Chemother. 2010, 21, 53–70. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Gennari, L.; Angelino, D.; Ninfali, P.; Castagnino, E. ORAC of chitosan and its derivatives. Food Hydrocoll. 2012, 28, 243–247. [Google Scholar] [CrossRef]
- Sims, K.R.; He, B.; Koo, H.; Benoit, D.S.W. Electrostatic Interactions Enable Nanoparticle Delivery of the Flavonoid Myricetin. ACS Omega 2020, 28, 12649–12659. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery system (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradble nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, S.; Liu, C.; Yang, C.; Dou, J.; Li, L.; Zhai, G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A 2013, 429, 24–30. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: A green approach. PLoS ONE 2012, 7, e41230. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Magnani, M.; Balestra, E.; Fraternale, A.; Aquaro, S.; Paiardini, M.; Cervasi, B.; Casabianca, A.; Garaci, E.; Perno, C.F. Drug-loaded red blood cell-mediated clearance of HIV-1 macrophage reservoir by selective inhibition of STAT1 expression. J. Leukoc. Biol. 2003, 74, 764–771. [Google Scholar] [CrossRef]
- Jeewantha, H.M.A.; Slivkin, A.I. The terpene-indole alkaloids loaded erythrocytes as a drug carrier: Design and assessment. Clin. Pharmacol. 2018, 7, e0406. [Google Scholar] [CrossRef]
- Trineeva, O.V.; Khalahakun, A.D. Study of desorbtion and exemption of terpeno-indole alkaloids of vinkristin and vinblastin from erythrocitary cell carriers. Drug Dev. Regist. 2019, 8, 16–21. [Google Scholar]
- Blasa, M.; Angelino, D.; Gennari, L.; Ninfali, P. The cellular antioxidant activity in red blood cells (CAA-RBC): A new approach to bioavailability and synergy of phytochemicals and botanical extracts. Food Chem. 2011, 125, 685–691. [Google Scholar] [CrossRef]
- Asgary, S.; Naderi, G.H.; Askari, N. Protective effect of flavonoids against red blood cell hemolysis by free radicals. Exp. Clin. Cardiol. 2005, 2, 10. [Google Scholar]
- Hou, L.; Zhou, B.; Yang, L.; Liu, Z.L. Inhibition of free radical initiated peroxidation of human erythrocyte ghosts by flavonols and their glycosides. Org. Biomol. Chem. 2004, 2, 1419–1423. [Google Scholar] [CrossRef]
- Fiorani, M.; Accorsi, A.; Cantoni, O. Human Red Blood Cell sas a Natural Flavonoid Reservoir. Free Radic. Res. 2003, 37, 1331–1338. [Google Scholar] [CrossRef]
- Serafini, S.; Rossi, L.; Antonelli, A.; Fraternale, A.; Cerasi, A.; Crinelli, R.; Chiarantini, L.; Schiavano, G.F.; Magnani, M. Drug delivery through phagocytosis of red blood cells. Transfus. Med. Hemotherapy 2004, 31, 92–101. [Google Scholar] [CrossRef]
- Lalani, S.; Poh, C.L. Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses 2020, 12, 184. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Magnani, M. Engineering erythrocytes for the modulation of drugs’ and contrasting agents’ pharmacokinetics and biodistribution. Adv. Drug Deliv. Rev. 2016, 106, 73–87. [Google Scholar]
- Magnani, M.; Serafini, S.; Fraternale, A.; Antonelli, A.; Biagiotti, S.; Pierigè, F.; Sfara, C.; Rossi, L. Red blood cell-based delivery of drugs and nanomaterials for therapeutic and diagnostic applications. In Encyclopedia of Nanoscience and Nanotechnology, 1st ed.; Nalwa, H.S., Ed.; ASP: Brussels, Belgium, 2011; Volume 22, pp. 309–354. [Google Scholar]
- Villa, C.H.; Pan, D.C.; Zaitsev, S.; Cines, D.B.; Siegel, D.L.; Muzykantov, V.R. Delivery of drugs bound to erythrocytes: New avenues for an old intravascular carrier. Ther. Deliv. 2015, 6, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.; Peiretti, E.; Magnani, M.; Rossi, L.; Serafini, S.; Gryczynski, Z.; Gryczynski, I. Observation of erythrocyte dynamics in the retinal capillaries and choriocapillaris using ICG-loaded erythrocyte ghost cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5510–5516. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.H.; Cines, D.B.; Siegel, D.L.; Muzykantov, V. Erythrocytes as carriers for drug delivery in blood transfusion and beyond. Transfus. Med. Rev. 2017, 31, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S. The potential impact of gut microbiota on your health: Current status and future challenges. Asian Pac. J. Allergy Immunol. 2016, 34, 429–464. [Google Scholar]
- Gibson, A.; Edgar, J.D.; Neville, C.E.; Gilchrist, S.; McKinley, M.C.; Patterson, C.; Young, I.S.; Woodside, J.V. Effect of fruit and vegetable consumption on immune function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1429–1436. [Google Scholar] [CrossRef]
- Koper, J.E.B.; Loonen, L.M.P.; Wells, J.M.; Troise, A.D.; Capuano, E.; Fogliano, V. Polyphenols and Tryptophan Metabolites Activate the Aryl Hydrocarbon receptor in an in vitro Model of Colonic Fermentation. Mol. Nutr. Food Res. 2019, 63, e1800722. [Google Scholar] [CrossRef]
- Dai, W.; Bi, J.; Li, F.; Wang, S.; Huang, X.; Meng, X.; Sun, B.; Wang, D.; Kong, W.; Jiang, C.; et al. Antiviral Efficacy of Flavonoids Against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses 2019, 11, 625. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, L.; You, Q.; Yang, Y.; Gu, H.; Song, G.; Lu, N.; Xin, J. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antivir. Res. 2007, 74, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, Y.; Gao, C.; Li, Y.; Chen, S.; Xiong, T.; Li, J.; Du, M.; Gong, Z.; Chen, H.; et al. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2014, 115, 125–131. [Google Scholar] [CrossRef]
- EC 2017. EC Regulation 258/97. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04728. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is There Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- Perez-Jimenez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary Intake of 337 Polyphenols in French Adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing Dentate Gyrus Function With Dietary Flavanols Improves Cognition in Older Adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of Flavonoid Ability to Cross the Blood-Brain Barrier of Rats by Co-Administration With α-tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Sanjuan, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids loaded in Nanocarriers: An Opportunity to Increase Oral Bioavailability and Bioefficacy. Food Nutr. Sci. 2014, 5, 1212–1227. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Botezat, D.; Gardikiotis, I.; Uritu, C.M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.I.; Mihai, C.T. Nanomaterials Designed for Antiviral Drug Delivery Transport across Biological Barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- EU 2015. Commission Regulation (EU) 2015/2283. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. OJL 2015, 327, 1–22. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).